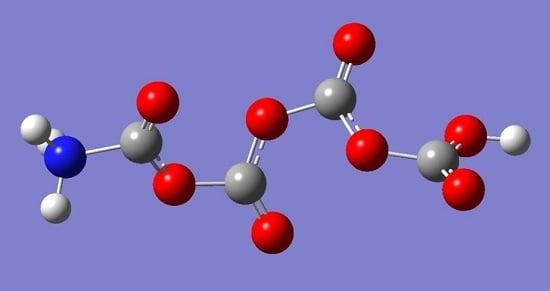
Is the Formation of Poly-CO2 Stabilized by Lewis Base Moieties in N- and S-Doped Porous Carbon?
Abstract
:1. Introduction
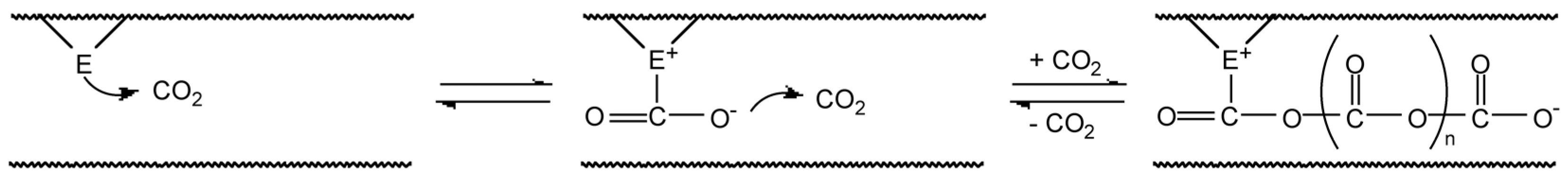
2. Results and Discussion


| LB | 1 | 2 | 3 | 4 | LA |
|---|---|---|---|---|---|
| NH3 | 170.9 | 177.7 | 177.2 | 177.2 | - |
| H2S | 179.9 | 179.8 | 179.9 | 179.0 | - |
| H2O | 171.7 | 176.4 | 178.4 | 178.4 | - |
| - | 177.1 | 176.8 | 175.9 | 173.9 | AlF3 b |
| - | 177.3 c | 178.9 | 179.5 | 174.7 | B4O6 |
| - | 179.6 | 179.4 | 178.1 d | 144.3 | H+ |
| NH3 e | 129.8 | 131.2 | 128.2 | 131.7 | AlF3 |
| NMe3 | 128.5 | 128.9 | 125.6 | 129.8 | B4O6 |
| NH3 | 134.4 | 131.9 | 130.9 | 129.9 | H+ |
| H2S | 135.4 | 132.2 | 131.0 | 129.9 | H+ |
| H2O | 141.1 | 133.8 | 131.7 | 130.3 | H+ |

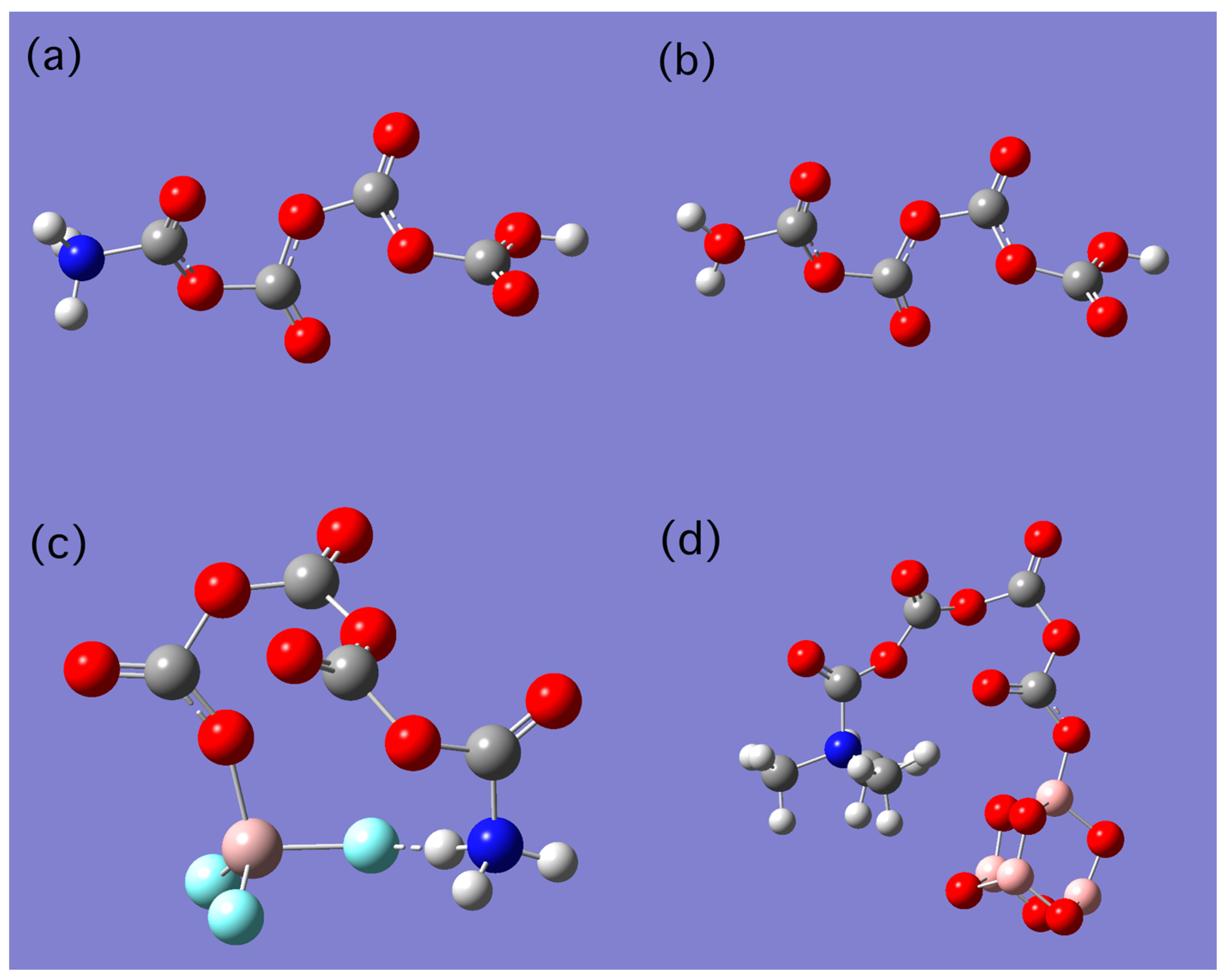
| LB | LB…C1 | O1…C2 | O2…C3 | O3…C4 | O4…LA | LA |
|---|---|---|---|---|---|---|
| NH3 | 1.467 | 1.404 | 1.409 | 1.435 | 1.744 | AlF3 |
| NMe3 | 1.479 | 1.385 | 1.404 | 1.385 | 1.466 | B4O6 |
| NH3 | 1.523 | 1.417 | 1.401 | 1.394 | 0.969 | H+ |
| H2S | 1.876 | 1.423 | 1.402 | 1.394 | 0.969 | H+ |
| H2O | 1.517 | 1.439 | 1.411 | 1.398 | 0.969 | H+ |



3. Experimental Section
3.1. Computational Methods
3.2. Synthesis and Characterization of N- and S-Doped Porous Carbons
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hwang, C.-C.; Tour, J.J.; Kittrell, C.; Espinal, L.; Alemany, L.B.; Tour, J.M. Capturing carbon dioxide as a polymer from natural gas. Nat. Commun. 2014, 5, 3961. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 7, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 14, 2781–2787. [Google Scholar] [CrossRef]
- Ello, A.S.; Yapo, J.A.; Trokourey, A. N-doped carbon aerogels for carbon dioxide (CO2) capture. Afr. J. Pure Appl. Chem. 2013, 7, 61–66. [Google Scholar]
- Chandra, V.; Yu, S.U.; Kim, S.H.; Yoon, Y.S.; Kim, D.Y.; Kwon, A.H.; Meyyappan, M.; Kim, K.S. Highly selective CO2 capture on N-doped carbon produced by chemical activation of polypyrrole functionalized graphene sheets. Chem. Commun. 2012, 48, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Highly porous S-doped carbons. Micropor. Mesopor. Mat. 2012, 158, 318–323. [Google Scholar] [CrossRef]
- Iota, V.; Yoo, C.S.; Cynn, H. Quartzlike carbon dioxide: An optically nonlinear extended solid at high pressures and temperatures. Science 1999, 283, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; González, F.; Poveda, M.L.; Marin, J.M. Reaction of cis-[Mo(N2)2(PMe3)4] with COP synthesis and characterization of products of disproportionation and the X-ray structure of a tetrametallic mixed-valence MoII–MoV carbonate with a novel mode of carbonate binding. J. Am. Chem. Soc. 1983, 105, 3365–3366. [Google Scholar]
- Langer, J.; Imhof, W.; Fabra, M.J.; García-Orduna, P.; Görls, H.; Lahoz, F.J.; Oro, L.A.; Westerhausen, M. Reversible CO2 fixation by iridium(I) complexes containing Me2PhP as ligand. Organometallics 2010, 29, 1642–1651. [Google Scholar] [CrossRef]
- Dreisbach, F.; Staudt, R.; Keller, J.U. High pressure adsorption data of methane, nitrogen, carbon dioxide and their binary and ternary mixtures on activated carbon. Adsorption 1999, 5, 215–227. [Google Scholar] [CrossRef]
- Krooss, B.M.; van Bergen, F.; Gensterblum, Y.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Bae, J.-S.; Bhatia, S.K. High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 2006, 20, 2599–2607. [Google Scholar] [CrossRef]
- Himeno, S.; Komatsu, T.; Fujita, S. High-pressure adsorption equilibria of methane and carbon dioxide on several activated carbons. J. Chem. Eng. Data 2005, 50, 369–376. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals radii for the whole main group. J. Phys. Chem. 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
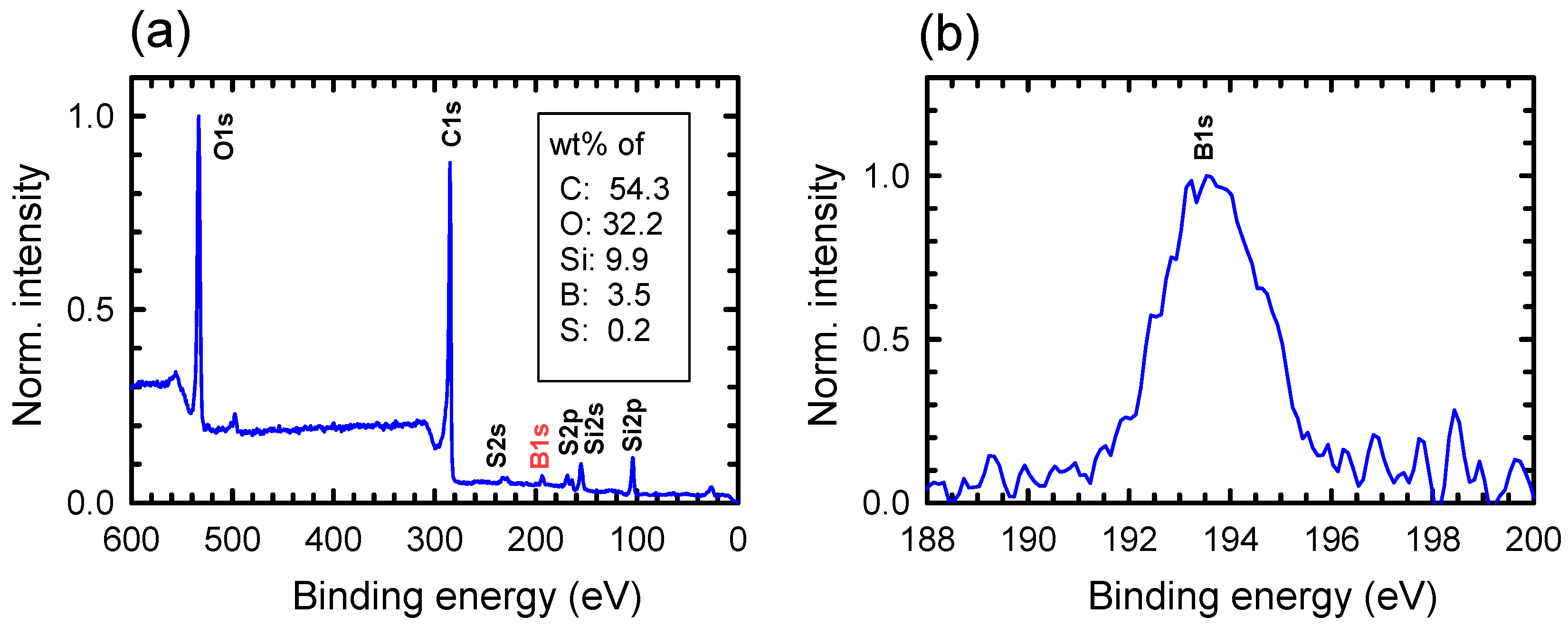
- Khattak, G.D.; Salim, M.A.; Wenger, L.E.; Gilani, A.H. X-ray photoelectron spectroscopy (XPS) and magnetization studies of iron-sodium borate glasses. J. Non-Cryst. Solids 1999, 244, 128–136. [Google Scholar] [CrossRef]
- Branch, C.S.; van Poppel, L.G.; Bott, S.G.; Barron, A.R. Molecular structure of (tBu)3Al[O=C(OPh)2]. J. Chem. Cryst. 1999, 29, 993–996. [Google Scholar] [CrossRef]
- Branch, C.S.; Bott, S.G.; Barron, A.R. Group 13 trihalide complexes of 9-fluorenone: A comparison of methods for assigning relative Lewis acidity. J. Organomet. Chem. 2003, 666, 23–34. [Google Scholar] [CrossRef]
- Power, M.B.; Bott, S.G.; Clark, D.L.; Atwood, J.L.; Barron, A.R. The interaction of organic carbonyls with sterically crowded aryloxide compounds of aluminum. Organometallics 1990, 9, 3086–3097. [Google Scholar] [CrossRef]
- Francis, J.A.; McMahon, C.N.; Bott, S.G.; Barron, A.R. Steric effects in aluminum compounds containing monoanionic potentially bidentate ligands: Towards a quantitative measure of steric bulk. Organometallics 1999, 18, 4399–4416. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Hydrogen-bond geometry in organic crystals. Acc. Chem. Res. 1984, 17, 320–326. [Google Scholar] [CrossRef]
- Vogelson, C.T.; Edwards, C.L.; Kobylivker, A.N.; Chacko, S.B.; Moran, C.E.; Dalton, K.; Stewart, S.M.; Werner, B.C.; Bott, S.G.; Barron, A.R. Molecular structure of M(tfac)3 (M = Al, Co) and Cu(H2O)(fod)2: Examples of unusual supramolecular architecture. J. Chem. Cryst. 1998, 28, 817–824. [Google Scholar] [CrossRef]
- Bishop, M.; Bott, S.G.; Barron, A.R. Structural characterization of borate esters in which sodium acts as a support to the structural framework. J. Chem. Soc. Dalton Trans. 2000, 18, 3100–3105. [Google Scholar] [CrossRef]
- Goddard, R.; Niemeyer, C.M.; Reetz, M.T. Structure of 1,3-xylyl-(18-crown-5)-ammonium catecholborate (1/1). Acta Cryst. 1993, 49, 402–404. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Barton, S.S.; Evans, M.J.B.; Halliop, E.; MacDonald, J.A.F. Acidic and basic sites on the surface of porous carbon. Carbon 1997, 35, 1361–1366. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Binkley, J.S.; Pople, J.A. Møller–Plesset theory for atomic ground state energies. Int. J. Quantum Chem. 1975, 9, 229–236. [Google Scholar] [CrossRef]
- Ogrin, D.; Bott, S.G.; Barron, A.R. Molecular structure of [Me2Al(µ-OPh)]2: A crystallographic and ab initio study. J. Chem. Cryst. 2008, 38, 397–401. [Google Scholar] [CrossRef]
- Keys, A.; Brain, P.T.; Morrison, C.A.; Callender, R.L.; Smart, B.A.; Wann, D.A.; Robinson, H.E.; Rankin, D.W.H.; Barron, A.R. Molecular structures of M(But)3 (M = Al, Ga, In) using gas-phase electron diffraction and ab initio calculations: Experimental and computational evidence for charge-transfer processes leading to photodissociation. Dalton Trans. 2008, 3, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.R. What is the reason for the anomalous C-substituent effects in the Lewis acid catalyzed thermal decomposition of [Me2Al(µ-OR)]2? Main Group Chem. 2015, 14, 87–96. [Google Scholar] [CrossRef]
- Andreoli, E.; Dillon, E.P.; Cullum, L.; Alemany, L.B.; Barron, A.R. Cross-linking amine-rich compounds into high performing selective CO2 absorbents. Sci. Rep. 2014, 4, 7304. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Barron, A.R. Is the Formation of Poly-CO2 Stabilized by Lewis Base Moieties in N- and S-Doped Porous Carbon? C 2016, 2, 5. https://doi.org/10.3390/c2010005
Ghosh S, Barron AR. Is the Formation of Poly-CO2 Stabilized by Lewis Base Moieties in N- and S-Doped Porous Carbon? C. 2016; 2(1):5. https://doi.org/10.3390/c2010005
Chicago/Turabian StyleGhosh, Saunab, and Andrew R. Barron. 2016. "Is the Formation of Poly-CO2 Stabilized by Lewis Base Moieties in N- and S-Doped Porous Carbon?" C 2, no. 1: 5. https://doi.org/10.3390/c2010005