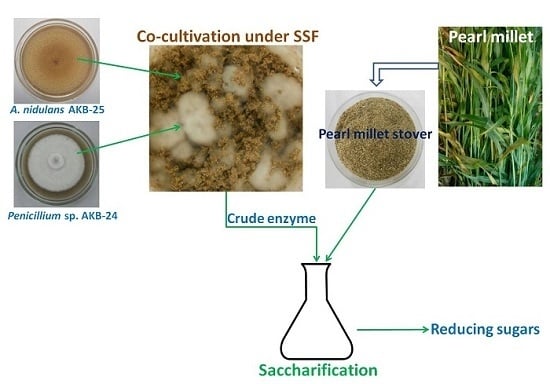
Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Fungi
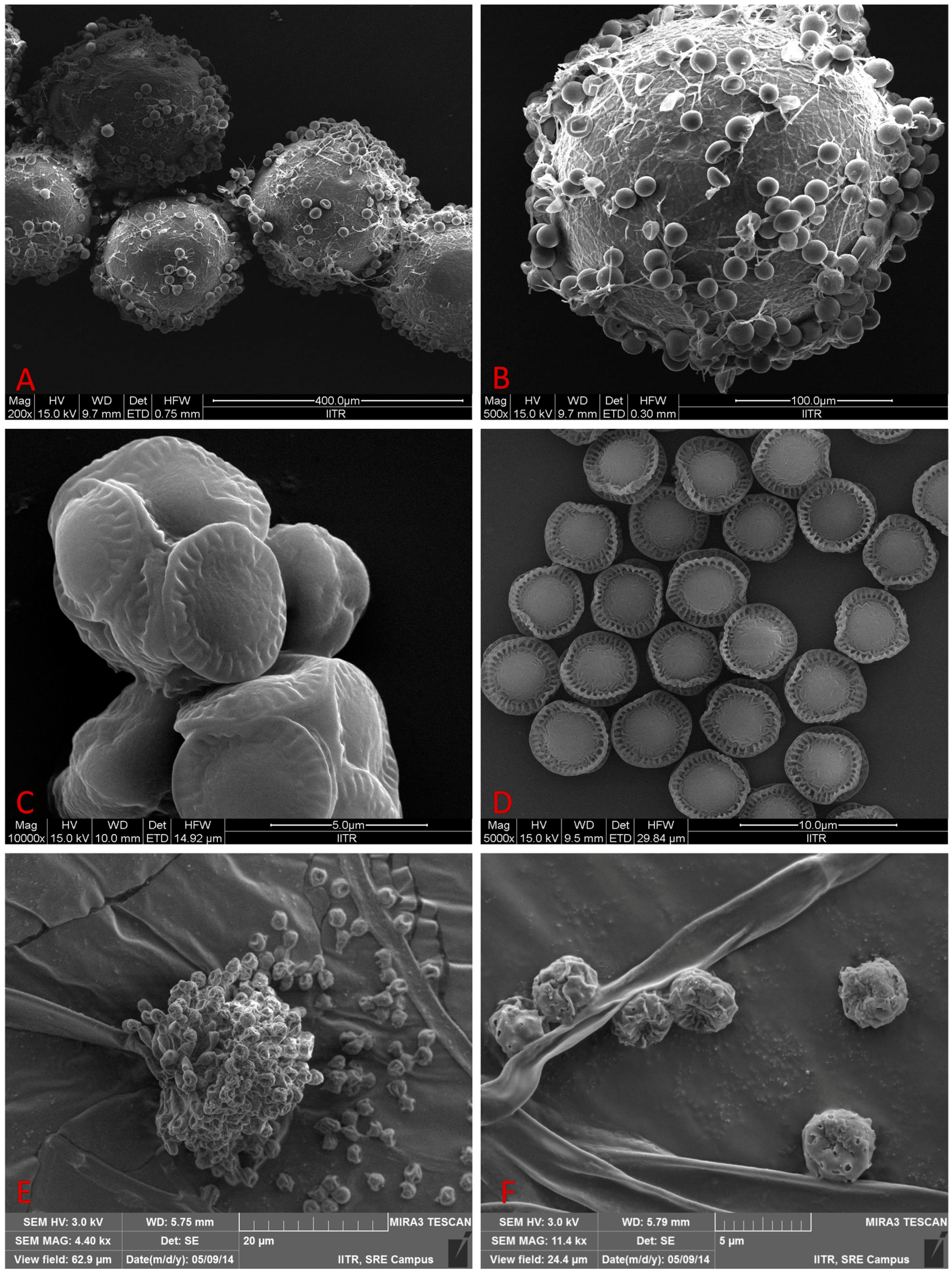
2.2. FE-SEM Analysis of Selected Fungal Strains
2.3. Preparation of Substrates
2.4. Enzyme Production under SSF
2.5. Optimization of Cultural Conditions
2.6. Enzyme Production with Co-cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25
2.7. Saccharification Studies
2.8. Enzyme Assays
2.9. Statistical Analysis
3. Results and Discussion
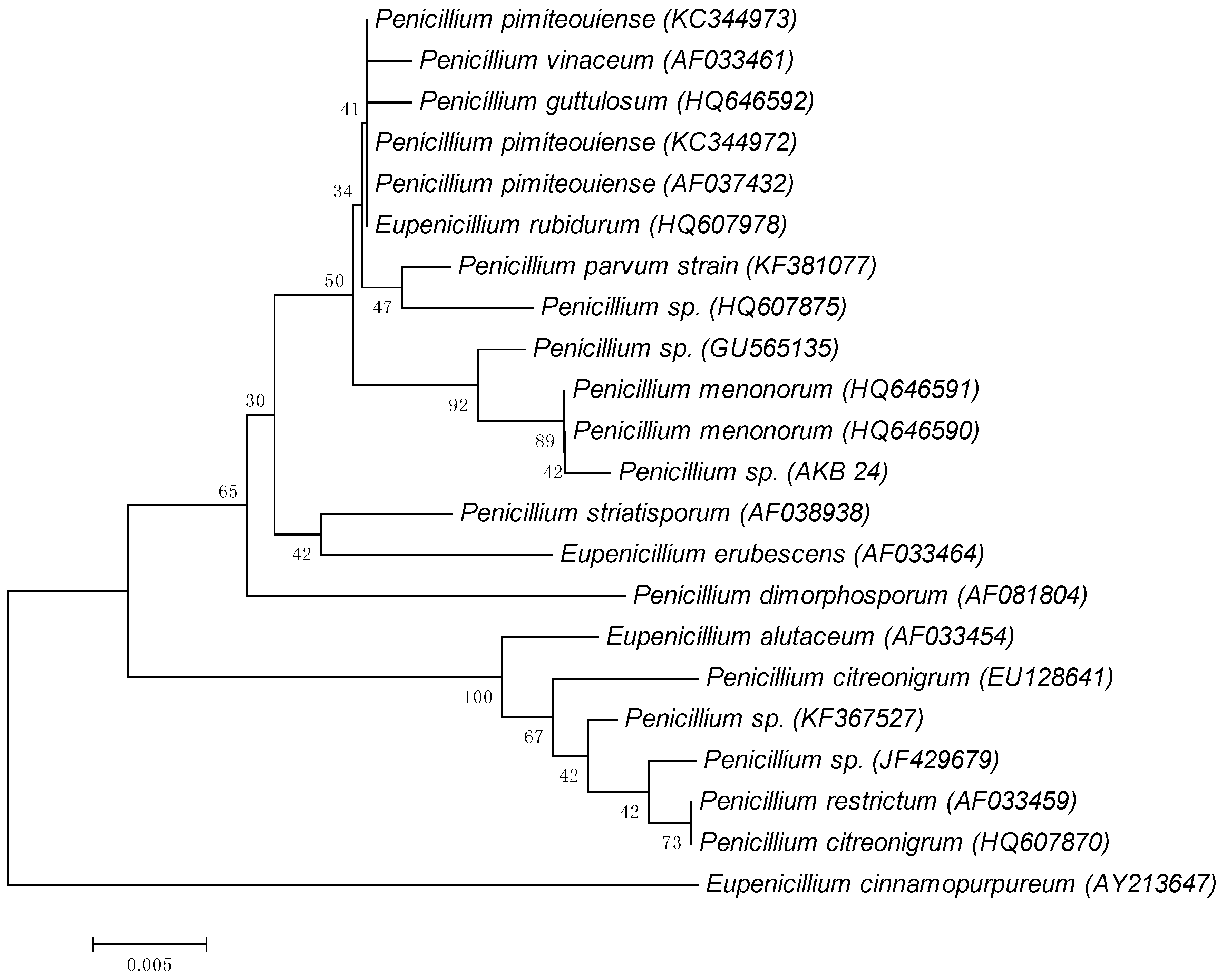
3.1. Identification of Microorganisms
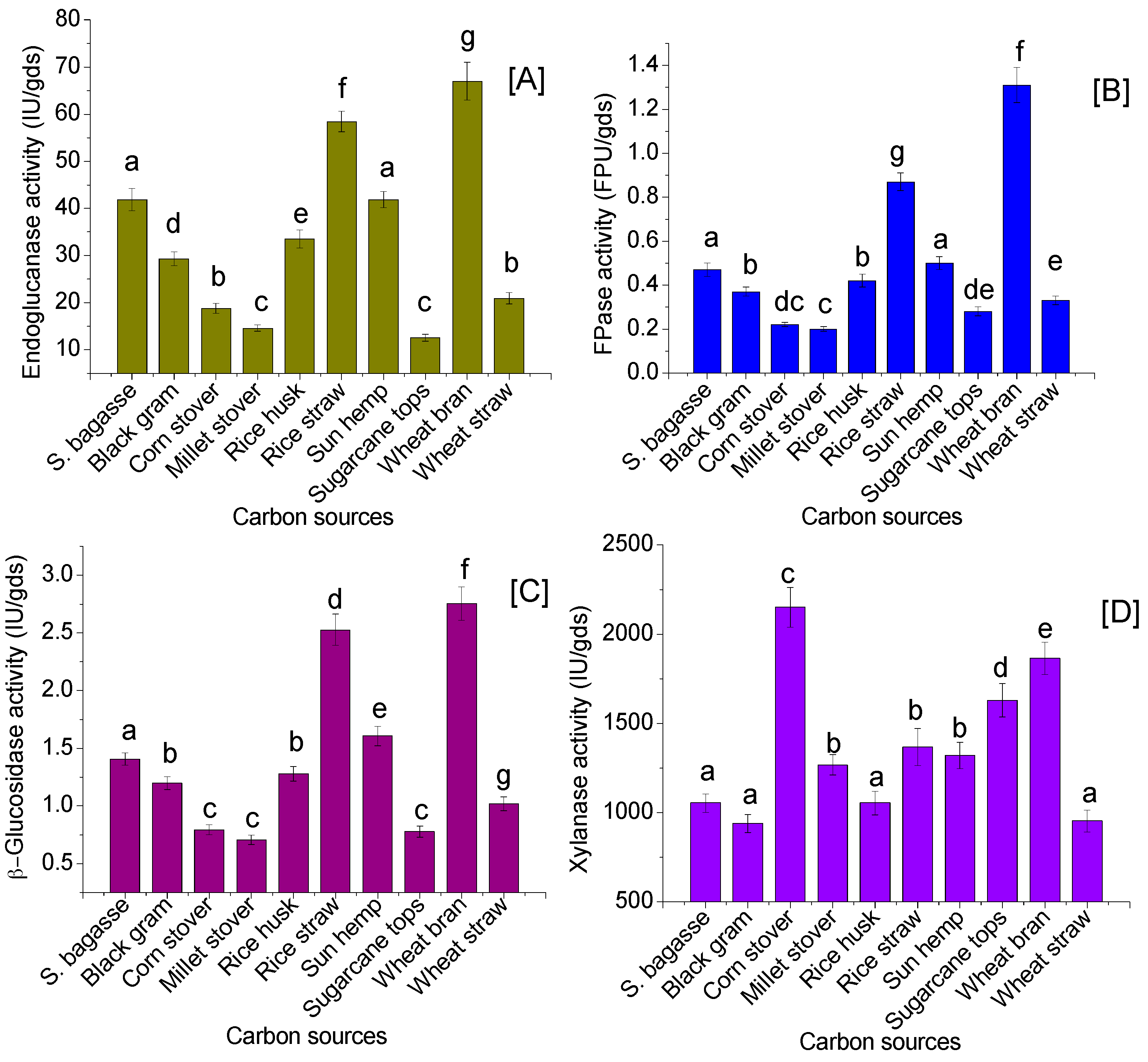
3.2. Agricultural Residues as the Carbon Sources for Enzyme Production
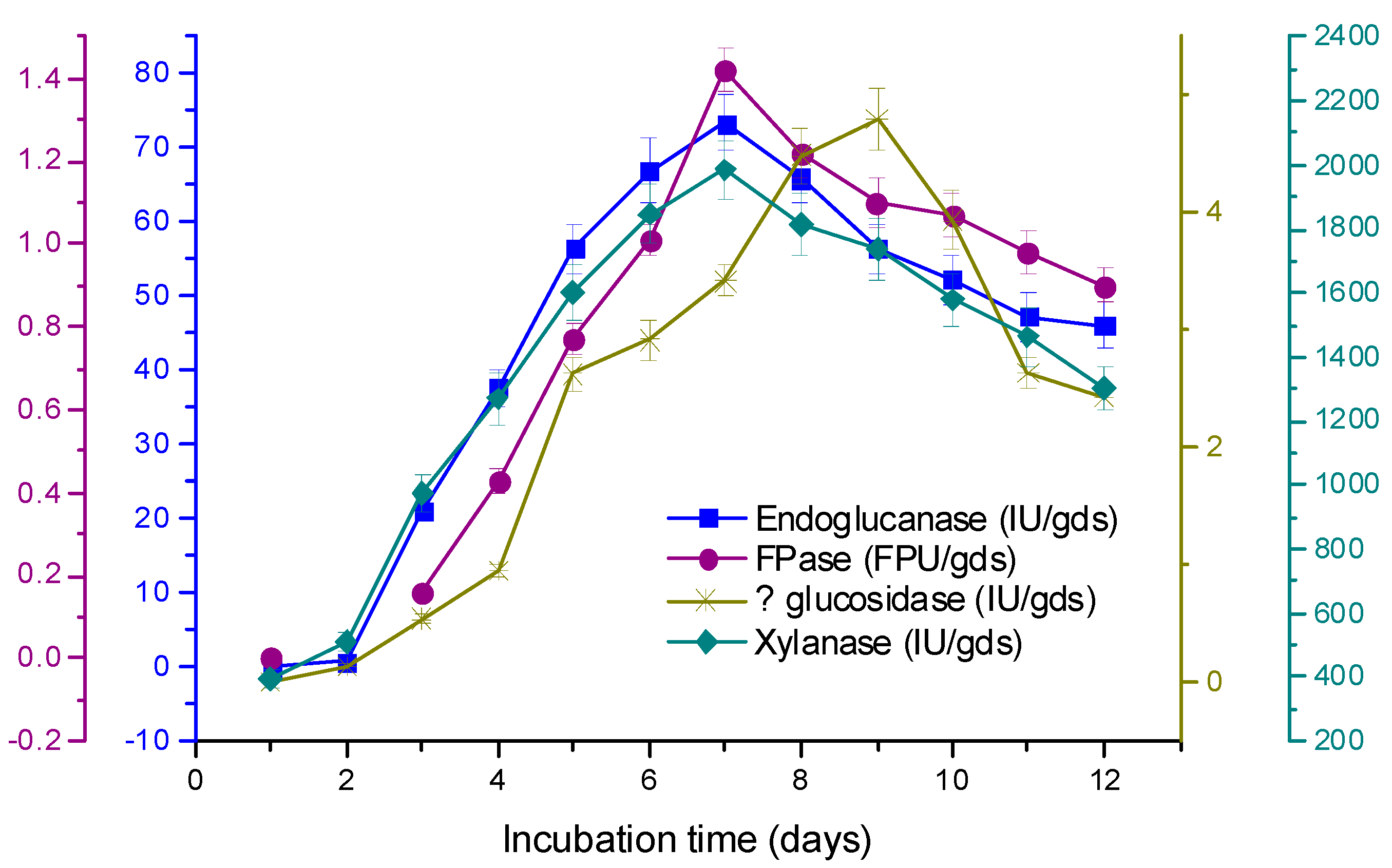
3.3. Time Course of Enzyme Production
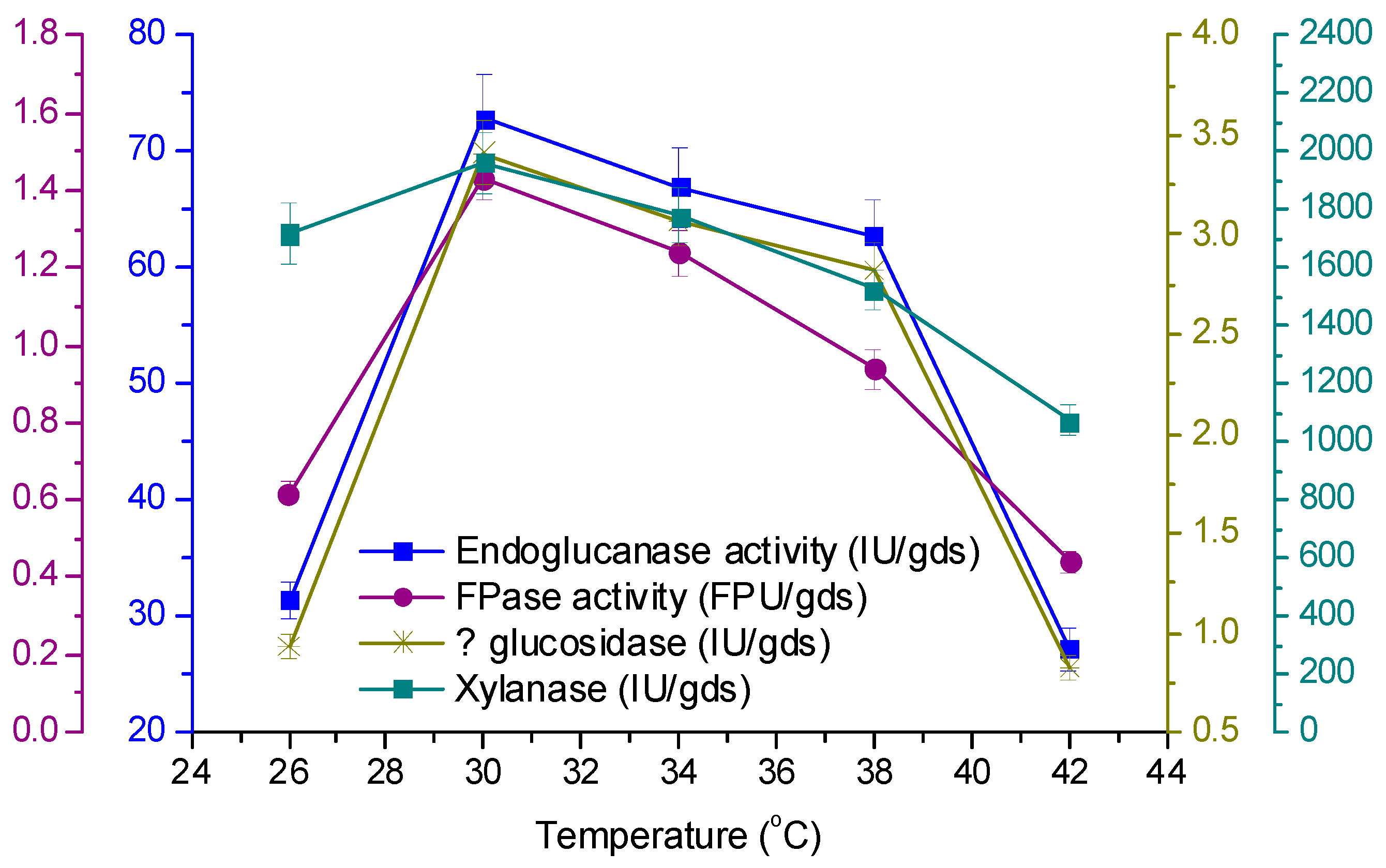
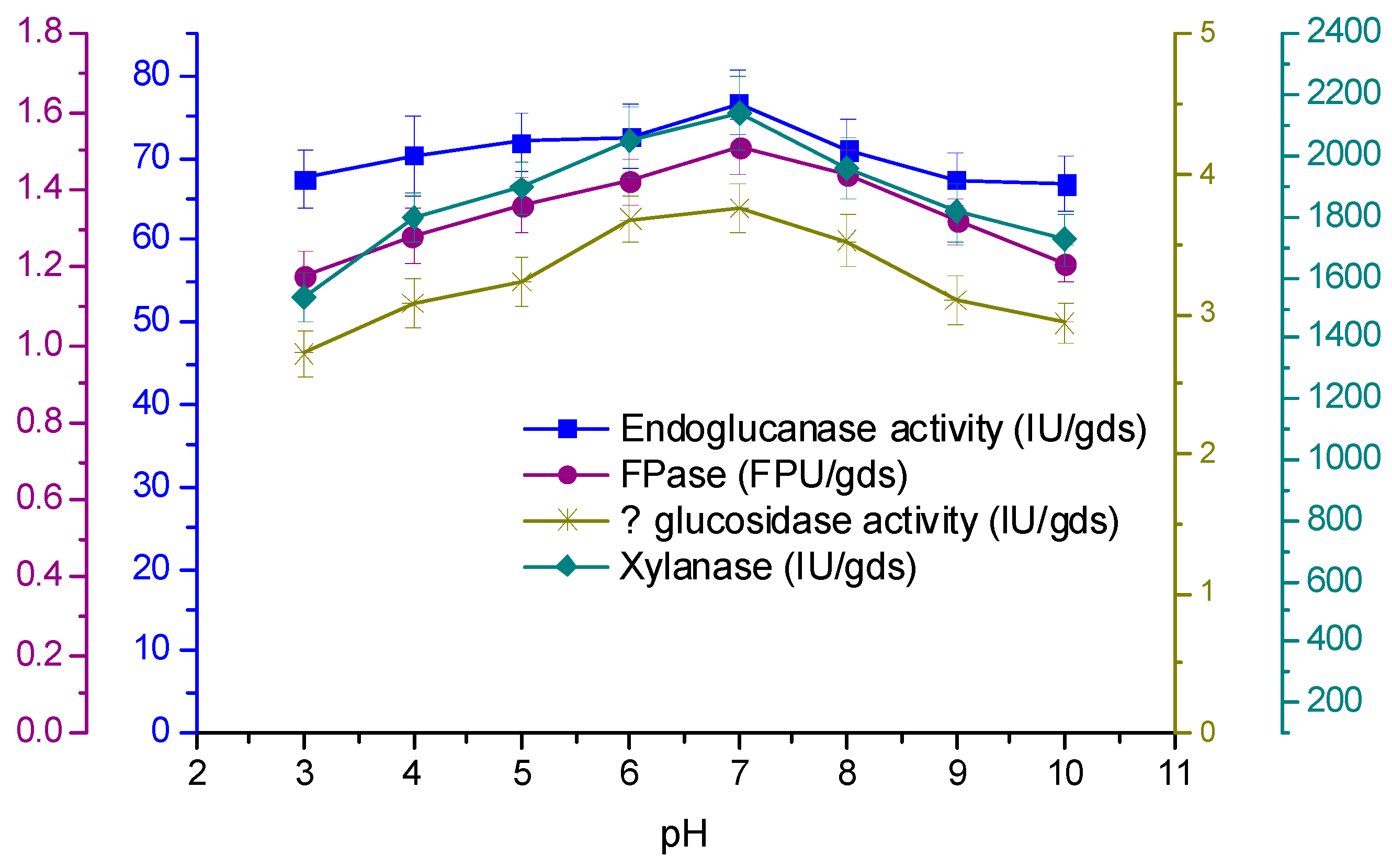
3.4. Effect of Temperature and Initial pH on Enzyme Production
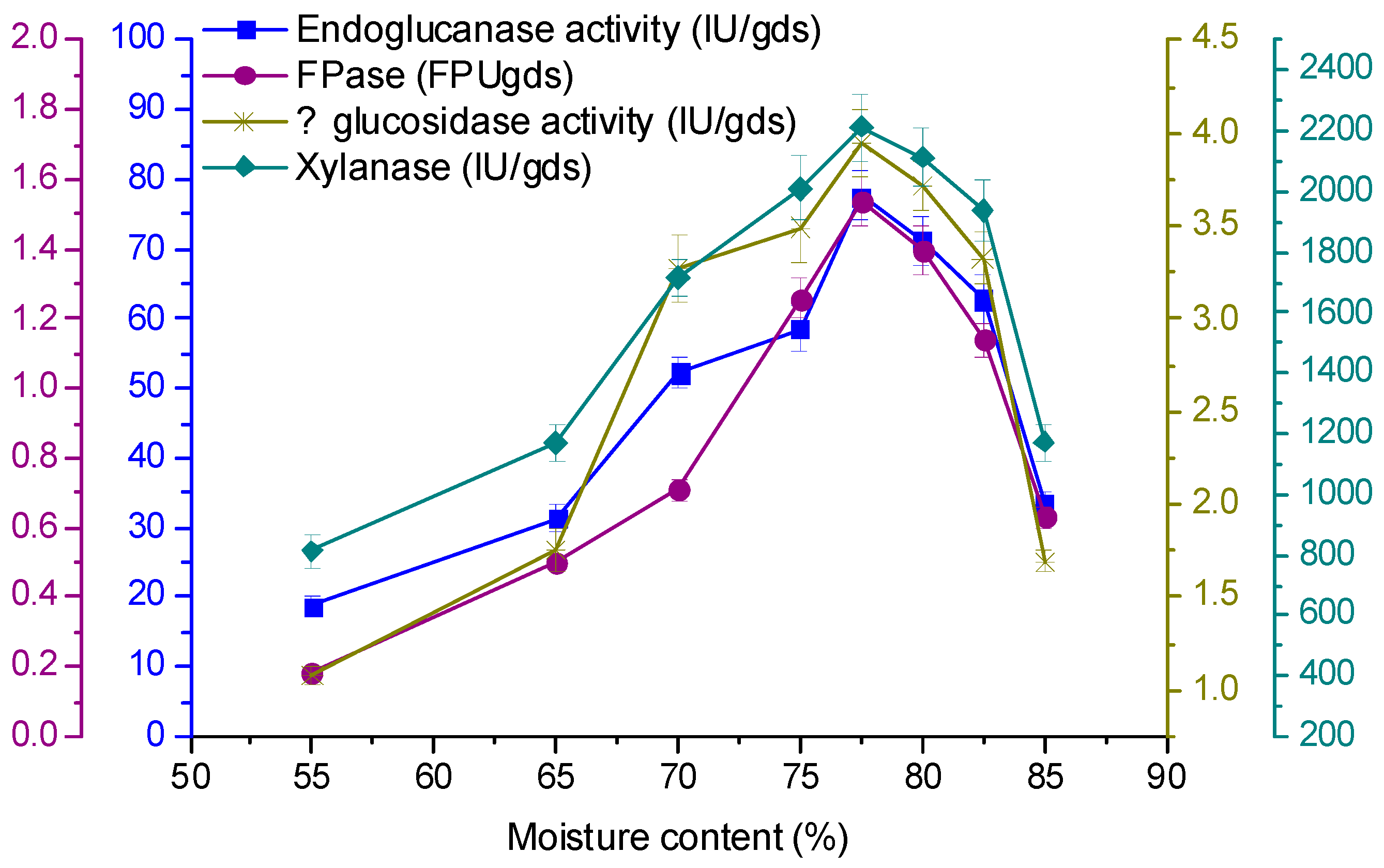
3.5. Effect of Initial Moisture Content on Enzyme Production
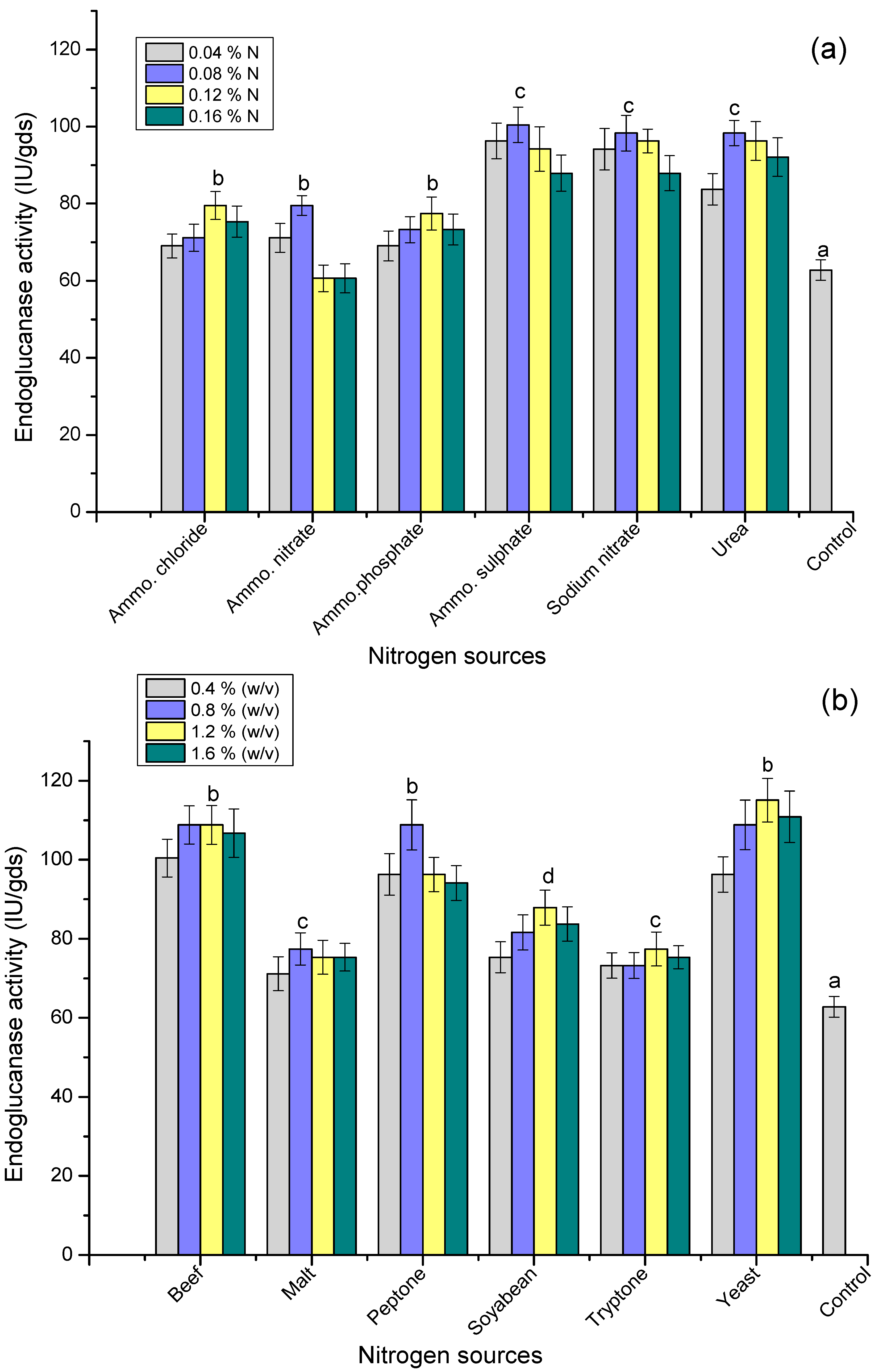
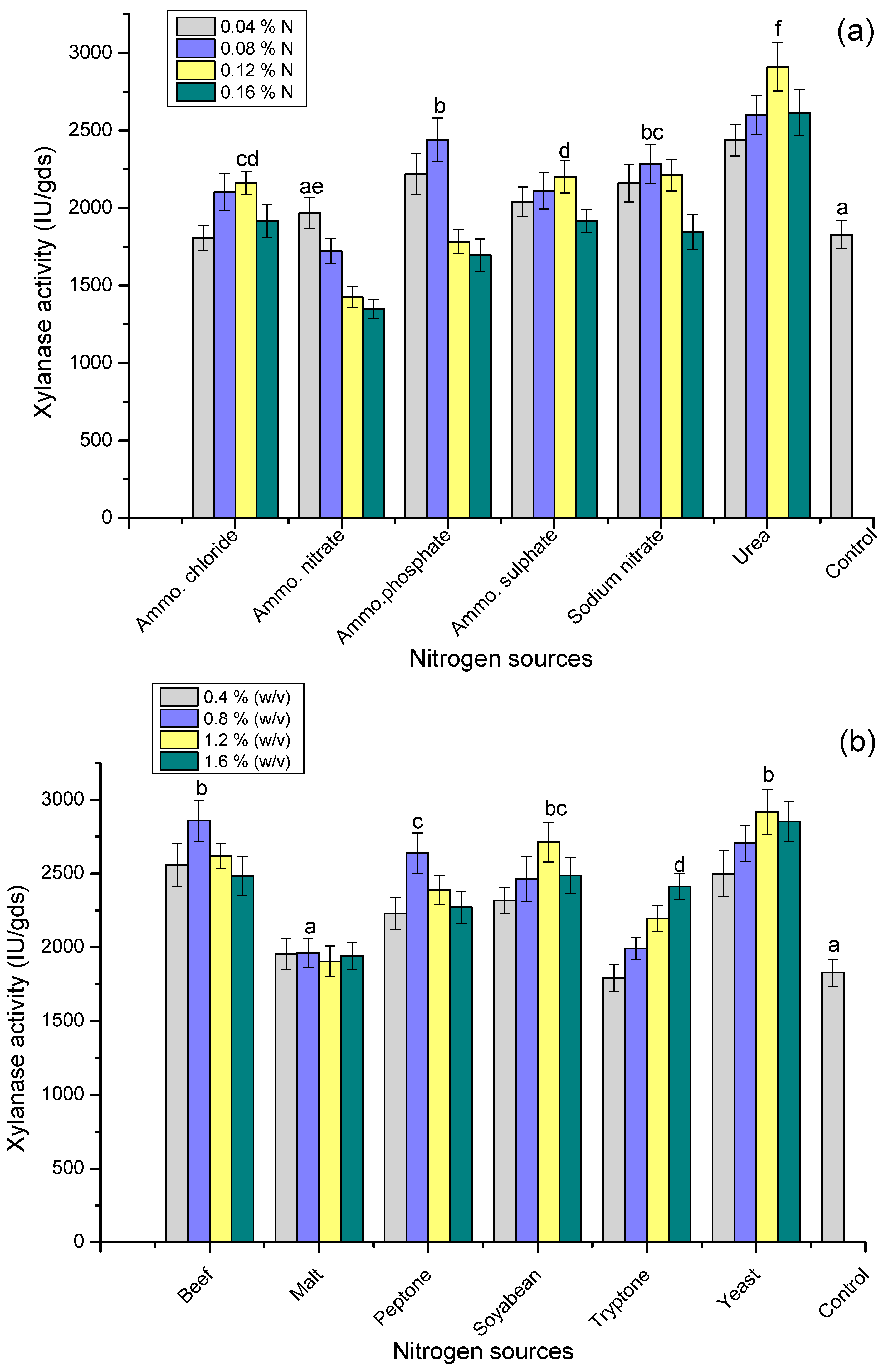
3.6. Effect of Nitrogen Sources
3.7. Effect of Surfactants
3.8. Improvement in Enzyme Production by Co-Cultivation
3.9. Saccharification by Crude Enzyme from Co-Culture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saini, R.; Saini, J.K.; Adsul, M.; Patel, A.K.; Mathur, A.; Tuli, D.; Singhania, R.R. Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresour. Technol. 2015, 188, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocelluloses. Enzyme Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Gregg, D.; Saddler, J.N. Factors affecting cellulose hydrolysis and the potential of enzyme recycle to enhance the efficiency of an integrated wood to ethanol process. Biotechnol. Bioeng. 1996, 51, 375–383. [Google Scholar] [CrossRef]
- Thompson, D.N.; Chen, H.-C. Comparison of pretreatment methods on the basis of available surface area. Bioresour. Technol. 1992, 39, 155–163. [Google Scholar] [CrossRef]
- Sanchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Detroy, R.W.; Stjulian, G. Biomass conversion—Fermentation chemicals and fuels. Crit. Rev. Microbiol. 1983, 10, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.T.; Cherry, J. Progress and challenges in enzyme development for biomass utilization. Adv. Biochem. Eng. Biotechnol. 2007, 108, 95–120. [Google Scholar] [PubMed]
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Delabona, P.d.S.; Pirota, R.D.P.B.; Codima, C.A.; Tremacoldi, C.R.; Rodrigues, A.; Farinas, C.S. Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass Bioenerg. 2012, 37, 243–250. [Google Scholar] [CrossRef]
- Gupte, A.; Madamwar, D. Solid-state fermentation of lignocellulosic waste for cellulase and β-glucosidase production by cocultivation of Aspergillus ellipticus and Aspergillus fumigatus. Biotechnol. Progress 1997, 13, 166–169. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenerg. 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Brijwani, K.; Oberoi, H.S.; Vadlani, P.V. Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 2010, 45, 120–128. [Google Scholar] [CrossRef]
- De Carvalho, M.L.A.; Carvalho, D.F.; Gomes, E.B.; Maeda, R.N.; Anna, L.M.M.S.; Castro, A.M.; Pereira, J.N. Optimisation of cellulase production by Penicillium funiculosum in a stirred tank bioreactor using multivariate response surface analysis. Enzyme Res. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Howard, R.L.; Abotsi, E.; Rensburg, J.V.E.L.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Oberoi, H.S.; Kaur, S.; Bansal, S.; Brar, S.K. Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind. Crop Prod. 2011, 34, 1160–1167. [Google Scholar] [CrossRef]
- Dutt, D.; Kumar, A. Optimization of cellulase production under solid-state fermentation by Aspergillus flavus (AT-2) and Aspergillus niger (AT-3) and its impact on stickies and ink particle size of sorted office paper. Cellul. Chem. Technol. 2014, 48, 285–298. [Google Scholar]
- Cheng, Q.; Wang, J.; McNeel, J.; Jacobson, P. Water retention value measurements of cellulosic materials using a centrifuge technique. Bioresources 2010, 5, 1945–1954. [Google Scholar]
- Botkova, M.; Šutý, S.; Jablonský, M.; Kucerkova, L.; Vrška, M. Monitoring of kraft pulps swelling in water. Cellul. Chem. Technol. 2013, 47, 95–102. [Google Scholar]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid-state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Production of cellulases and xylanases and their applications in industrial processes. Ph.D. Thesis, Department of Paper Technology, IIT Roorkee, Roorkee, India, 2015. [Google Scholar]
- Miller, G.L. Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Wood, T.M.; Bhat, K.M. Methods for measuring cellulase activities. Methods Enzymol. 1988, 160, 87–112. [Google Scholar]
- Kumar, A.; Gautam, A.; Dutt, D. Screening of fungal resources for the production of cellulases and xylanases. Br. Biotechnol. J. 2015, 9, 1–13. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Brijwani, K.; Vadlani, P.V. Cellulolytic enzymes production via solid-state Fermentation: Effect of pretreatment methods on physicochemical characteristics of substrate. Enzyme Res. 2011, 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.L.; Veeken, A.H.M.; de Wilde, V.; Hamelers, H.V.M. Air-filled porosity and permeability relationships during solid-state fermentation. Biotechnol. Progr. 2004, 20, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Rahardjo, Y.S.P.; Jolink, F.; Haemers, S.; Tramper, J.; Rinzema, A. Significance of bed porosity, bran and specific surface area in solid-state cultivation of Aspergillus oryzae. Biomol. Eng. 2005, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Delabona, P.dS.; Pirota, R.D.P.B.; Codima, C.A.; Tremacoldi, C.l.R.; Rodrigues, A.; Farinas, C.S. Effect of initial moisture content on two Amazon rainforest Aspergillus strains cultivated on agro-industrial residues: Biomass-degrading enzymes production and characterization. Ind. Crop Prod. 2013, 42, 236–242. [Google Scholar] [CrossRef]
- Ellouz, C.S.; Belguith, H.; Hassairi, I.; M’Rad, K.; Ellouz, R. Optimization of cellulase production by Penicillium occitanis. Appl. Microbiol. Biotechnol. 1995, 43, 267–269. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Dornez, E.; Gebruers, K.; Wiame, S.; Delcour, J.A.; Courtin, C.M. Insight into the distribution of arabinoxylans, endoxylanases, and endoxylanase inhibitors in industrial wheat roller mill streams. J. Agric Food Chem. 2006, 54, 8521–8529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sang, Q. Statistical optimization of cellulases production by Penicillium chrysogenum QML-2 under solid-state fermentation and primary application to chitosan hydrolysis. World J. Microbiol. Biotechnol. 2012, 28, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Shendye, A.; Rao, M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 1999, 23, 411–456. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, S.; Wei, Z.; Shen, Q.; Xu, Y. Insights into high-efficiency lignocellulolytic enzyme production by Penicillium oxalicum GZ-2 induced by a complex substrate. Biotechnol. Biofuels 2014, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Todero Ritter, C.E.; Camassola, M.; Zampieri, D.; Silveira, M.M.; Dillon, A.J.P. Cellulase and xylanase production by Penicillium echinulatum in submerged media containing cellulose amended with sorbitol. Enzyme Res. 2013, 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Soni, R.; Janveja, C.; Soni, S.K. Production of xylanase-cellulase complex by Bacillus subtilis NS7 for the biodegradation of agro-waste residues. Lignocellulose 2012, 1, 196–209. [Google Scholar]
- Sehnem, N.; de Bittencourt, L.; Camassola, M.; Dillon, A.P. Cellulase production by Penicillium echinulatum on lactose. Appl. Microbiol. Biotechnol. 2006, 72, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhao, C.; Song, X.Y. Optimization of enzymatic hydrolysis of steam-exploded corn stover by two approaches: Response surface methodology or using cellulase from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger NL02. Bioresour. Technol. 2010, 101, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Sabu, A.; Sarita, S.; Pandey, A.; Bogar, B.; Szakacs, G.; Soccol, C.R. Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl. Biochem. Biotechnol. 2002, 102–103, 251–260. [Google Scholar] [CrossRef]
- Ng, I.S.; Li, C.-W.; Chan, S.-P.; Chir, J.-L.; Chen, P.T.; Tong, C.-G.; Yu, S.-M.; Ho, T.-H.D. High-level production of a thermoacidophilic β-glucosidase from Penicillium citrinum YS40-5 by solid-state fermentation with rice bran. Bioresour. Technol. 2010, 101, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Luo, Z.-Y.; Long, C.-N.; Wang, H.-D.; Long, M.-N.; Hu, Z. Cellulase production in a new mutant strain of Penicillium decumbens ML-017 by solid state-fermentation with rice bran. New Biotechnol. 2011, 28, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Mamma, D.; Kourtoglou, E.; Christakopoulos, P. Fungal multienzyme production on industrial by-products of the citrus-processing industry. Bioresour. Technol. 2008, 99, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Dutt, D.; Tyagi, C.H. Production of novel alkali-thermo-tolerant cellulase-poor xylanases from Coprinopsis Cinerea HK-1 NFCCI-2032. Bioresources 2011, 6, 1376–1391. [Google Scholar]
- Maeda, R.; da Silva, M.; Santa Anna, L.M.M.; Pereira, N.J. Nitrogen source optimization for cellulase production by Penicillium funiculosum, using a sequential experimental design methodology and the desirability function. Appl. Biochem. Biotechnol. 2010, 161, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Adsul, M.G.; Terwadkar, A.P.; Varma, A.J.; Gokhale, D. Cellulases from Penicillium janthinellum mutants: Solid-state production and their stability in ionic liquids. Bioresources 2009, 4, 1670–1681. [Google Scholar]
- Pardo, A. Effect of surfactants on cellulase production by Nectria catalinensis. Curr. Microbiol. 1996, 33, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Niranjane, A.P.; Madhou, P.; Stevenson, T.W. The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantea. Enzyme Microb. Technol. 2007, 40, 1464–1468. [Google Scholar] [CrossRef]
- Vu, V.H.; Pham, T.A.; Kim, K. Improvement of fungal cellulase production by mutation and optimization of solid state-fermentation. Mycobiology 2011, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Suprabha, N.G.; Shashidhar, S. Media engineering for the production of cellulase from Penicillium species (SBSS 30) under solid state-fermentation. Biotechnol. Bioinf. Bioeng. 2011, 1, 343–249. [Google Scholar]
- Pirota, R.P.B.; Delabona, P.; Farinas, C. Simplification of the biomass to ethanol conversion process by using the whole medium of filamentous fungi cultivated under solid-state fermentation. Bioenerg. Res. 2014, 7, 744–752. [Google Scholar] [CrossRef]
- Yang, R.; Meng, D.; Hu, X.; Ni, Y.; Li, Q. Saccharification of pumpkin residues by coculturing of Trichoderma reesei RUT-C30 and Phanerochaete chrysosporium burdsall with delayed inoculation timing. J. Agric. Food Chem. 2013, 61, 9192–9199. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Car, S.; Scott-Craig, J.S.; Borrusch, M.S.; Bongers, M.; Walton, J.D. Synthetic multi-component enzyme mixtures for deconstuction of lignocellulosic biomass. Bioresour. Technol. 2010, 101, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Progr. 2009, 25, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Singh, O.V.; Rao, V.L.; Chandrasekhar, G.; Narasu, M.L. Bioconversion of novel substrate Saccharum spontaneum, a weedy material, into ethanol by Pichia stipitis NCIM3498. Bioresour. Technol. 2011, 102, 1709–1714. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Substrates | C % | N % | C/N Ratio | WRV (%) |
|---|---|---|---|---|---|
| 1 | Sugarcane bagasse | 43.47 | 0.53 | 82.02 | 126.73 ± 3.17 a |
| 2 | Black gram | 42.59 | 0.98 | 43.46 | 145.15 ± 3.50 b |
| 3 | Corn stover | 43.93 | 0.74 | 59.36 | 160.12 ± 2.31 d |
| 4 | Pearl millet stover | 40.37 | 0.48 | 84.10 | 128.27 ± 3.23 a |
| 5 | Rice husk | 41.32 | 0.63 | 65.59 | 92.05 ± 3.60 e |
| 6 | Rice straw | 36.58 | 0.66 | 55.42 | 148.79 ± 3.61 b |
| 7 | Sunhemp | 43.27 | 0.54 | 80.13 | 133.24 ± 3.52 a |
| 8 | Sugarcane tops | 42.39 | 0.61 | 69.49 | 171.12 ± 4.19 c |
| 9 | Wheat bran | 44.12 | 1.95 | 22.63 | 174.94 ± 3.42 c |
| 10 | Wheat straw | 43.12 | 0.68 | 63.41 | 144.78 ± 3.09 b |
| Variables | Endoglucanase (IU/gds) | FPase (FPU/gds) | β-Glucosidase (IU/gds) | Xylanase (IU/gds) |
|---|---|---|---|---|
| Incubation time, 4 days | 65.79 ± 3.19 | 1.58 ± 0.08 | 23.58 ± 1.30 | 1449.95 ± 81.63 |
| Temperature, 30 °C | 65.79 ± 3.88 | 1.60 ± 0.09 | 23.93 ± 1.50 | 1472.17 ± 76.25 |
| Initial pH, 8.0 | 79.00 ± 3.19 | 1.73 ± 0.10 | 27.50 ± 1.33 | 1832.25 ± 125.50 |
| Moisture contents, 80.0% | 104.85 ± 6.98 | 2.06 ± 0.13 | 28.94 ± 1.78 | 1955.24 ± 126.30 |
| Surfactants | Endoglucanase (IU/gds) | FPase (FPU/gds) | Xylanase (IU/gds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Penicillium sp. AKB-24 | |||||||||
| 0.05% | 0.10% | 0.20% | 0.05% | 0.10% | 0.20% | 0.05% | 0.10% | 0.20% | |
| T-20 | 108.82 ± 5.69 | 123.47 ± 6.03 b | 115.10 ± 5.47 | 2.47 ± 0.09 | 2.70 ± 0.10 b | 2.24 ± 0.10 | 2978.88 ± 109.02 | 3261.69 ± 151.66 b | 2855.71 ± 147.35 |
| T-40 | 110.92 ± 5.71 | 125.57 ± 6.60 bc | 115.10 ± 4.48 | 2.50 ± 0.11 | 2.64 ± 0.12 b | 2.18 ± 0.12 | 2924.84 ± 141.26 | 3136.00 ± 162.13 b | 2541.48 ± 133.68 |
| T-60 | 108.82 ± 4.32 | 123.47 ± 5.96 b | 113.01 ± 5.69 | 2.56 ± 0.11 | 2.79 ± 0.13 bc | 2.41 ± 0.13 | 3002.76 ± 140.82 | 3262.95 ± 162.82 b | 3049.27 ± 154.90 |
| T-80 | 113.01 ± 5.81 | 129.75 ± 6.27 bc | 121.38 ± 4.24 | 2.68 ± 0.12 | 2.91 ± 0.15 bc | 2.78 ± 0.14 | 3002.76 ± 112.00 | 3335.85 ± 170.12 b | 3081.95 ± 146.39 |
| T-x-100 | 117.19 ± 5.10 b | 113.01 ± 4.46 | 100.45 ± 4.90 | 2.70 ± 0.14 b | 2.54 ± 0.13 | 2.45 ± 0.045 | 2435.90 ± 94.75 a | 2185.77 ± 126.77 | 1690.55 ± 61.53 |
| SDS | 125.57 ± 5.95 | 133.94 ± 7.19 c | 113.01 ± 3.76 | 2.63 ± 0.12 | 2.96 ± 0.14 c | 2.67 ± 0.13 | 3094.52 ± 160.29 | 3592.26 ± 170.99 c | 2379.34 ± 117.30 |
| EDTA | 98.36 ± 4.61 a | 92.08 ± 3.48 | 85.80 ± 4.34 | 1.78 ± 0.11 d | 1.61 ± 0.09 | 1.56 ± 0.08 | 2469.83 ± 130.16 a | 2297.64 ± 81.79 | 2048.77 ± 88.71 |
| Control | 106.73 ± 5.14 a | 2.08 ± 0.8 a | 2510.06 ± 126.75 a | ||||||
| Aspergillus nidulans AKB-25 | |||||||||
| T-x-100 | 152.14 ± 8.29 | 3.42 ± 0.18 | 2441.03 ± 144.99 | ||||||
| Particulars | FPase, FPU/gds | Endoglucanase, IU/gds | |
|---|---|---|---|
| Carbon sources * | Wheat bran (WB) | 2.93 ± 0.10 | 137.19 ± 5.93 |
| Black gram (BG) | 3.48 ± 0.14 | 155.89 ± 5.19 | |
| WB:BG (1:1) | 3.64 ± 0.14 | 172.52 ± 6.80 | |
| pH ** | 6 | 3.38 ± 0.12 | 166.29 ± 8.13 |
| 7 | 3.67 ± 0.17 | 174.60 ± 8.87 | |
| 8 | 3.83 ± 0.16 | 187.07 ± 9.09 | |
| Moisture content *** | 77.5 | 3.84 ± 0.15 | 187.07 ± 8.19 |
| 80.0 | 4.04 ± 0.19 | 203.70 ± 9.41 |
| Particulars | A. nidulans AKB-25 | Penicillium sp. AKB-24 | Co-culture of A. nidulans AKB-25 and Penicillium sp. AKB-24 |
|---|---|---|---|
| Optimal cultural parameters | |||
| Carbon source | Black gram | Wheat bran | Black gram + Wheat bran |
| Incubation time (days) | 4 | 7 | 7 |
| Temperature (°C) | 30 | 30 | 30 |
| pH | 8.0 | 7.0 | 8.0 |
| Moisture content (%) | 80.0 | 77.5 | 80.0 |
| Nitrogen source (%) | Ammonium sulphate (0.12% N) | Yeast extract (1.2% w/v) | Ammonium sulphate (0.6% N) + Yeast extract (0.6% w/v) |
| Surfactant (%) | Triton-x-100 (0.05% w/v) | SDS (0.10% w/v) | Triton-x-100 (0.05% w/v) |
| Enzyme activities | |||
| Endoglucanase (IU/gds) | 152.14 ± 8.29 | 133.94 ± 7.19 | 203.70 ± 9.41 [(+33.88%) *, (+52.08%) **] |
| FPase (FPU/gds) | 3.42 ± 0.18 | 2.96 ± 0.14 | 4.04 ± 0.19 [(+18.12%) *, (+36.48%) **] |
| Exoglucanase (IU/gds) | 3.34 ± 0.14 | 2.48 ± 0.09 | 3.70 ± 0.13 [(+10.77%) *, (+49.19%) **] |
| β-Glucosidase (IU/gds) | 44.05 ± 2.17 | 5.88 ± 0.26 | 35.28 ± 1.15 [(−19.90%) *, (+500%) **] |
| Xylanase (IU/gds) | 2441.03 ± 144.99 | 3592.26 ± 170.99 | 3674.94 ± 183.12 [(+50.54%) *] |
| Amylase (IU/gds) | 37.41 ± 1.95 | 194.90 ± 11.05 | 83.25 ± 4.30 [(+122.53%) *, (−57.28%) **] |
| Crude enzyme from co-cultures of A. nidulans AKB-25 and Penicillium sp. AKB-24 | ||||
| Enzyme activity (IU/mL) | 0.42 | |||
| Enzyme dosing (FPU/g) | 5 (11.90 mL) * | 10 (23.80 mL) * | 15 (35.71 mL) * | 20 (47.61 mL) * |
| Hydrolysis time (h) | ||||
| 12 | 259.24 ± 8.92 | 323.55 ± 16.50 | 406.52 ± 18.05 | 420.13 ± 15.84 |
| 24 | 336.08 ± 14.25 | 396.25 ± 13.79 | 476.11 ± 20.57 | 490.55 ± 24.92 |
| 36 | 389.96 ± 16.11 | 460.37 ± 19.98 | 532.54 ± 17.25 | 547.62 ± 21.25 |
| 48 | 419.49 ± 16.32 | 514.66 ± 22.23 | 575.27 ± 22.72 | 582.85 ± 25.35 |
| 60 | 446.17 ± 13.30 | 537.16 ± 21.11 | 602.39 ± 29.34 | 606.92 ± 22.09 |
| 72 | 455.64 ± 16.58 | 550.36 ± 26.75 | 612.45 ± 24.17 | 616.75 ± 27.57 |
| 84 | 458.82 ± 15.31 | 554.15 ± 24.05 | 615.38 ± 28.92 | 618.44 ± 27.77 |
| Crude enzyme from A. nidulans AKB-25 | ||||
| Enzyme activity (IU/mL) | 0.36 | |||
| Enzyme dosing (FPU/g) | 5 (13.88 mL) * | 10 (27.77 mL) * | 15 (41.66 mL) * | 20 (55.55 mL) * |
| Hydrolysis time (h) | ||||
| 72 | 451.72 ± 14.44 | 556.14 ± 25.85 | 613.17 ± 20.42 | 610.35 ± 24.56 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Gautam, A.; Dutt, D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover. Fermentation 2016, 2, 12. https://doi.org/10.3390/fermentation2020012
Kumar A, Gautam A, Dutt D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover. Fermentation. 2016; 2(2):12. https://doi.org/10.3390/fermentation2020012
Chicago/Turabian StyleKumar, Amit, Archana Gautam, and Dharm Dutt. 2016. "Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover" Fermentation 2, no. 2: 12. https://doi.org/10.3390/fermentation2020012