Bacterial Nanocellulose Hydrogel for the Green Cleaning of Copper Stains from Marble
Abstract
:1. Introduction
2. Results and Discussion
2.1. BC Hydrogel Characterisation
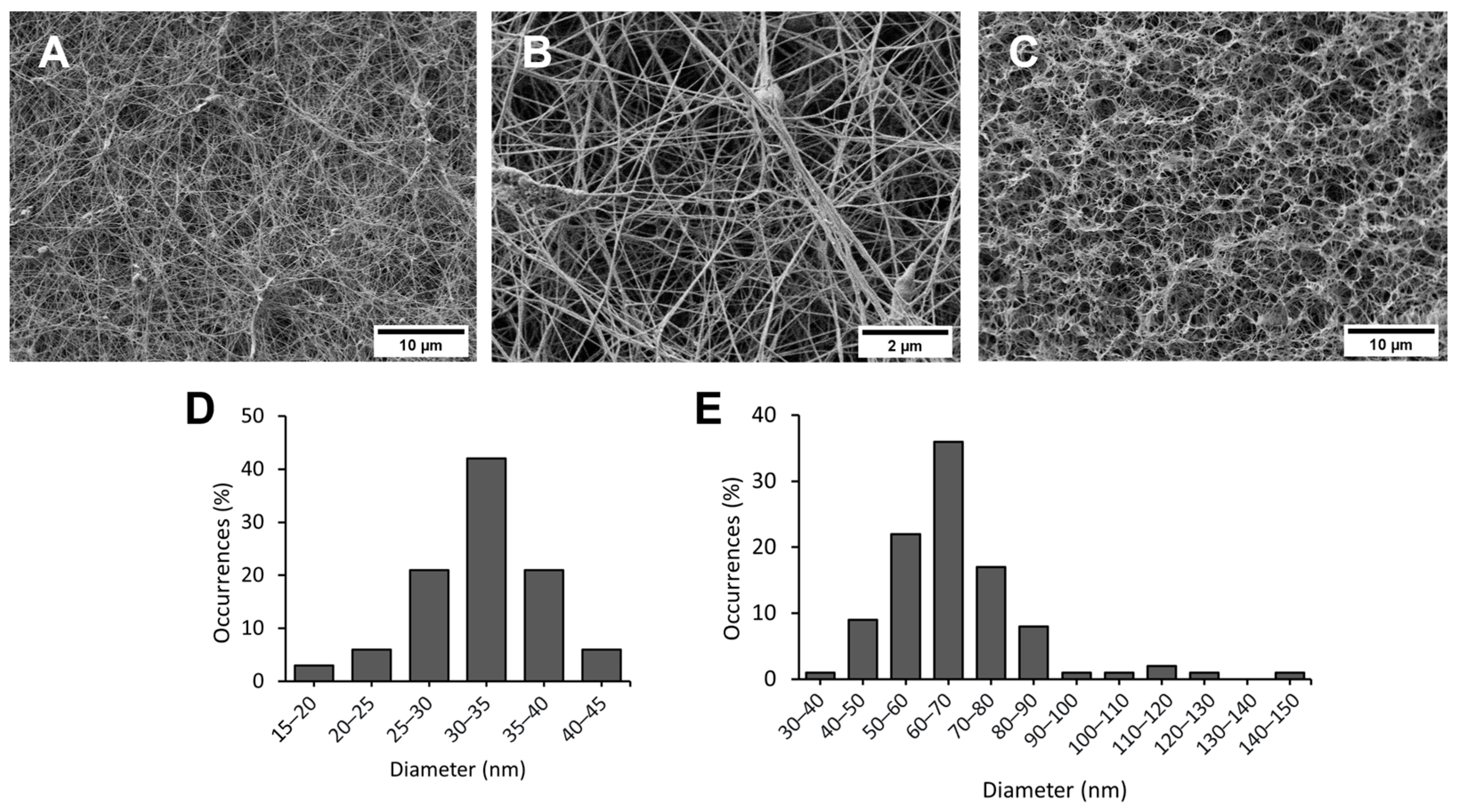
2.1.1. Microstructure
2.1.2. Crystallinity and Chemical Structure
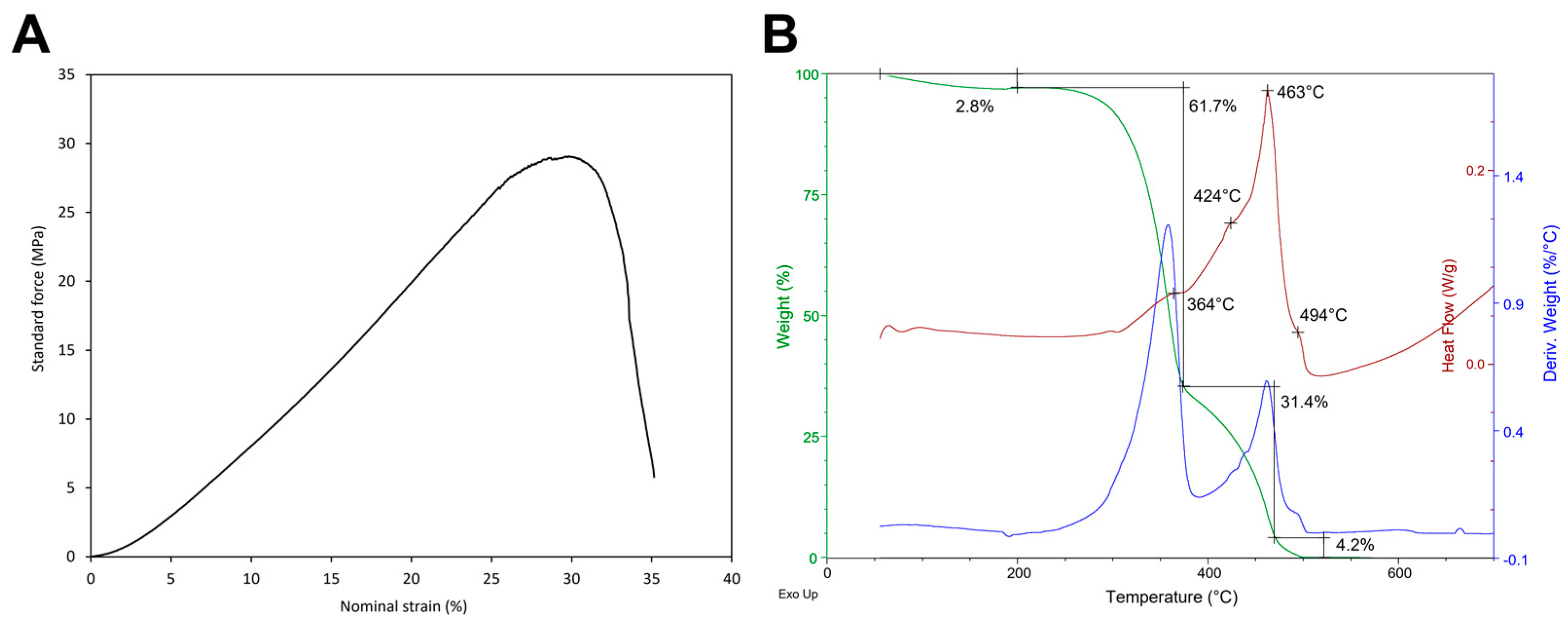
2.1.3. Tensile and Thermal Behaviours
2.1.4. Water Holding Capacity (WHC) and Water Release Rate (WRR)
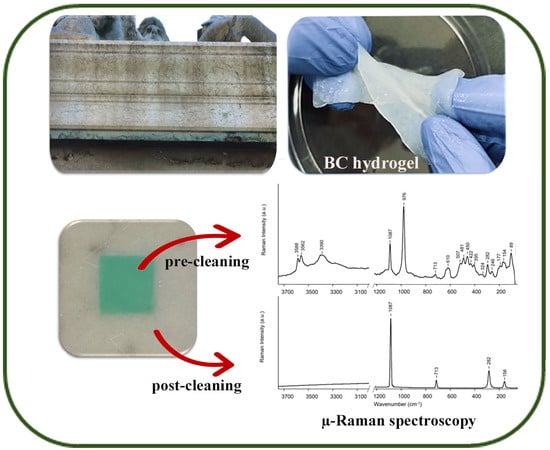
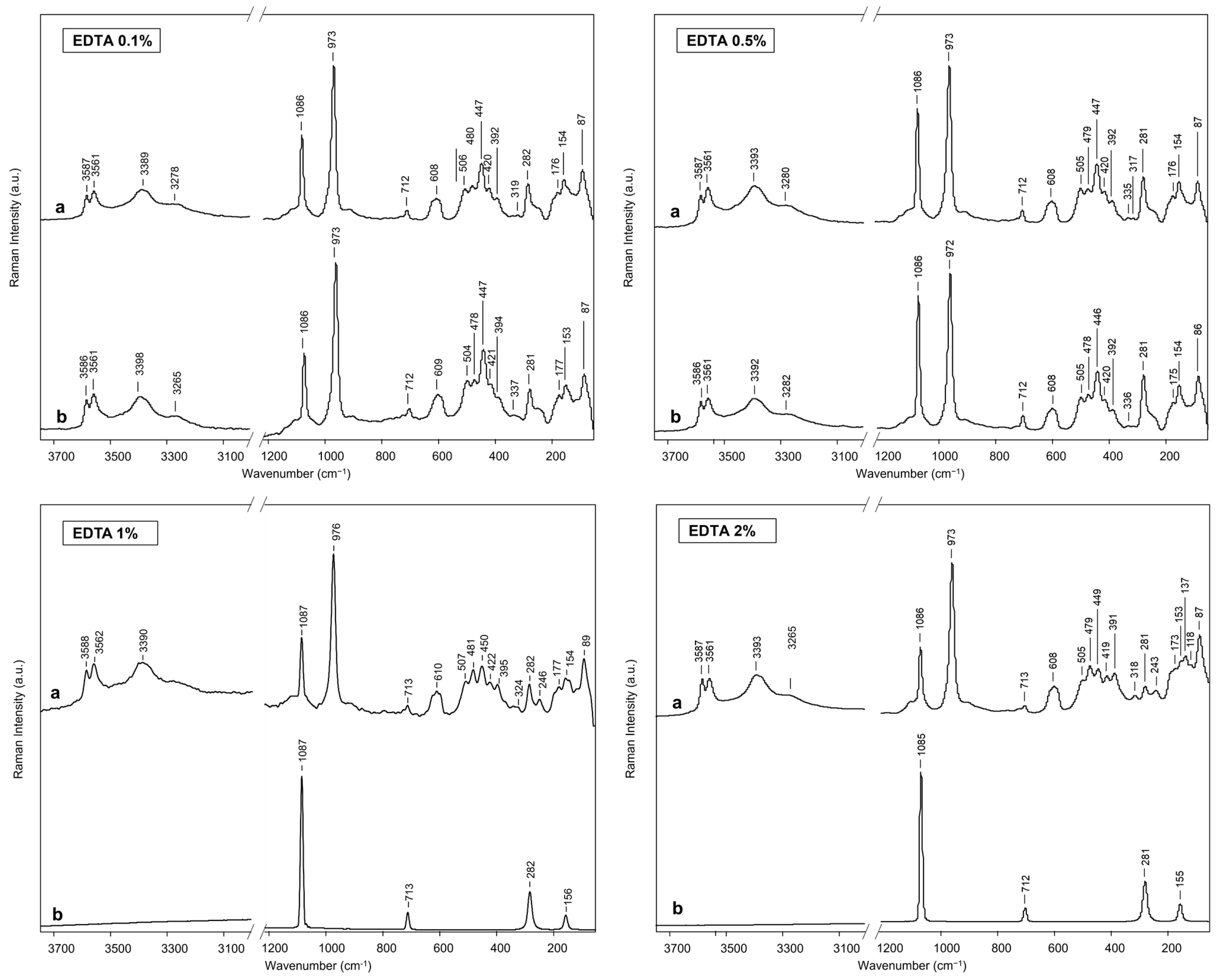
2.2. EDTA-Loaded BC Hydrogel and Cleaning Efficiency Evaluation
3. Conclusions
4. Materials and Methods
4.1. Bacterial Nanocellulose (BC) Hydrogel Characterisation
4.1.1. BC Purification
4.1.2. BC Microstructure Analysis
4.1.3. BC Crystallinity Analysis
4.1.4. BC Chemical Structure Analysis
4.1.5. BC Water Holding Capacity (WHC)
4.1.6. BC Water Release Rate (WRR)
4.1.7. BC Thermal Behaviour
4.1.8. BC Tensile Behaviour
4.2. BC Hydrogel Loading with EDTA Solution
4.3. Preparation of Laboratory Specimens
4.4. Evaluation of the Cleaning Efficacy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric Corrosion of Copper and the Colour, Structure and Composition of Natural Patinas on Copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Andrea Macchia, L.C.; Colasanti, I.A.; Rivaroli, L.; Favero, G.; de Caro, T.; Munoz, L.P.; Russa, M.F.L. Natural Based Products for Cleaning Copper and Copper Alloys Artefacts. Nat. Prod. Res. 2023, 37, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Bertasa, M.; Canevali, C.; Sansonetti, A.; Lazzari, M.; Malandrino, M.; Simonutti, R.; Scalarone, D. An In-Depth Study on the Agar Gel Effectiveness for Built Heritage Cleaning. J. Cult. Herit. 2021, 47, 12–20. [Google Scholar] [CrossRef]
- Leygraf, C.; Chang, T.; Herting, G.; Wallinder, I.O. The Origin and Evolution of Copper Patina Colour. Corros. Sci. 2019, 157, 337–346. [Google Scholar] [CrossRef]
- Manti, P.; Watkinson, D. Corrosion Phenomena and Patina on Archaeological Low-Tin Wrought Bronzes: New Data. J. Cult. Herit. 2022, 55, 158–170. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Gaylarde, P.M.; Beech, I.B. Deterioration of Limestone Structures Associated with Copper Staining. Int. Biodeterior. Biodegrad. 2008, 62, 179–185. [Google Scholar] [CrossRef]
- Canevali, C.; Fasoli, M.; Bertasa, M.; Botteon, A.; Colombo, A.; Tullio, V.D.; Capitani, D.; Proietti, N.; Scalarone, D.; Sansonetti, A. A Multi-Analytical Approach for the Study of Copper Stain Removal by Agar Gels. Microchem. J. 2016, 129, 249–258. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Corti, C.; Rampazzi, L.; Monticelli, D.; Scalarone, D.; Sassella, A.; Canevali, C. Optimization of Copper Stain Removal from Marble through the Formation of Cu (II) Complexes in Agar Gels. Gels 2021, 7, 111. [Google Scholar] [CrossRef]
- Boccalon, E.; Nocchetti, M.; Pica, M.; Romani, A.; Sterflinger, K. Hydrogels: A ‘Stepping Stone’ towards New Cleaning Strategies for Biodeteriorated Surfaces. J. Cult. Herit. 2021, 47, 1–11. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir ACS J. Surf. Colloids 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- Gabriele, F.; Vetrano, A.; Bruno, L.; Casieri, C.; Germani, R.; Rugnini, L.; Spreti, N. New Oxidative Alginate-Biocide Hydrogels against Stone Biodeterioration. Int. Biodeterior. Biodegrad. 2021, 163, 105281. [Google Scholar] [CrossRef]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Hydrogels for the Cleaning of Art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Sciutto, G.; Guilminot, E.; Joseph, E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Appl. Sci. 2021, 11, 3405. [Google Scholar] [CrossRef]
- Cuvillier, L.; Passaretti, A.; Guilminot, E.; Joseph, E. Agar and Chitosan Hydrogels’ Design for Metal-Uptaking Treatments. Gels 2024, 10, 55. [Google Scholar] [CrossRef]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in Art Conservation: A Novel Bio-Based Organogel for the Cleaning of Water Sensitive Works of Art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The Green Attitude in Art Conservation: Polyhydroxybutyrate–Based Gels for the Cleaning of Oil Paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Přádnỳ, M.; Šlouf, M.; Martinová, L.; Michálek, J. Macroporous Hydrogels Based on 2-Hydroxyethyl Methacrylate. Part 7: Methods of Preparation and Comparison of Resulting Physical Properties. E-Polymers 2010, 10, 043. [Google Scholar] [CrossRef]
- Gao, X.; Shi, Z.; Kuśmierczyk, P.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Time-Dependent Rheological Behaviour of Bacterial Cellulose Hydrogel. Mater. Sci. Eng. C 2016, 58, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M.; Zhu, Y.; Abidi, N.; Zhou, X.; Wang, J.; Liang, N. Engineering of Porous Bacterial Cellulose toward Human Fibroblasts Ingrowth for Tissue Engineering. J. Mater. Res. 2014, 29, 2682–2693. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and By-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of Bacterial Cellulose Using Hot Water Extracted Wood Sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Wada, M.; Okano, T. Localization of Iα and Iβ Phases in Algal Cellulose Revealed by Acid Treatments. Cellulose 2001, 8, 183–188. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; Filho, M.d.S.M.d.S.; de Rosa, M.F. Bacterial Cellulose Nanocrystals Produced under Different Hydrolysis Conditions: Properties and Morphological Features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin Jr, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR Study on Cellulose with the Varied Moisture Contents: Insight into the Supramolecular Structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Higgins, H.; Stewart, C.; Harrington, K. Infrared Spectra of Cellulose and Related Polysaccharides. J. Polym. Sci. 1961, 51, 59–84. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined Infrared and Electron Diffraction Study of the Polymorphism of Native Celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- Mohd, N.; Draman, S.F.S.; Salleh, M.S.N.; Yusof, N.B. Dissolution of Cellulose in Ionic Liquid: A Review; AIP Publishing: Istanbul, Turkey, 2017; p. 020035. [Google Scholar]
- Gao, X.; Shi, Z.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Inelastic Behaviour of Bacterial Cellulose Hydrogel: In Aqua Cyclic Tests. Polym. Test. 2015, 44, 82–92. [Google Scholar] [CrossRef]
- Astley, O.M.; Chanliaud, E.; Donald, A.M.; Gidley, M.J. Tensile Deformation of Bacterial Cellulose Composites. Int. J. Biol. Macromol. 2003, 32, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, Z.N.; Gromovykh, T.I.; Grachev, V.S.; Traskin, V.Y. Physicochemical Mechanics of Bacterial Cellulose. Colloid J. 2019, 81, 366–376. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Lopez-Sanchez, P.; Wang, D.; Mikkelsen, D.; Gidley, M.J. Mechanical Properties of Bacterial Cellulose Synthesised by Diverse Strains of the Genus Komagataeibacter. Food Hydrocoll. 2018, 81, 87–95. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tammelin, T.; Schulfter, K.; Kiiskinen, H.; Samela, J.; Bismarck, A. High Performance Cellulose Nanocomposites: Comparing the Reinforcing Ability of Bacterial Cellulose and Nanofibrillated Cellulose. ACS Appl. Mater. Interfaces 2012, 4, 4078–4086. [Google Scholar] [CrossRef] [PubMed]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Physicochemical and Mechanical Characterization of Bacterial Cellulose Produced with an Excellent Productivity in Static Conditions Using a Simple Fed-Batch Cultivation Strategy. Carbohydr. Polym. 2010, 82, 173–180. [Google Scholar] [CrossRef]
- Bertasa, M.; Poli, T.; Riedo, C.; Tullio, V.D.; Capitani, D.; Proietti, N.; Canevali, C.; Sansonetti, A.; Scalarone, D. A Study of Non-Bounded/Bounded Water and Water Mobility in Different Agar Gels. Microchem. J. 2018, 139, 306–314. [Google Scholar] [CrossRef]
- Enev, V.; Sedláček, P.; Řihák, M.; Kalina, M.; Pekař, M. IR-Supported Thermogravimetric Analysis of Water in Hydrogels. Front. Mater. 2022, 9, 931303. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water Holding and Release Properties of Bacterial Cellulose Obtained by in Situ and Ex Situ Modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Ana, R.; Rebelo, G.Y.; Andrew, J.; Chen, A.X.; Liu, C.; Liu, Y. Dehydration of Bacterial Cellulose and the Water Content Effects on Its Viscoelastic and Electrochemical Properties. Sci. Technol. Adv. Mater. 2018, 19, 203–211. [Google Scholar] [CrossRef]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of Polyvinyl Alcohol-Borax/Agarose (PVA-B/AG) Double Network Hydrogel Utilized for the Cleaning of Works of Art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]
- Coccato, A.; Bersani, D.; Coudray, A.; Sanyova, J.; Moens, L.; Vandenabeele, P. Raman Spectroscopy of Green Minerals and Reaction Products with an Application in Cultural Heritage Research. J. Raman Spectrosc. 2016, 47, 1429–1443. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. Highlights Mineral. Crystallogr. 2015, 1, 25. [Google Scholar]
- Szymańska-Chargot, M.; Cybulska, J.; Zdunek, A. Sensing the Structural Differences in Cellulose from Apple and Bacterial Cell Wall Materials by Raman and FT-IR Spectroscopy. Sensors 2011, 11, 5543–5560. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Sbardella, F.; Pronti, L.; Santarelli, M.; Asua Gonzàlez, J.; Bracciale, M. Waterborne Acrylate-Based Hybrid Coatings with Enhanced Resistance Properties on Stone Surfaces. Coatings 2018, 8, 283. [Google Scholar] [CrossRef]
- García, O.; Malaga, K. Definition of the Procedure to Determine the Suitability and Durability of an Anti-Graffiti Product for Application on Cultural Heritage Porous Materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]
- Ortiz, P.; Antúnez, V.; Ortiz, R.; Martín, J.M.; Gómez, M.A.; Hortal, A.R.; Martínez-Haya, B. Comparative Study of Pulsed Laser Cleaning Applied to Weathered Marble Surfaces. Appl. Surf. Sci. 2013, 283, 193–201. [Google Scholar] [CrossRef]
- Becerra, J.; Mateo, M.; Ortiz, P.; Nicolás, G.; Zaderenko, A.P. Evaluation of the Applicability of Nano-Biocide Treatments on Limestones Used in Cultural Heritage. J. Cult. Herit. 2019, 38, 126–135. [Google Scholar] [CrossRef]
- Guilminot, E. The Use of Hydrogels in the Treatment of Metal Cultural Heritage Objects. Gels 2023, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Muzio, L.D.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S.; et al. Design of a Tunable Nanocomposite Double Network Hydrogel Based on Gellan Gum for Drug Delivery Applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Bakhtiari, F.; Darezereshki, E. One-Step Synthesis of Tenorite (CuO) Nano-Particles from Cu4 (SO4) (OH) 6 by Direct Thermal-Decomposition Method. Mater. Lett. 2011, 65, 171–174. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial Effect of Zinc Oxide-Based Nanomaterials on Environmental Biodeteriogens Affecting Historical Buildings. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef]



| Pristine | Stained | Cleaned | |
|---|---|---|---|
| L* | 88.75 ± 0.67 | 81.86 ± 0.60 | 87.56 ± 0.43 |
| a* | −0.12 ± 0.02 | −16.99 ± 1.13 | −1.18 ± 0.42 |
| b* | 0.40 ± 0.12 | −0.03 ± 1.75 | 0.29 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglia, E.; Schifano, E.; Sharbaf, M.; Uccelletti, D.; Felici, A.C.; Santarelli, M.L. Bacterial Nanocellulose Hydrogel for the Green Cleaning of Copper Stains from Marble. Gels 2024, 10, 150. https://doi.org/10.3390/gels10020150
Sonaglia E, Schifano E, Sharbaf M, Uccelletti D, Felici AC, Santarelli ML. Bacterial Nanocellulose Hydrogel for the Green Cleaning of Copper Stains from Marble. Gels. 2024; 10(2):150. https://doi.org/10.3390/gels10020150
Chicago/Turabian StyleSonaglia, Erica, Emily Schifano, Mohammad Sharbaf, Daniela Uccelletti, Anna Candida Felici, and Maria Laura Santarelli. 2024. "Bacterial Nanocellulose Hydrogel for the Green Cleaning of Copper Stains from Marble" Gels 10, no. 2: 150. https://doi.org/10.3390/gels10020150