On the Evolution of the Cardiac Pacemaker
Abstract
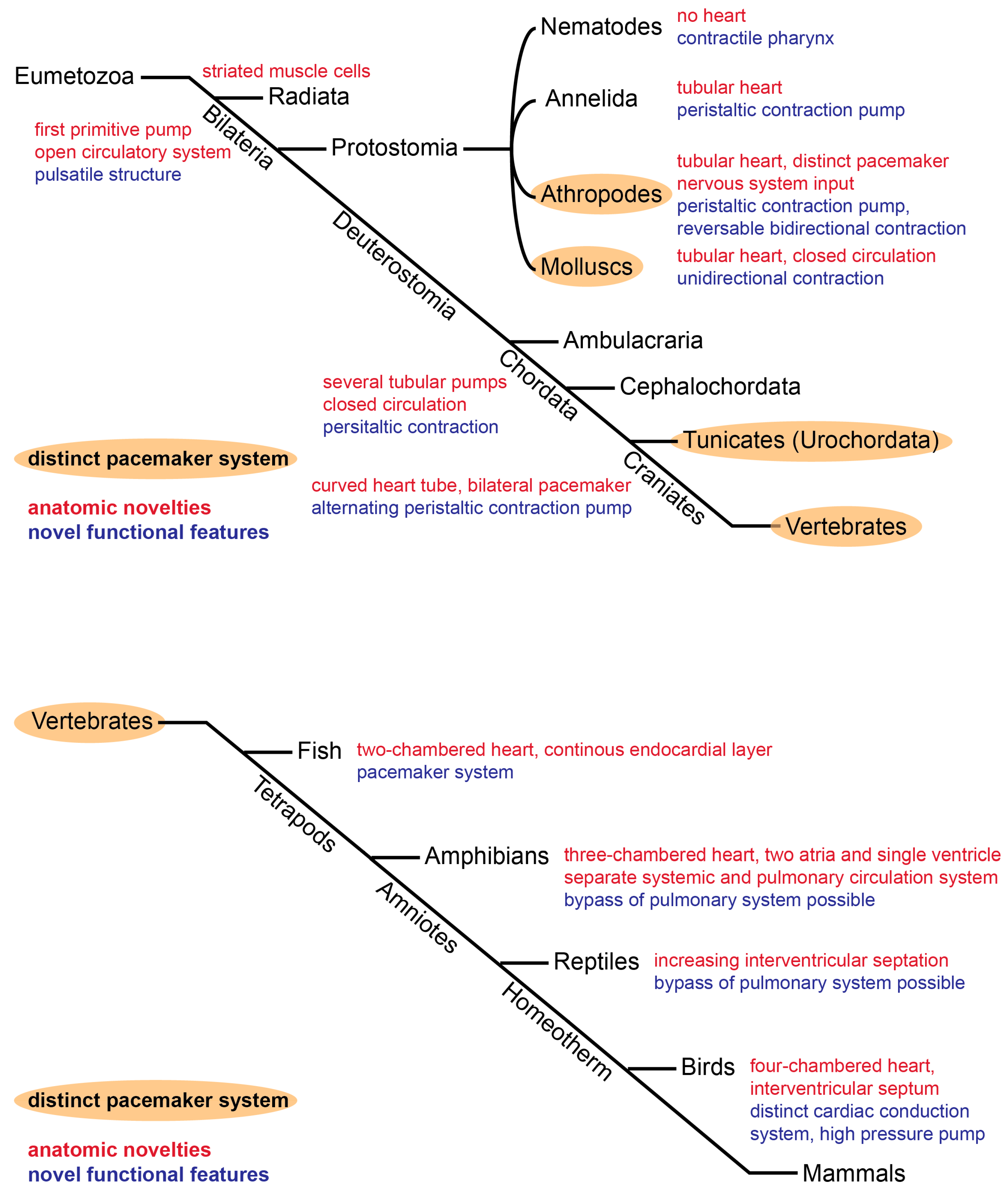
:1. Introduction
2. Origin of the Basic Tubular Heart
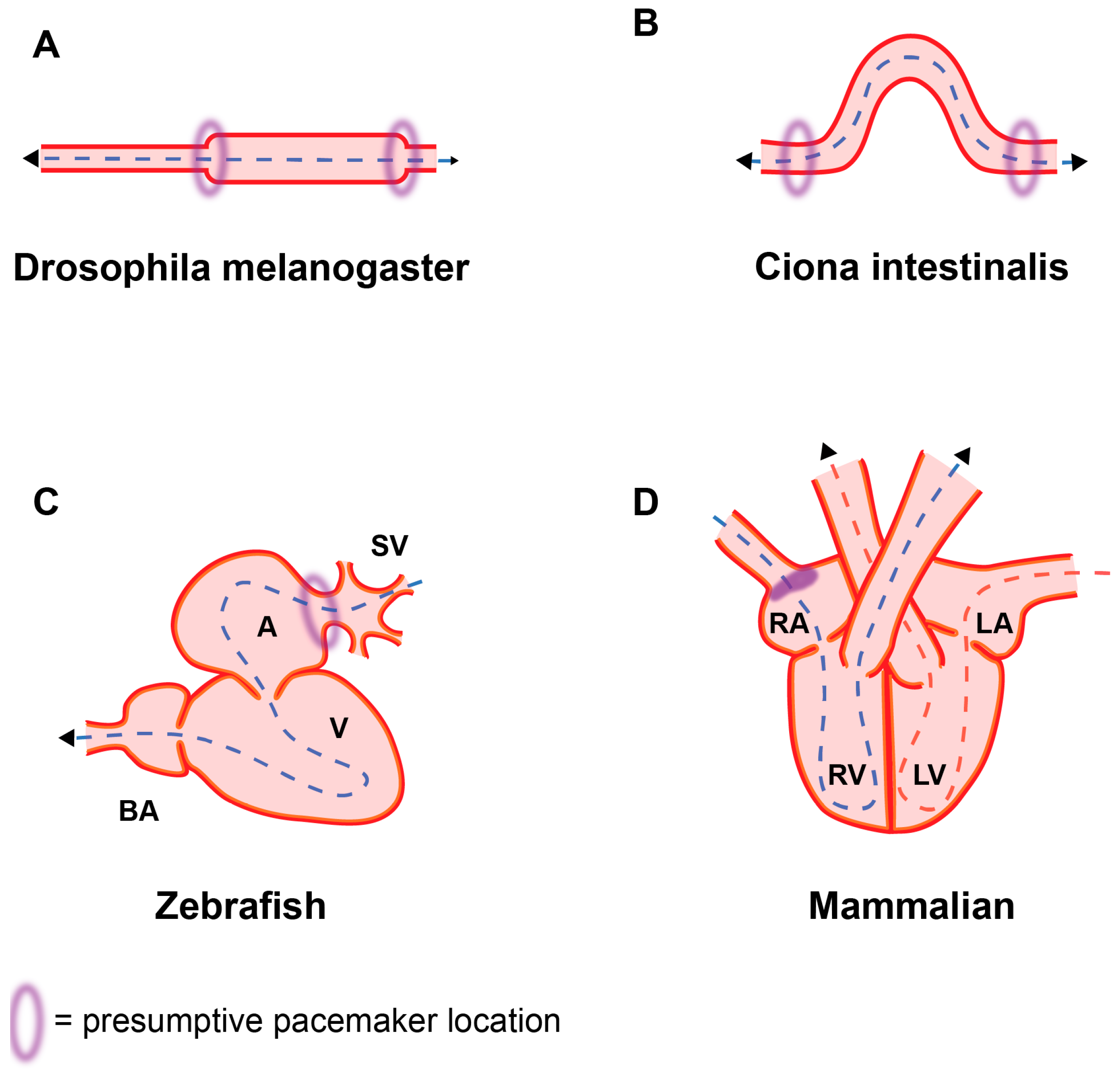
3. Bilateral Pacemaker System in Drosophila Melanogaster
4. Basic Circulation System in Early Deuterostomia
5. Transition to a Sequential Contraction Pattern in Lower Vertebrates
6. Two-Chambered Heart in Zebrafish
7. Septation and Conduction System Development in Amphibians
8. Complex Pacemaker and Cardiac Conduction System in Birds and Mammals
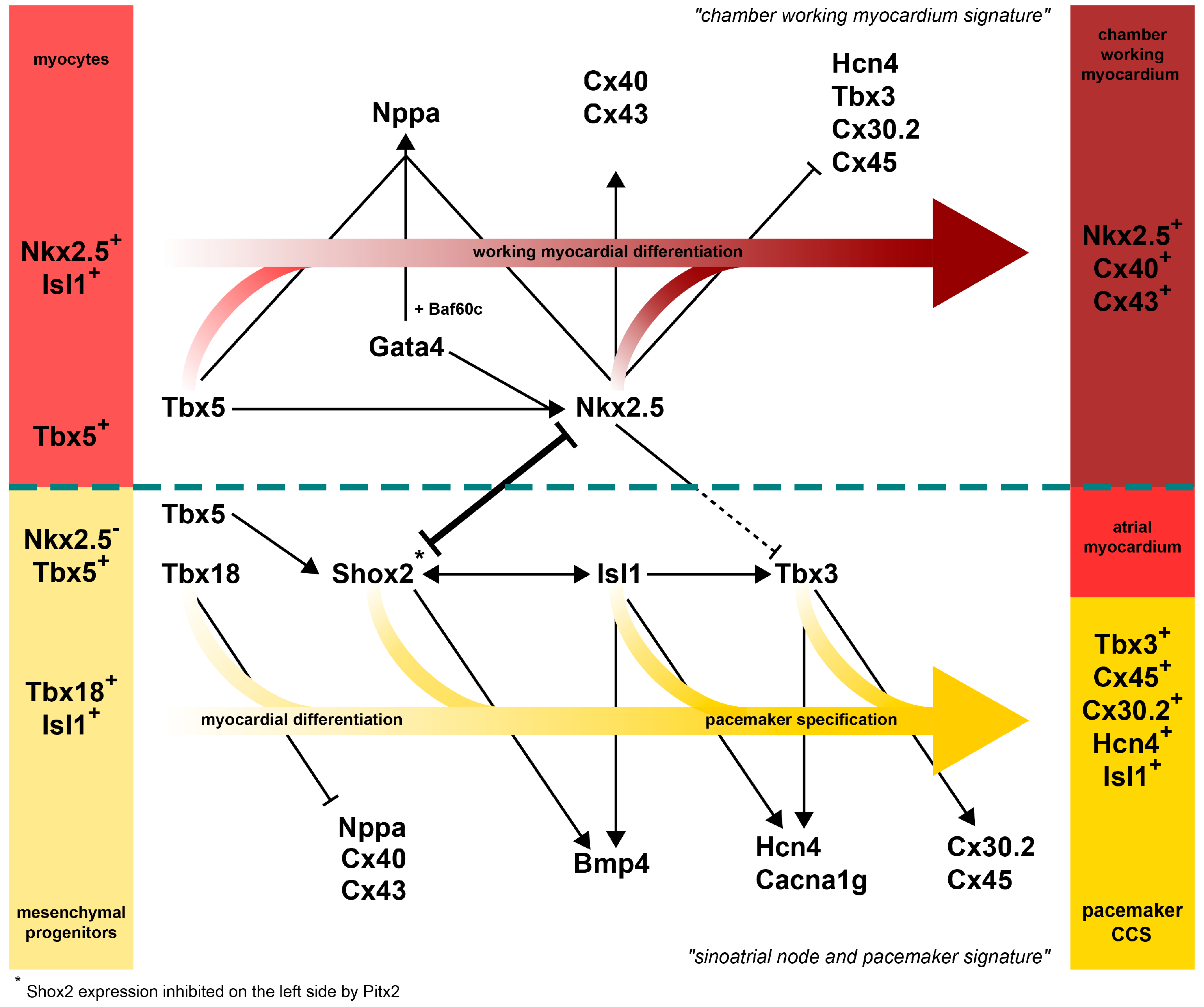
9. Transcription Factor Network Patterns the Mammalian SAN
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bishopric, N.H. Evolution of the heart from bacteria to man. Ann. N. Y. Acad. Sci. 2005, 1047, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, C.J.; Moorman, A.F.; Barnett, P. Protein interactions at the heart of cardiac chamber formation. Ann. Anat. 2009, 191, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.W.; Pinder, A.W. Ontogeny of cardiovascular and respiratory physiology in lower vertebrates. Annu. Rev. Physiol. 1991, 53, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.M.; Olson, E.N. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev. Biol. 2002, 246, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.C.; Olson, E.N. Parsing the heart: Genetic modules for organ assembly. Cell 1997, 91, 153–156. [Google Scholar] [CrossRef]
- Hertel, W.; Pass, G. An evolutionary treatment of the morphology and physiology of circulatory organs in insects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 555–575. [Google Scholar] [CrossRef]
- Meijler, F.L.; Meijler, T.D. Archetype, adaptation and the mammalian heart. Neth. Heart J. 2011, 19, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Satoh, N. Gene regulatory networks for the development and evolution of the chordate heart. Genes Dev. 2006, 20, 2634–2638. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Costa, M.S.; Vasconcelos, M.; Sampaio, A.C.; Cravo, R.M.; Linhares, V.L.; Hochgreb, T.; Yan, C.Y.; Davidson, B.; Xavier-Neto, J. The evolutionary origin of cardiac chambers. Dev. Biol. 2005, 277, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Solc, D. The heart and heart conducting system in the kingdom of animals: A comparative approach to it’s evolution. Exp. Clin. Cardiol. 2007, 12, 113–118. [Google Scholar] [PubMed]
- Xavier-Neto, J.; Davidson, B.; Simoes-Costa, M.S.; Castro, R.A.; Castillo, H.A.; Sampaio, A.C. Evolutionary Origins of Hearts. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Elsevier: London, UK, 2010; pp. 3–46. [Google Scholar]
- Greener, I.D.; Monfredi, O.; Inada, S.; Chandler, N.J.; Tellez, J.O.; Atkinson, A.; Taube, M.A.; Billeter, R.; Anderson, R.H.; Efimov, I.R.; et al. Molecular architecture of the human specialised atrioventricular conduction axis. J. Mol. Cell. Cardiol. 2011, 50, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Manner, J.; Wessel, A.; Yelbuz, T.M. How does the tubular embryonic heart work? Looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 2010, 239, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Schulz, R.A. Heart development in Drosophila. Semin. Cell Dev. Biol. 2007, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Ya, J.; de Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F.M. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; van Borren, M.M.; Peters, R.J.; Broekhuis, E.; Lam, K.; Coronel, R.; de Bakker, J.M.; Tan, H.L. Pacemaker current (If) in the human sinoatrial node. Eur. Heart J. 2007, 28, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Honjo, H.; Kodama, I.; Boyett, M.R. Heterogeneous expression of the delayed-rectifier K+ currents iK,r and iK,s in rabbit sinoatrial node cells. J. Physiol. 2001, 535, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Lockhead, D.; Larson, E.D.; Proenza, C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J. Gen. Physiol. 2010, 136, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Vinogradova, T.M.; Lakatta, E.G. The emergence of a general theory of the initiation and strength of the heartbeat. J. Pharmacol. Sci. 2006, 100, 338–369. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; Difrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation 2009, 119, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, C.; Dupont, E.; Coppen, S.R.; Yeh, H.I.; Severs, N.J. Chamber-related differences in connexin expression in the human heart. J. Mol. Cell. Cardiol. 1999, 31, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Desplantez, T.; Dupont, E.; Severs, N.J.; Weingart, R. Gap junction channels and cardiac impulse propagation. J. Membr. Biol. 2007, 218, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Boyett, M.R.; Morris, G.M. Biology of the Sinus Node and its Disease. Arrhythm. Electrophysiol. Rev. 2015, 4, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, H.; Boyett, M.R.; Andersoon, R.H. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation 2007, 115, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Fabritz, L.; Layh, B.; Kirchhof, P.; Ludwig, A. Insights into sick sinus syndrome from an inducible mouse model. Cardiovasc. Res. 2011, 90, 38–48. [Google Scholar] [CrossRef] [PubMed]
- OOta, S.; Saitou, N. Phylogenetic relationship of muscle tissues deduced from superimposition of gene trees. Mol. Biol. Evol. 1999, 16, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Venkatesh, T.V.; Holland, L.Z.; Jacobs, D.K.; Bodmer, R. AmphiNk2-tin, an amphioxus homeobox gene expressed in myocardial progenitors: Insights into evolution of the vertebrate heart. Dev. Biol. 2003, 255, 128–137. [Google Scholar] [CrossRef]
- Bodmer, R.; Frasch, M. Development and Aging of the Drosophila Heart. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 47–86. [Google Scholar]
- Medioni, C.; Sénatore, S.; Salmand, P.A.; Lalevée, N.; Perrin, L.; Sémériva, M. The fabulous destiny of the Drosophila heart. Curr. Opin. Genet. Dev. 2009, 19, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Wasserthal, L.T. Drosophila flies combine periodic heartbeat reversal with a circulation in the anterior body mediated by a newly discovered anterior pair of ostial valves and ’venous’ channels. J. Exp. Biol. 2007, 210, 3707–3719. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.R.; Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef]
- Curtis, N.J.; Ringo, J.M.; Dowse, H.B. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, Drosophila melanogaster. J. Morphol. 1999, 240, 225–235. [Google Scholar] [CrossRef]
- Bodmer, R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 1993, 118, 719–729. [Google Scholar] [PubMed]
- Azpiazu, N.; Frasch, M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.; Skeath, J.B.; Gajewski, K.; Schulz, R.A.; Frasch, M. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Dev. Biol. 2002, 251, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.; Frasch, M. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech. Dev. 2001, 104, 49–60. [Google Scholar] [CrossRef]
- Reim, I.; Frasch, M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 2005, 132, 4911–4925. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Bodmer, R.; Pandur, P. The Drosophila homolog of vertebrate Islet1 is a key component in early cardiogenesis. Development 2009, 136, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, K.; Zhang, Q.; Choi, C.Y.; Fossett, N.; Dang, A.; Kim, Y.H.; Kim, Y.; Schulz, R.A. Pannier is a transcriptional target and partner of Tinman during Drosophila cardiogenesis. Dev. Biol. 2001, 233, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Mohler, J.P.; Frasch, M. Tbx20-related genes, mid and H15, are required for tinman expression, proper patterning, and normal differentiation of cardioblasts in Drosophila. Mech. Dev. 2005, 122, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, S.; Xu, X.; Lo, P.C.; Lee, H.H.; Frasch, M. Cardiogenesis in the Drosophila model: Control mechanisms during early induction and diversification of cardiac progenitors. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dowse, H.; Ringo, J.; Power, J.; Johnson, E.; Kinney, K.; White, L. A congenital heart defect in Drosophila caused by an action-potential mutation. J. Neurogenet. 1995, 10, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.G.; Singh, S. Pharmacological analysis of heartbeat in Drosophila. J. Neurobiol. 1995, 28, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B. Glutamatergic innervation of the heart initiates retrograde contractions in adult Drosophila melanogaster. J. Neurosci. 2005, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J. Comp. Neurol. 2003, 465, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Ringo, J.; Bray, N.; Dowse, H. Genetic and pharmacological identification of ion channels central to the Drosophila cardiac pacemaker. J. Neurogenet. 1998, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gisselmann, G.; Gamerschlag, B.; Sonnenfeld, R.; Marx, T.; Neuhaus, E.M.; Wetzel, C.H.; Hatt, H. Variants of the Drosophila melanogaster Ih-channel are generated by different splicing. Insect Biochem. Mol. Biol. 2005, 35, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Lalevee, N.; Monier, B.; Sénatore, S.; Perrin, L.; Sémériva, M. Control of cardiac rhythm by ORK1, a Drosophila two-pore domain potassium channel. Curr. Biol. 2006, 16, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Sasaki, A.; Nakayama, A.; Takamura, K.; Satoh, N. Development of Ciona intestinalis juveniles (through 2nd ascidian stage). Zool. Sci. 2004, 21, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B. Ciona intestinalis as a model for cardiac development. Semin. Cell Dev. Biol. 2007, 18, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Electrophysiological studies on initiation and reversal of the heart beat in Ciona intestinalis. J. Exp. Biol. 1968, 49, 363–385. [Google Scholar] [PubMed]
- Kriebel, M.E. Conduction velocity and intracellular action potentials of the tunicate heart. J. Gen. Physiol. 1967, 50, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, M.E. Pacemaker properties of tunicate heart cells. Biol. Bull. 1968, 135, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, M.E. Studies on cardiovascular physiology of tunicates. Biol. Bull. 1968, 134, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, M.E. Electrical characteristics of tunicate heart cell membranes and nexuses. J. Gen. Physiol. 1968, 52, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, M.E. Cholinoceptive and adrenoceptive properties of the tunicate heart pacemaker. Biol. Bull. 1968, 135, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pomares, J.M.; Gonzalez-Rosa, J.M.; Munoz-Chapuli, R. Building the vertebrate heart—An evolutionary approach to cardiac development. Int. J. Dev. Biol. 2009, 53, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Levine, M. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc. Natl. Acad. Sci. USA 2003, 100, 11469–11473. [Google Scholar] [CrossRef] [PubMed]
- Pope, E.C.; Rowley, A.F. The heart of Ciona intestinalis: Eicosanoid-generating capacity and the effects of precursor fatty acids and eicosanoids on heart rate. J. Exp. Biol. 2002, 205, 1577–1583. [Google Scholar] [PubMed]
- Keefner, K.R.; Akers, T.K. Sodium localization in digitalis- and epinephrine-treated tunicate myocardium. Comp. Gen. Pharmacol. 1971, 2, 415–422. [Google Scholar] [CrossRef]
- Di Gregorio, A. T-Box Genes and Developmental Gene Regulatory Networks in Ascidians. Curr. Top. Dev. Biol. 2017, 122, 55–91. [Google Scholar] [PubMed]
- Stolfi, A.; Gainous, T.B.; Young, J.J.; Mori, A.; Levine, M.; Christiaen, L. Early chordate origins of the vertebrate second heart field. Science 2010, 329, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.C.; Chien, K.R. Fashioning the vertebrate heart: Earliest embryonic decisions. Development 1997, 124, 2099–2117. [Google Scholar] [PubMed]
- Christoffels, V.M.; Smits, G.J.; Kispert, A.; Moorman, A.F. Development of the pacemaker tissues of the heart. Circ. Res. 2010, 106, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Reckova, M.; de Almeida, A.; Sedmerova, M.; Biermann, M.; Volejnik, J.; Sarre, A.; Raddatz, E.; McCarthy, R.A.; Gourdie, R.G.; et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1152–H1160. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Moorman, A.F. Development of the cardiac conduction system: Why are some regions of the heart more arrhythmogenic than others? Circ. Arrhythm. Electrophysiol. 2009, 2, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Forouhar, A.S.; Liebling, M.; Hickerson, A.; Nasiraei-Moghaddam, A.; Tsai, H.J.; Hove, J.R.; Fraser, S.E.; Dickinson, M.E.; Gharib, M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 2006, 312, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.C.; Shaw, R.M.; Jungblut, B.; Huisken, J.; Ferrer, T.; Arnaout, R.; Scott, I.; Beis, D.; Xiao, T.; Baier, H.; et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008, 6, e109. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Higashijima, S.; Miyawaki, A.; Okamura, Y. Visualizing voltage dynamics in zebrafish heart. J. Physiol. 2010, 588, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, J.; Vornanen, M. Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1023–R1032. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and functional characterization of cardiac pacemaker cells in zebrafish. PLoS ONE 2012, 7, e47644. [Google Scholar] [CrossRef] [PubMed]
- De Pater, E.; Clijsters, L.; Marques, S.R.; Lin, Y.F.; Garavito-Aguilar, Z.V.; Yelon, D.; Bakkers, J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 2009, 136, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Stoyek, M.R.; Croll, R.P.; Smith, F.M. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J. Comp. Neurol. 2015, 523, 1683–1700. [Google Scholar] [CrossRef] [PubMed]
- De Pater, E.; Ciampricotti, M.; Priller, F.; Veerkamp, J.; Strate, I.; Smith, K.; Lagendijk, A.K.; Schilling, T.F.; Herzog, W.; Abdelilah-Seyfried, S.; et al. Bmp signaling exerts opposite effects on cardiac differentiation. Circ. Res. 2012, 110, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Chocron, S.; Verhoeven, M.C.; Rentzsch, F.; Hammerschmidt, M.; Bakkers, J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev. Biol. 2007, 305, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Berger, I.M.; Glaser, A.; Bacon, C.; Li, L.; Gretz, N.; Steinbeisser, H.; Rottbauer, W.; Just, S.; Rappold, G. Islet1 is a direct transcriptional target of the homeodomain transcription factor Shox2 and rescues the Shox2-mediated bradycardia. Basic Res. Cardiol. 2013, 108, 339. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, C.H.; Espinoza-Lewis, R.A.; Jiao, Z.; Sheu, I.; Hu, X.; Lin, M.; Zhang, Y.; Chen, Y. Functional redundancy between human SHOX and mouse Shox2 genes in the regulation of sinoatrial node formation and pacemaking function. J. Biol. Chem. 2011, 286, 17029–17038. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, E.J.; McEwen, G.K.; Callaway, H.; Elgar, G. Functional analysis of conserved non-coding regions around the short stature hox gene (shox) in whole zebrafish embryos. PLoS ONE 2011, 6, e21498. [Google Scholar] [CrossRef] [PubMed]
- Stoyek, M.R.; Quinn, T.A.; Croll, R.P.; Smith, F.M. Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H676–H688. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Boukens, B.J.; Postma, A.V.; Gunst, Q.D.; van den Hoff, M.J.; Moorman, A.F.; Wang, T.; Christoffels, V.M. Identifying the evolutionary building blocks of the cardiac conduction system. PLoS ONE 2012, 7, e44231. [Google Scholar] [CrossRef] [PubMed]
- Brette, F.; Luxan, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Nemtsas, P.; Wettwer, E.; Christ, T.; Weidinger, G.; Ravens, U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J. Mol. Cell. Cardiol. 2010, 48, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Alday, A.; Alonso, H.; Gallego, M.; Urrutia, J.; Letamendia, A.; Callol, C.; Casis, O. Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol. Res. 2014, 84, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.S.; Baker, K.; Fishman, M.C. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1711–H1719. [Google Scholar] [PubMed]
- Baker, K.; Warren, K.S.; Yellen, G.; Fishman, M.C. Defective ”pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 1997, 94, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Fouquet, B.; Chen, J.N.; Warren, K.S.; Weinstein, B.M.; Meiler, S.E.; Mohideen, M.A.; Neuhauss, S.C.; Solnica-Krezel, L.; Schier, A.F.; et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996, 123, 285–292. [Google Scholar] [PubMed]
- Chi, N.C.; Bussen, M.; Brand-Arzamendi, K.; Ding, C.; Olgin, J.E.; Shaw, R.M.; Martin, G.R.; Stainier, D.Y. Cardiac conduction is required to preserve cardiac chamber morphology. Proc. Natl. Acad. Sci. USA 2010, 107, 14662–14667. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Chin, A.J.; Valdimarsson, G.; Finis, C.; Sonntag, J.M.; Choi, B.Y.; Tao, L.; Balasubramanian, K.; Bell, C.; Krufka, A.; et al. Developmental regulation and expression of the zebrafish connexin43 gene. Dev. Dyn. 2005, 233, 890–906. [Google Scholar] [CrossRef] [PubMed]
- Christie, T.L.; Mui, R.; White, T.W.; Valdimarsson, G. Molecular cloning, functional analysis, and RNA expression analysis of connexin45.6: A zebrafish cardiovascular connexin. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1623–H1632. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.J.; Liao, C.F. Zebrafish M2 muscarinic acetylcholine receptor: Cloning, pharmacological characterization, expression patterns and roles in embryonic bradycardia. Br. J. Pharmacol. 2002, 137, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Mercola, M.; Guzzo, R.M.; Foley, A.C. Cardiac Development in the Frog. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 87–102. [Google Scholar]
- Bartlett, H.L.; Scholz, T.D.; Lamb, F.S.; Weeks, D.L. Characterization of embryonic cardiac pacemaker and atrioventricular conduction physiology in Xenopus laevis using noninvasive imaging. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2035–H2041. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, H.L.; Escalera, R.B., 2nd; Patel, S.S.; Wedemeyer, E.W.; Volk, K.A.; Lohr, J.L.; Reinking, B.E. Echocardiographic assessment of cardiac morphology and function in Xenopus. Comp. Med. 2010, 60, 107–113. [Google Scholar] [PubMed]
- Arbel, E.R.; Liberthson, R.; Langendorf, R.; Pick, A.; Lev, M.; Fishman, A.P. Electrophysiological and anatomical observations on the heart of the African lungfish. Am. J. Physiol. 1977, 232, H24–H34. [Google Scholar] [PubMed]
- Koshiba-Takeuchi, K.; Mori, A.D.; Kaynak, B.L.; Cebra-Thomas, J.; Sukonnik, T.; Georges, R.O.; Latham, S.; Beck, L.; Henkelman, R.M.; Black, B.L.; et al. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature 2009, 461, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wyneken, J. Normal reptile heart morphology and function. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; van den Berg, G.; van den Doel, R.; Oostra, R.J.; Wang, T.; Moorman, A.F. Development of the hearts of lizards and snakes and perspectives to cardiac evolution. PLoS ONE 2013, 8, e63651. [Google Scholar] [CrossRef] [PubMed]
- Kik, M.J.L.; Mitchell, M.A. Reptile cardiology: A review of anatomy and physiology, diagnostic approaches, and clinical disease. Semin. Avian Exot. Pet Med. 2005, 14, 52–60. [Google Scholar] [CrossRef]
- Mitchell, M.A. Reptile cardiology. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A., 2nd; Burggren, W.W. Development of cardiac form and function in ectothermic sauropsids. J. Morphol. 2009, 270, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.; Crossley, D.A., 2nd. Comparative cardiovascular development: Improving the conceptual framework. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 661–674. [Google Scholar] [CrossRef]
- Geddes, L.A. Electrocardiograms from the turtle to the elephant that illustrate interesting physiological phenomena. Pacing Clin. Electrophysiol. 2002, 25, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Koprla, E.C. The ultrastructure of alligator conductive tissue: An electron microscopic study of the sino-atrial node. Acta Physiol. Hung. 1987, 69, 71–84. [Google Scholar] [PubMed]
- Taylor, E.W.; Leite, C.A.; Skovgaard, N. Autonomic control of cardiorespiratory interactions in fish, amphibians and reptiles. Braz. J. Med. Biol. Res. 2010, 43, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Franklin, C.E. Physiological mechanisms of thermoregulation in reptiles: A review. J. Comp. Physiol. B 2005, 175, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.W.; Leite, C.A.; Levings, J.J. Central control of cardiorespiratory interactions in fish. Acta Histochem. 2009, 111, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.W.; Jordan, D.; Coote, J.H. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol. Rev. 1999, 79, 855–916. [Google Scholar] [PubMed]
- Bressan, M.; Liu, G.; Mikawa, T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Steijn, R.; Kolditz, D.P.; Mahtab, E.A.; Askar, S.F.; Bax, N.A.; VAN DER Graaf, L.M.; Wisse, L.J.; Passier, R.; Pijnappels, D.A.; Schalij, M.J.; et al. Electrical activation of sinus venosus myocardium and expression patterns of RhoA and Isl-1 in the chick embryo. J. Cardiovasc. Electrophysiol. 2010, 21, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kamino, K. Functiogenesis of cardiac pacemaker activity. J. Physiol. Sci. 2015, 66, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189. [Google Scholar] [PubMed]
- Opthof, T. The mammalian sinoatrial node. Cardiovasc. Drugs Ther. 1988, 1, 573–597. [Google Scholar] [CrossRef] [PubMed]
- Challice, C.E.; Viragh, S. Origin and early differentiation of the sinus node in the mouse embryo heart. Adv. Myocardiol. 1980, 1, 267–277. [Google Scholar] [PubMed]
- Reecy, J.M.; Li, X.; Yamada, M.; DeMayo, F.J.; Newman, C.S.; Harvey, R.P.; Schwartz, R.J. Identification of upstream regulatory regions in the heart-expressed homeobox gene Nkx2-5. Development 1999, 126, 839–849. [Google Scholar] [PubMed]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.; et al. Formation of the venous pole of the heart from an Nkx2–5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 dynamically marks the first heart field and conduction system precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, Q.; Cattaneo, P.; Zhuang, S.; Gong, X.; Spann, N.J.; Jiang, C.; Cao, X.; Zhao, X.; Zhang, X.; et al. Transcription factor ISL1 is essential for pacemaker development and function. J. Clin. Investig. 2015, 125, 3256–3268. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.; Martin, J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.; Domínguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.; Kispert, A.; Brown, N.A.; Moorman, A.F.; Christoffels, V.M. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Begemann, G.; Gibert, Y.; Meyer, A.; Ingham, P.W. Cloning of zebrafish T-box genes tbx15 and tbx18 and their expression during embryonic development. Mech. Dev. 2002, 114, 137–141. [Google Scholar] [CrossRef]
- Kapoor, N.; Liang, W.; Marbán, E.; Cho, H.C. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 2013, 31, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Dawkins, J.F.; Cho, H.C.; Marbán, E.; Cingolani, E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 2014, 6, 245ra94. [Google Scholar] [CrossRef] [PubMed]
- Greulich, F.; Trowe, M.O.; Leffler, A.; Stoetzer, C.; Farin, H.F.; Kispert, A. Misexpression of Tbx18 in cardiac chambers of fetal mice interferes with chamber-specific developmental programs but does not induce a pacemaker-like gene signature. J. Mol. Cell. Cardiol. 2016, 97, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes. Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Boink, G.J.; Boukens, B.J.; Verkerk, A.O.; van den Boogaard, M.; den Haan, A.D.; Hoogaars, W.M.; Buermans, H.P.; de Bakker, J.M.; Seppen, J.; et al. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc. Res. 2012, 94, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Lewis, R.A.; Liu, H.; Sun, C.; Chen, C.; Jiao, K.; Chen, Y. Ectopic expression of Nkx2.5 suppresses the formation of the sinoatrial node in mice. Dev. Biol. 2011, 356, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, D.; Ye, W.; Liu, C.; Gu, S.; Sinsheimer, N.R.; Song, Z.; Li, X.; Chen, C.; Song, Y.; et al. The short stature homeobox 2 (Shox2)-bone morphogenetic protein (BMP) pathway regulates dorsal mesenchymal protrusion development and its temporary function as a pacemaker during cardiogenesis. J. Biol. Chem. 2015, 290, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, R.J.; Hahurij, N.D.; Kuijper, S.; Just, S.; Wisse, L.J.; Deissler, K.; Maxelon, T.; Anastassiadis, K.; Spitzer, J.; Hardt, S.E.; et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation 2007, 115, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.D.; Zhu, Y.; Vahora, I.; Nieman, B.; Koshiba-Takeuchi, K.; Davidson, L.; Pizard, A.; Seidman, J.G.; Seidman, C.E.; Chen, X.J.; et al. Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev. Biol. 2006, 297, 566–586. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Vedantham, V.; Galang, G.; Evangelista, M.; Deo, R.C.; Srivastava, D. RNA sequencing of mouse sinoatrial node reveals an upstream regulatory role for Islet-1 in cardiac pacemaker cells. Circ. Res. 2015, 116, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Devalla, H.D.; Anderson, R.H.; Passier, R.; Christoffels, V.M.; Moorman, A.F. Molecular analysis of patterning of conduction tissues in the developing human heart. Circ. Arrhythm. Electrophysiol. 2011, 4, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Mehrkens, D.; Friedrich, F.W.; Stubbendorff, M.; Hua, X.; Müller, J.C.; Schrepfer, S.; Evans, S.M.; Carrier, L.; Eschenhagen, T. Localization of Islet-1-positive cells in the healthy and infarcted adult murine heart. Circ. Res. 2012, 110, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Barnett, P.; van Duijvenboden, K.; Weber, D.; Gessler, M.; Christoffels, V.M. GATA—Dependent regulatory switches establish atrioventricular canal specificity during heart development. Nat. Commun. 2014, 5, 3680. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, M.C.; Haase, C.; Christoffels, V.M.; Weidinger, G.; Bakkers, J. Wnt signaling regulates atrioventricular canal formation upstream of BMP and Tbx2. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Davis, C.A.; Potthoff, M.J.; Haberland, M.; Fielitz, J.; Qi, X.; Hill, J.A.; Richardson, J.A.; Olson, E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007, 21, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, V.; Evangelista, M.; Huang, Y.; Srivastava, D. Spatiotemporal regulation of an Hcn4 enhancer defines a role for Mef2c and HDACs in cardiac electrical patterning. Dev. Biol. 2013, 373, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Craig, C.; Lamothe, M.; Sarunic, M.V.; Beg, M.F.; Tibbits, G.F. Construction and use of a zebrafish heart voltage and calcium optical mapping system, with integrated electrocardiogram and programmable electrical stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R755–R768. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Rottbauer, W.; Just, S. Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert Opin. Drug Discov. 2015, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.N.; Jopling, C.; van Eeden, F.J. Zebrafish as a model of cardiac disease. Prog. Mol. Biol. Transl. Sci. 2014, 124, 65–91. [Google Scholar] [PubMed]
- Lodder, E.M.; De Nittis, P.; Koopman, C.D.; Wiszniewski, W.; Moura de Souza, C.F.; Lahrouchi, N.; Guex, N.; Napolioni, V.; Tessadori, F.; Beekman, L.; et al. GNB5 Mutations Cause an Autosomal-Recessive Multisystem Syndrome with Sinus Bradycardia and Cognitive Disability. Am. J. Hum. Genet. 2016, 99, 704–710. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhard, S.; Van Eif, V.; Garric, L.; Christoffels, V.M.; Bakkers, J. On the Evolution of the Cardiac Pacemaker. J. Cardiovasc. Dev. Dis. 2017, 4, 4. https://doi.org/10.3390/jcdd4020004
Burkhard S, Van Eif V, Garric L, Christoffels VM, Bakkers J. On the Evolution of the Cardiac Pacemaker. Journal of Cardiovascular Development and Disease. 2017; 4(2):4. https://doi.org/10.3390/jcdd4020004
Chicago/Turabian StyleBurkhard, Silja, Vincent Van Eif, Laurence Garric, Vincent M. Christoffels, and Jeroen Bakkers. 2017. "On the Evolution of the Cardiac Pacemaker" Journal of Cardiovascular Development and Disease 4, no. 2: 4. https://doi.org/10.3390/jcdd4020004
APA StyleBurkhard, S., Van Eif, V., Garric, L., Christoffels, V. M., & Bakkers, J. (2017). On the Evolution of the Cardiac Pacemaker. Journal of Cardiovascular Development and Disease, 4(2), 4. https://doi.org/10.3390/jcdd4020004






