In Vitro Viability Tests of New Ecofriendly Nanosystems Incorporating Essential Oils for Long-Lasting Conservation of Stone Artworks
Abstract
:1. Introduction
2. Results and Discussion
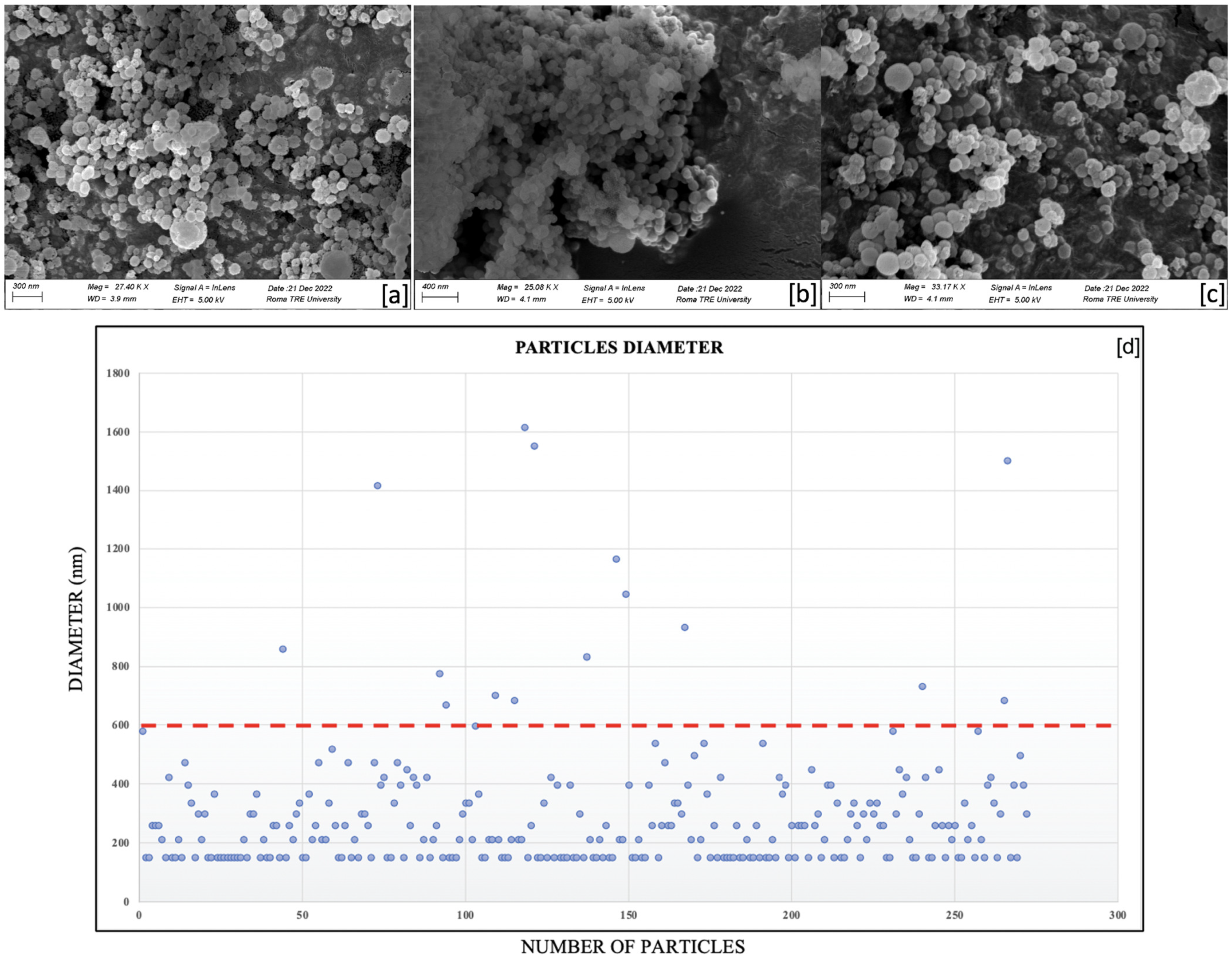
2.1. SEM Characterization of the Nanocapsules
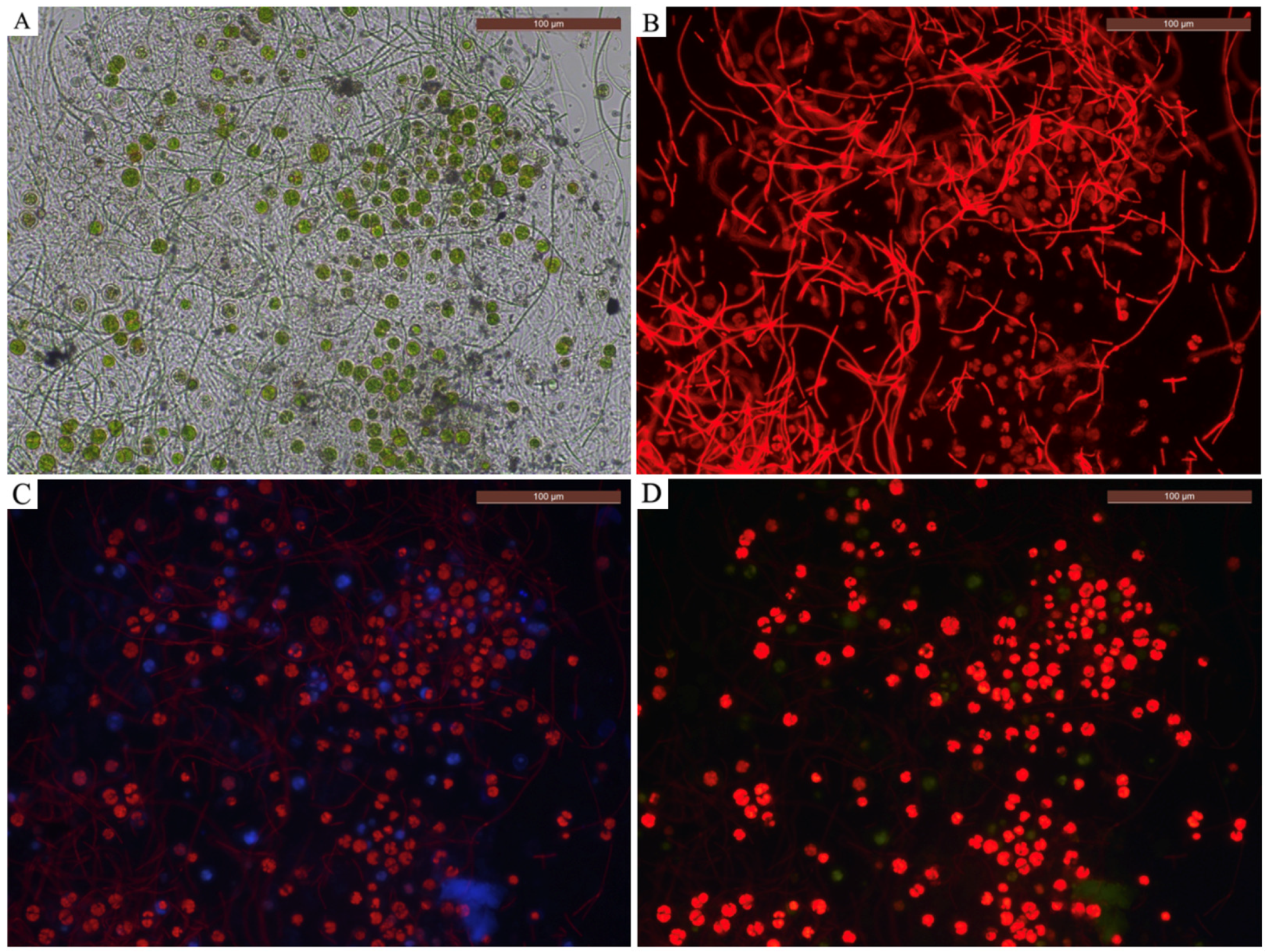
2.2. Identification of Microorganisms—Morphological Evaluations
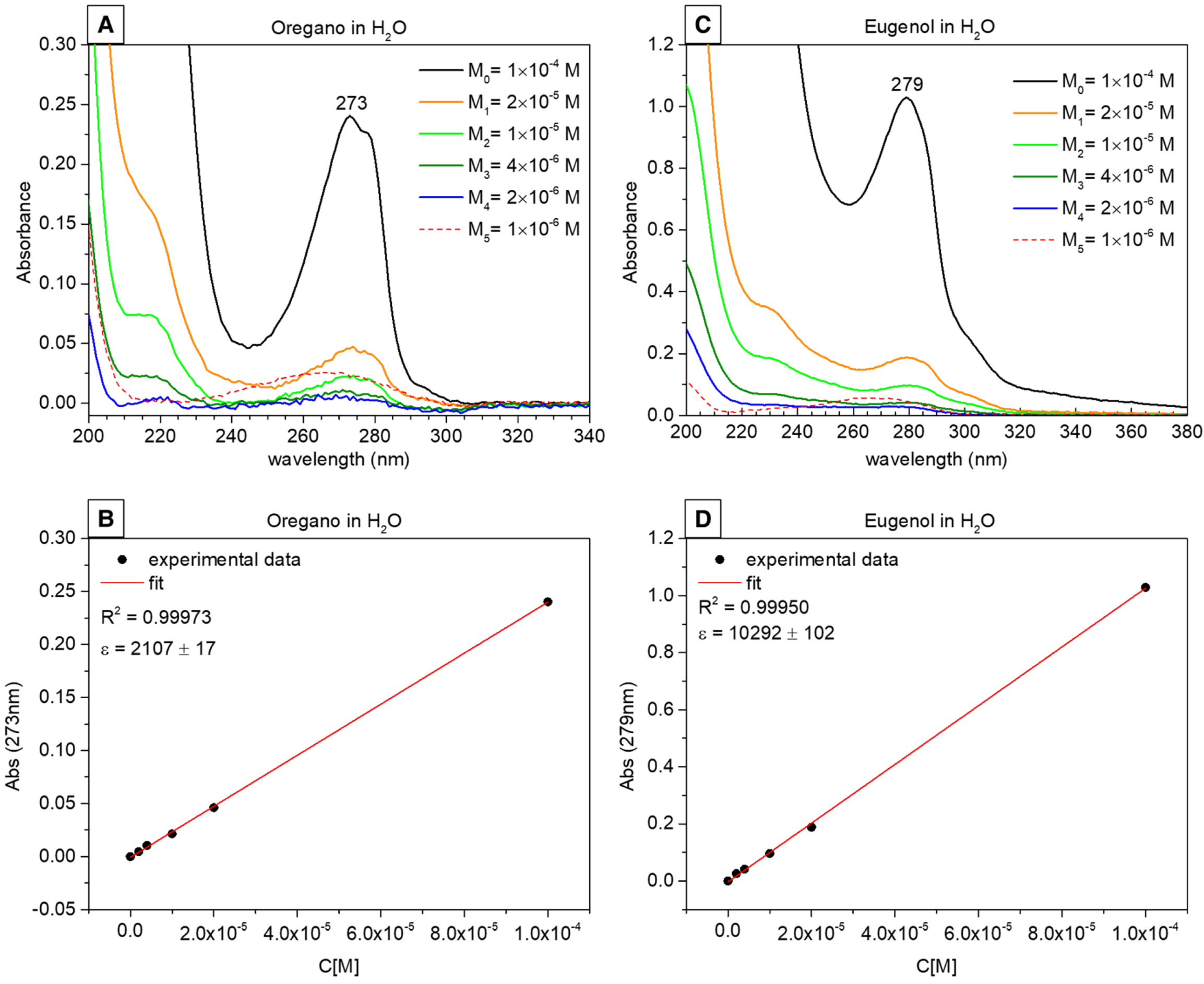
2.3. UV–Vis Spectroscopy Results and Release Profiles
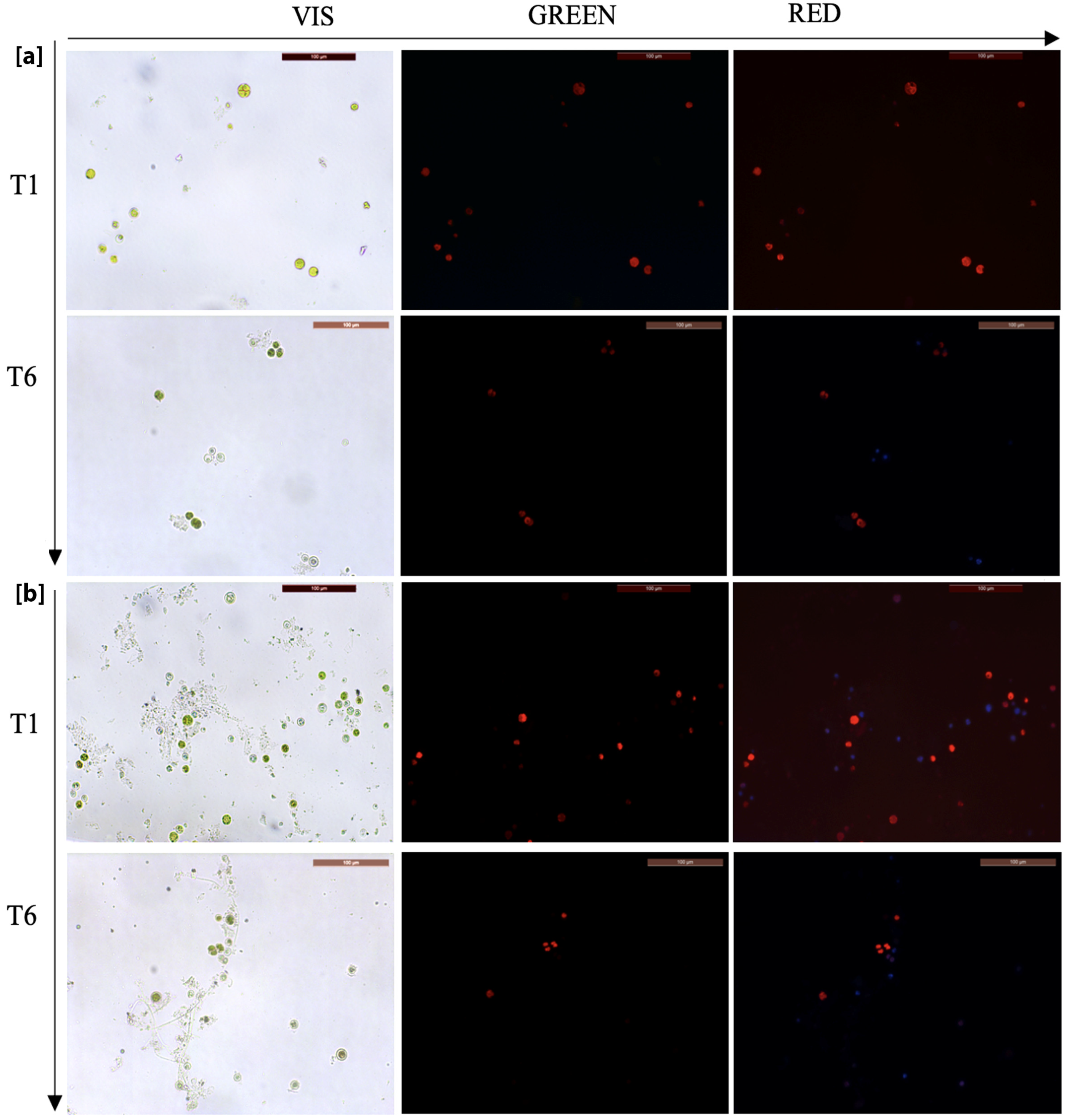
2.4. Analysis of the Efficacy of Biocides—Viability Tests
3. Conclusions
4. Materials and Methods
4.1. Biocidal Description
4.2. Nanoencapsulation Step
4.3. Release Study
4.4. Cultivation and Identification of Microorganisms
4.5. In Vitro Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Carlo, E.; Barresi, G.; Palla, F. Biodeterioration. In Biotechnology and Conservation of Cultural Heritage; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–30. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Santunione, G.; Libbra, A.; Muscio, A.; Sgarbi, E.; Siligardi, C.; Barozzi, G.S. Review on the influence of biological deterioration on the surface properties of building materials: Organisms, materials, and methods. Int. J. Des. Nat. Ecodyn. 2015, 10, 21–39. [Google Scholar] [CrossRef]
- Pinna, D. Microorganisms in the Deterioration and Preservation of Cultural Heritage. In Microbial Growth and Its Effects on Inorganic Heritage Materials; Joseph, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–36. [Google Scholar] [CrossRef]
- Negi, A.; Sarethy, I.P. Microbial biodeterioration of cultural heritage: Events, colonization, and analyses. Microb. Ecol. 2019, 78, 1014–1029. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Sáiz-Jiménez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Tomaselli, L.; Lamenti, G.; Bosco, M.; Tiano, P. Biodiversity of photosynthetic micro-organisms dwelling on stone monuments. Int. Biodeterior. Biodegrad. 2000, 46, 251–258. [Google Scholar] [CrossRef]
- Crispim, C.A.; Gaylarde, C.C. Cyanobacteria and biodeterioration of cultural heritage: A review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microb. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; De Hoog, G.S.; Selbmann, L. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Biodeterioration and weathering effects on rock decay. Corros. Rev. 2004, 22, 341–364. [Google Scholar] [CrossRef]
- Ortega-Morales, O.; Montero-Muñoz, J.L.; Neto, J.A.B.; Beech, I.B.; Sunner, J.; Gaylarde, C. Deterioration and microbial colonization of cultural heritage stone buildings in polluted and unpolluted tropical and subtropical climates: A meta-analysis. Int. Biodeterior. Biodegrad. 2019, 143, 104734. [Google Scholar] [CrossRef]
- De Leo, F.; Antonelli, F.; Pietrini, A.M.; Ricci, S.; Urzì, C. Study of the euendolithic activity of black meristematic fungi isolated from a marble statue in the Quirinale Palace’s Gardens in Rome, Italy. Facies 2019, 65, 18. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microb. Biotech. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, O.; Casanova Municchia, A. The role of fungi and lichens in the biodeterioration of stone monuments. IOP Conf. Ser. Mater. Sci. Eng. 2016, 7, 39–54. [Google Scholar] [CrossRef]
- de Los Ríos, A.; Cámara, B.; Del Cura, M.A.G.; Rico, V.J.; Galván, V.; Ascaso, C. Deteriorating effects of lichen and microbial colonization of carbonate building rocks in the Romanesque churches of Segovia (Spain). Sci. Total Environ. 2009, 407, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Oakville, ON, Canada, 2017. [Google Scholar]
- Caneva, G.; Nugari, M.P.; Pinna, D.; Salvadori, O. Il Controllo del Degrado Biologico; Nardini Editore: Florence, Italy, 1996; pp. 1–200. [Google Scholar]
- Cappitelli, F.; Cattò, C.; Villa, F. The control of cultural heritage microbial deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Palla, F. Plant Essential Oils as Biocides in Sustainable Strategies for the Conservation of Cultural Heritage. Sustainbility 2023, 15, 8522. [Google Scholar] [CrossRef]
- Pinna, D. Can we do without biocides to cope with biofilms and lichens on stone heritage? Int. Biodeterior. Biodegrad. 2022, 172, 105437. [Google Scholar] [CrossRef]
- Warscheid, T. The evaluation of biodeterioration processes on cultural objects and approaches for their effective control. In Art, Biology, and Conservation: Biodeterioration of Works of Art; The Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 14–27. [Google Scholar]
- Tiano, P. Biodeterioration of monumental rocks: Decay mechanisms and control methods. Sci. Technol. Cult. Herit. 1998, 7, 19–38. [Google Scholar]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Gustavs, L.; Pandolfini, G.; Tretiach, M. Heat shock treatments for the control of lithobionts: A case study with epilithic green microalgae. Int. Biodeterior. Biodegrad. 2017, 123, 236–243. [Google Scholar] [CrossRef]
- Pfendler, S.; Borderie, F.; Bousta, F.; Alaoui-Sosse, L.; Alaoui-Sosse, B.; Aleya, L. Comparison of biocides, allelopathic substances and UV-C as treatments for biofilm proliferation on heritage monuments. J. Cult. Herit. 2018, 33, 117–124. [Google Scholar] [CrossRef]
- Geweely, N.S. A novel comparative review between chemical, natural essential oils and physical (ozone) conservation of archaeological objects against microbial deterioration. Geomicrob. J. 2022, 39, 531–540. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and future perspectives for biocides and antifouling products for stone-built cultural heritage: Ionic liquids as a challenging alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sorlini, C. New environmentally friendly approaches against biodeterioration of outdoor cultural heritage. In Biocolonization of Stone: Middle Missouri Plains Control and Preventive Village Sites Methods; Proceedings of the MCI Workshop Series; Charola, E.A., Ed.; Smithsonian Contributions to Museum Conservation; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011; pp. 51–58. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bartoli, F.; Isola, D.; Casanova Municchia, A.; Kumbaric, A.; Caneva, G. Science for art: Multi-years’ evaluations of biocidal efficacy in support of artwork conservation. Front. Microbiol. 2023, 14, 1178900. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Casanova Municchia, A.; Fidanza, M.R.; Caneva, G. Advances in testing the interference of biocides on stone materials: A comparative analysis and guidelines for a standardised approach. J. Cult. Herit. 2023, 64, 23–41. [Google Scholar] [CrossRef]
- Silva, M.; Salvador, C.; Candeias, M.F.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Toxicological assessment of novel green biocides for cultural heritage. Int. J. Consum. Sci. 2016, 7, 265–272. [Google Scholar]
- Marin, E.; Vaccaro, C.; Leis, M. Biotechnology applied to historic stoneworks conservation: Testing the potential harmfulness of two biological biocides. Int. J. Consum. Sci. 2016, 7, 227–238. [Google Scholar]
- Guiamet, P.S.; De Saravia, G. Natural products isolated from plants used in biodeterioration control. Pharmacologyonline 2006, 3, 537–544. [Google Scholar]
- Jeong, S.H.; Lee, H.J.; Kim, D.W.; Chung, Y.J. New biocide for eco-friendly biofilm removal on outdoor stone monuments. Int. Biodeterior. Biodegrad. 2018, 131, 19–28. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Tabasso, M.L.; Brigadeci, F.; Capua, M.C.; Morelli, A.; Pastorello, P.; Sohrabi, M.; Chaverdi, A.A.; Callieri, P. A first assessment of the biocidal efficacy of plant essential oils against lichens on stone cultural heritage, and the importance of evaluating suitable application protocols. J. Cult. Herit. 2022, 55, 68–77. [Google Scholar] [CrossRef]
- Rotolo, V.; Barresi, G.; Di Carlo, E.; Giordano, A.; Lombardo, G.; Crimi, E.; Costa, E.; Bruno, M.; Palla, F. Plant extracts as green potential strategies to control the biodeterioration of cultural heritage. Int. J. Consum. Sci. 2016, 2, 839–846. [Google Scholar]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oils as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Gazzano, C.; Favero-Longo, S.E.; Iacomussi, P.; Piervittori, R. Biocidal effect of lichen secondary metabolites against rock-dwelling microcolonial fungi, cyanobacteria and green algae. Int. Biodeterior. Biodegrad. 2013, 84, 300–306. [Google Scholar] [CrossRef]
- Gabriele, F.; Ranaldi, R.; Bruno, L.; Casieri, C.; Rugnini, L.; Spreti, N. Biodeterioration of stone monuments: Studies on the influence of bioreceptivity on cyanobacterial biofilm growth and on the biocidal efficacy of essential oils in natural hydrogel. Sci. Total Environ. 2023, 870, 161901. [Google Scholar] [CrossRef]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential oils mixtures as an eco-friendly biocidal solution for a marble statue restoration. Int. Biodeterior. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- Veneranda, M.; Blanco-Zubiaguirre, L.; Roselli, G.; Di Girolami, G.; Castro, K.; Madariaga, J.M. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchem. J. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The green attitude in art conservation: Polyhydroxybutyrate–based gels for the cleaning of oil paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.; Karapanagiotis, I.; Ortiz, P. Evaluation of silver nanoparticles effectiveness as biocide by multi-spectral imaging. In Science and Digital Technology for Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2019; pp. 307–311. [Google Scholar]
- Allen, N.S.; Edge, M.; Sandoval, G.; Verran, J.; Stratton, J.; Maltby, J. Photocatalytic Coatings for Environmental Applications. J. Photochem. Photobiol. 2005, 81, 279–290. [Google Scholar] [CrossRef]
- Hegedus, C.R.; Pepe, F.R.; Lindenmuth, D.L.; Burgard, D. Zinc Oxide Nanoparticle Dispersion as Unique Additives for Coatings. JCT CoatingsTech 2008, 5, 42–52. [Google Scholar]
- Serafini, I.; Ciccola, A. Nanotechnologies and Nanomaterials. Nanotechnologies and Nanomaterials for Diagnostic. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–380. [Google Scholar]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Chelazzi, D.; Baglioni, P. From nanoparticles to gels: A breakthrough in art conservation science. Langmuir 2023, 39, 10744–10755. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Baglioni, M.; Berti, D.; Baglioni, P. New methodologies for the conservation of cultural heritage: Micellar solutions, microemulsions, and hydroxide nanoparticles. Acc. Chem. Res. 2010, 43, 695–704. [Google Scholar] [CrossRef]
- Kanth, A.P.; Soni, A.K. Application of nanocomposites for conservation of materials of cultural heritage. J. Cult. Herit. 2023, 59, 120–130. [Google Scholar] [CrossRef]
- Saji, V.S. Supramolecular concepts and approaches in corrosion and biofouling prevention. Corros. Rev. 2019, 37, 187–230. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L.; Bartoli, F.; Fidanza, M.R.; Zurlo, F.; Marconi, E.; Gasperi, T.; Tuti, S.; Crociani, L.; Di Bartolomeo, E.; Caneva, G.; et al. Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl. Surf. Sci. 2020, 514, 145908. [Google Scholar] [CrossRef]
- Bartoli, F.; Zuena, M.; Sodo, A.; Caneva, G. The Efficiency of Biocidal Silica Nanosystems for the Conservation of Stone Monuments: Comparative In Vitro Tests against Epilithic Green Algae. Appl. Sci. 2021, 11, 6804. [Google Scholar] [CrossRef]
- Zuena, M.; Ruggiero, L.; Caneva, G.; Bartoli, F.; Della Ventura, G.C.; Ricci, M.A.; Sodo, A. Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide. Coatings 2021, 11, 1109. [Google Scholar] [CrossRef]
- Bartoli, F.; Hosseini, Z.; Graziani, V.; Zuena, M.; Venettacci, C.; Della Ventura, G.; Tortora, L.; Sodo, A.; Caneva, G. In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent the Biodeterioration of Stone Monuments. Coatings 2024, 14, 163. [Google Scholar] [CrossRef]
- Ruggiero, L.; Di Bartolomeo, E.; Gasperi, T.; Luisetto, I.; Talone, A.; Zurlo, F.; Peddis, D.; Ricci, M.A.; Sodo, A. Silica nanosystems for active antifouling protection: Nanocapsules and mesoporous nanoparticles in controlled release applications. J. Alloys Compd. 2019, 798, 144–148. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Tang, H.; Yan, C. Porous Silica Nanocapsules and Nanospheres: Dynamic Self-Assembly Synthesis and Application in Controlled Release. Chem. Mater. 2008, 20, 5894–5900. [Google Scholar] [CrossRef]
- Ruggiero, L.; Crociani, L.; Zendri, E.; El Habra, N.; Guerriero, P. Incorporation of the zosteric sodium salt in silica nanocapsules: Synthesis and characterization of new fillers for antifouling coatings. Appl. Surf. Sci. 2018, 439, 705–711. [Google Scholar] [CrossRef]
- Marconi, E.; Luisetto, I.; Di Carlo, G.; Staccioli, M.P.; Tuti, S.; Tortora, L. 3-APTES on Dendritic Fibrous Mesoporous Silica Nanoparticles for the pH-Controlled Release of Corrosion Inhibitors. Nanomaterials 2023, 13, 2543. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Banerjee, S.; Yalkowsky, S.H.; Valvani, C. Water solubility and octanol/water partition coefficients of organics. Limitations of the solubility-partition coefficient correlation. Environ. Sci. Technol. 1980, 14, 1227–1229. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Dresler, C.; Saladino, M.L.; Demirbag, C.; Caponetti, E.; Martino, D.F.C.; Alduina, R. Development of controlled release systems of biocides for the conservation of cultural heritage. Int. Biodeterior. Biodegrad. 2017, 125, 150–156. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles–polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Genova, C.; Fuentes, E.; Favero, G.; Prieto, B. Evaluation of the cleaning effect of natural-based biocides: Application on different phototropic biofilms colonizing the same granite wall. Coatings 2023, 13, 520. [Google Scholar] [CrossRef]
- Gatti, L.; Troiano, F.; Vacchini, V.; Cappitelli, F.; Balloi, A. An in vitro evaluation of the biocidal effect of oregano and cloves’ volatile compounds against microorganisms colonizing an oil painting—A pioneer study. Appl. Sci. 2020, 11, 78. [Google Scholar] [CrossRef]
- Baratta, M.T.; Dorman, H.J.; Deans, S.G.; Biondi, D.M.; Ruberto, G. Chemical Composition, Antimicrobial and Antioxidative Activity of Laurel, Sage, Rosemary, Oregano and Coriander Essential Oils. J. Essent. Oil Res. 1998, 10, 618–627. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Palla, F.; Barresi, G.; Giordano, A.; Trapani, M.; Rotolo, V.; Parisi, M.; Cammarata, M. Cold-active molecules for a sustainable preservation and restoration of historic-artistic manufacts. Int. J. Cons. Sci. 2016, 7, 239–246. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Lee, H.J.; Chung, Y.-J. Antifungal, Antibacterial, and Interference Effects of Plant-Extracted Essential Oils Used for Mural Conservation at Buyeo Royal Tomb No. 1. Appl. Sci. 2023, 13, 3645. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Privitera, A.; Ruggiero, L.; Venditti, I.; Laverdura, U.P.; Tuti, S.; De Felicis, D.; Mastro, S.L.; Duranti, L.; Di Bartolomeo, E.; Gasperi, T.; et al. One step nanoencapsulation of corrosion inhibitors for gradual release application. Mater. Today Chem. 2022, 24, 100851. [Google Scholar] [CrossRef]
- UNI 10923; Beni Culturali: Materiali Lapidei Naturali ed Artificiali—Allestimento di Preparati Biologici per L’osservazione al Microscopio Ottico. UNI: Milano, Italy, 2001.
- Guiry, M.D.; Guiry, G.M. AlgaeBase Version 4.2. World-Wide Electronic Publication; National University of Ireland: Maynooth, Ireland, 2007; Available online: https://www.algaebase.org/ (accessed on 15 January 2021).


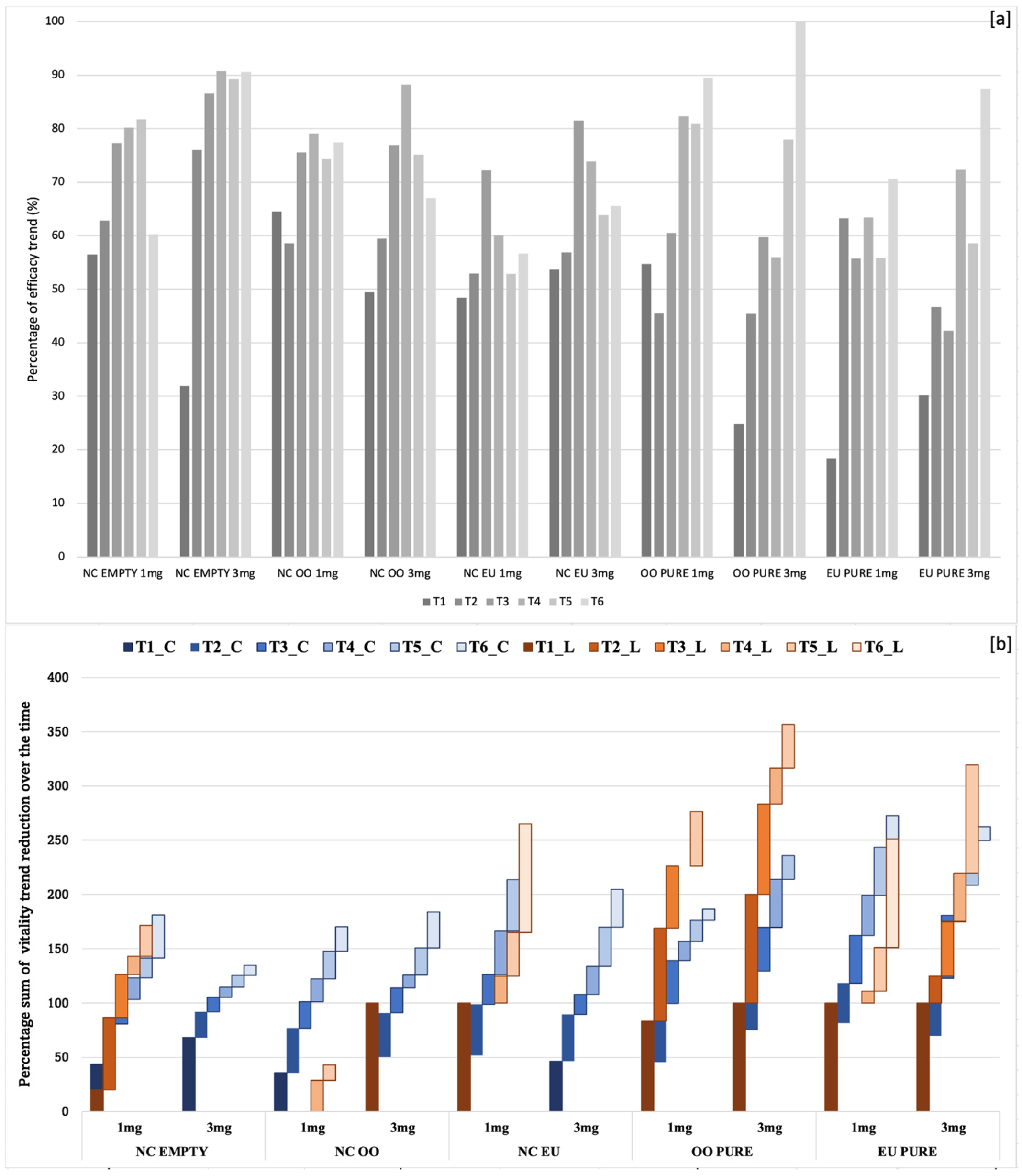
| Time | Filter | Cells | NC EMPTY 1 mg | NC EMPTY 3 mg | NC OO 1 mg | NC OO 3 mg | NC EU 1 mg | NC EU 3 mg | OO PURE 1 mg | OO PURE 3 mg | EU PURE 1 mg | EU PURE 3 mg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Green | Red | 43.5 | 68.1 | 35.5 | 50.5 | 51.6 | 46.3 | 45.3 | 75.1 | 81.6 | 69.8 |
| Red | Red | 40.8 | 63.3 | 29.9 | 48.5 | 51.1 | 42.6 | 42.1 | 75.9 | 81.6 | 68.5 | |
| Blue | 38.7 | 97.0 | 29.8 | 40.7 | 36.0 | 30.6 | 35.8 | 27.0 | 11.5 | 16.7 | ||
| Blue | Red | 49.5 | 64.5 | 36.1 | 49.0 | 55.9 | 50.4 | 41.1 | 75.5 | 80.9 | 68.5 | |
| Green | 31.0 | 86.7 | 26.0 | 29.4 | 36.0 | 27.3 | 72.6 | 29.0 | 13.9 | 19.1 | ||
| T2 | Green | Red | 37.2 | 24.0 | 41.4 | 40.5 | 47.1 | 43.1 | 54.4 | 54.5 | 36.8 | 53.3 |
| Red | Red | 39.8 | 23.6 | 41.4 | 35.8 | 45.3 | 41.8 | 55.1 | 54.0 | 34.2 | 47.5 | |
| Blue | 40.1 | 51.2 | 35.2 | 43.9 | 36.6 | 41.2 | 42.2 | 41.7 | 41.0 | 36.1 | ||
| Blue | Red | 37.2 | 27.2 | 44.1 | 43.2 | 51.7 | 44.4 | 46.9 | 50.6 | 34.2 | 47.5 | |
| Green | 36.6 | 48.0 | 31.7 | 37.2 | 36.0 | 39.2 | 44.9 | 36.2 | 27.4 | 21.3 | ||
| T3 | Green | Red | 22.7 | 13.4 | 24.4 | 23.1 | 27.7 | 18.5 | 39.4 | 40.2 | 44.3 | 57.8 |
| Red | Red | 22.7 | 14.8 | 26.2 | 24.1 | 27.9 | 17.3 | 48.0 | 57.1 | 41.0 | 52.6 | |
| Blue | 70.0 | 71.0 | 64.5 | 49.5 | 52.7 | 43.9 | 43.7 | 35.0 | 42.6 | 54.5 | ||
| Blue | Red | 24.9 | 16.9 | 29.1 | 26.2 | 28.3 | 22.2 | 37.3 | 35.3 | 26.6 | 46.8 | |
| Green | 55.4 | 60.2 | 41.0 | 27.7 | 34.3 | 22.7 | 33.6 | 38.7 | 32.4 | 33.1 | ||
| T4 | Green | Red | 19.8 | 9.3 | 20.9 | 11.8 | 39.9 | 26.1 | 17.6 | 44.1 | 36.6 | 27.7 |
| Red | Red | 18.9 | 9.8 | 20.9 | 11.8 | 38.8 | 24.7 | 9.1 | 43.3 | 36.1 | 27.7 | |
| Blue | 45.4 | 73.2 | 60.1 | 67.0 | 57.7 | 64.0 | 73.9 | 63.5 | 52.3 | 61.5 | ||
| Blue | Red | 29.8 | 13.4 | 25.8 | 16.8 | 40.3 | 31.8 | 10.8 | 25.1 | 36.6 | 23.1 | |
| Green | 30.9 | 55.6 | 33.3 | 47.2 | 39.2 | 37.8 | 44.9 | 56.5 | 25.9 | 36.9 | ||
| T5 | Green | Red | 18.2 | 10.7 | 25.6 | 24.8 | 47.1 | 36.2 | 19.1 | 22.0 | 44.2 | 41.4 |
| Red | Red | 18.2 | 11.4 | 25.0 | 24.1 | 47.1 | 35.7 | 12.8 | 5.1 | 15.5 | 27.6 | |
| Blue | 52.8 | 79.9 | 57.1 | 58.4 | 48.8 | 63.4 | 87.2 | 86.4 | 72.1 | 75.9 | ||
| Blue | Red | 27.5 | 11.4 | 28.2 | 27.0 | 48.8 | 36.2 | 10.6 | 5.1 | 16.3 | 27.6 | |
| Green | 27.2 | 59.2 | 35.4 | 46.0 | 34.3 | 46.9 | 78.7 | 81.4 | 63.6 | 51.7 | ||
| T6 | Green | Red | 39.7 | 9.4 | 22.5 | 33.0 | 43.4 | 34.5 | 10.5 | 0.0 | 29.4 | 12.5 |
| Red | Red | 39.7 | 10.4 | 21.6 | 35.2 | 54.0 | 39.5 | 10.5 | 0.0 | 29.4 | 25.0 | |
| Blue | 55.4 | 89.6 | 73.0 | 48.4 | 28.3 | 55.5 | 89.5 | 90.9 | 52.9 | 62.5 | ||
| Blue | Red | 42.1 | 22.6 | 24.3 | 39.6 | 66.4 | 42.9 | 10.5 | 0.0 | 26.5 | 12.5 | |
| Green | 14.9 | 58.5 | 38.7 | 19.8 | 4.4 | 35.3 | 65.8 | 45.5 | 38.2 | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, F.; Corradi, L.; Hosseini, Z.; Privitera, A.; Zuena, M.; Kumbaric, A.; Graziani, V.; Tortora, L.; Sodo, A.; Caneva, G. In Vitro Viability Tests of New Ecofriendly Nanosystems Incorporating Essential Oils for Long-Lasting Conservation of Stone Artworks. Gels 2024, 10, 132. https://doi.org/10.3390/gels10020132
Bartoli F, Corradi L, Hosseini Z, Privitera A, Zuena M, Kumbaric A, Graziani V, Tortora L, Sodo A, Caneva G. In Vitro Viability Tests of New Ecofriendly Nanosystems Incorporating Essential Oils for Long-Lasting Conservation of Stone Artworks. Gels. 2024; 10(2):132. https://doi.org/10.3390/gels10020132
Chicago/Turabian StyleBartoli, Flavia, Leonora Corradi, Zohreh Hosseini, Antonella Privitera, Martina Zuena, Alma Kumbaric, Valerio Graziani, Luca Tortora, Armida Sodo, and Giulia Caneva. 2024. "In Vitro Viability Tests of New Ecofriendly Nanosystems Incorporating Essential Oils for Long-Lasting Conservation of Stone Artworks" Gels 10, no. 2: 132. https://doi.org/10.3390/gels10020132