Management of Arrhythmias in Heart Failure
Abstract
:1. Introduction
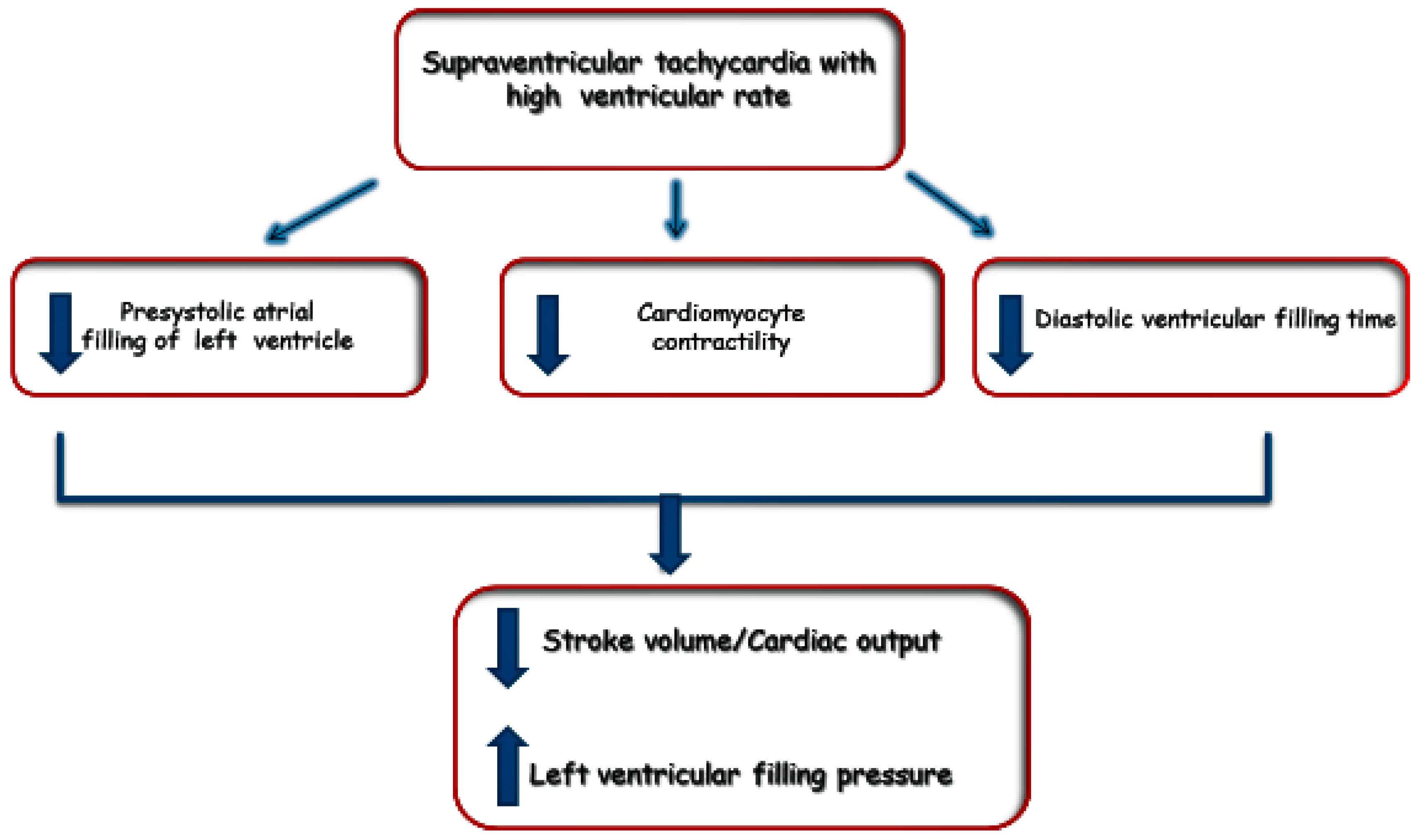
2. Pathophysiology of Tachyarrhytmias in HF
2.1. Structural and Hemodynamic Abnormalities
2.1.1. Myocardial
2.1.2. Chamber Hypertrophy and Stretch
2.2. Metabolic Abnormalities
2.2.1. Neurohormonal Activation
2.2.2. Electrolyte Abnormalities
2.3. Pharmacologic Agents
2.4. Electrophysiologic Changes in Heart Failure
2.4.1. Action Potential Prolongation
2.4.2. Calcium Handling
2.4.3. Role of the Sodium–Calcium Exchanger
2.4.4. Voltage-Dependent Potassium Channels
3. Pathophysiology of Bradyarrhytmias in HF
3.1. Sinus Node Dysfunction
3.2. Atrio-Ventricular Node Dysfunction
4. Clinical Impact of Tachyarrhythmias in HF
5. Clinical Impact of Bradyarrhythmias in HF
6. Management of Supraventricular Tachyarrhythmias in HF
6.1. Management of Atrial Fibrillation
6.1.1. Rate Control
6.1.2. Rhythm Control
6.1.3. Rate Control versus Rhythm Control
6.1.4. Anticoagulation
6.1.5. Catheter Ablation
6.2. Management of Atrial Flutter
6.3. Management of Other Type of Supraventricular Arrhytmias
7. Management of Ventricular Tachyarrhythmias in HF
7.1. Pharmacologic Management
7.2. Catheter Ablation
7.2.1. Ablation of Premature Ventricular Complex and Non-Sustained Ventricular Tachycardia
7.2.2. Ablation of Ischemic Cardiomyopathy
7.2.3. Ablation of Non Ischemic Cardiomyopathy
8. Sudden Cardiac Death
8.1. Sudden Cardiac Death in Patients with HFrEF
8.1.1. Left Ventricular Systolic Dysfunction
8.1.2. T-Wave Alternans
8.1.3. Signal-Averaged Electrocardiogram
8.1.4. Study of Autonomic Tone
8.1.5. Electrophysiologic Study
8.2. Sudden Cardiac Death in Patients with HFpEF
9. Management of Bradyarrhythmias in HF
10. Conclusions
Conflicts of Interest
Abbreviations
| ACE-inhibitors | Angiotensin Converting Enzyme inhibitors |
| ARBs | Angiotensin Receptor Blockers |
| AF | Atrial Fibrillation |
| AFFIRM | Atrial Fibrillation Follow-up Investigation of Rhythm Management |
| AFL | Atrial Flutter |
| ARNI | Angiotensin Receptor/Neprilysin Inhibitors |
| AT | Atrial Tachycardia |
| AVRT | Atrio-Ventricular reentrant (or reciprocating) tachycardia |
| BRS | Baroreflex Sensitivity |
| CHARM | Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity |
| COR | Class of Recommendation |
| DCM | Dilated Cardiomyopathy |
| ESC | European Society of Cardiology |
| HF | Heart Failure |
| HFrEF | Heart Failure Reduced Ejection Fraction |
| HFmEF | Heart Failure mid reduction Ejection Fraction |
| HFpEF | Heart Failure Preserved Ejection Fraction |
| ICD | Implantable Cardioverter Defibrillator |
| I-PRESERVE | Irbesartan in Heart Failure with Preserved Ejection Fraction Study |
| MADIT II | Multicenter Automatic Defibrillator Implantation Trial Investigator II |
| MRAs | Mineral Receptor Antagonists |
| MTWA | Microvolt T-wave alternans |
| NYHA | New York Heart Association |
| NSVT | Non Sustained Ventricular Tachycardia |
| PABA-CHF | Pulmonary Vein Antrum Isolation vs. AV Node Ablation With Biventricular Pacing for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure |
| PARAGON-HF | Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction |
| PVCs | Premature Ventricular Complexes |
| RACE II | The Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient versus Strict Rate Control II |
| SR | Sinus Rhythm |
| SVAs | Supraventricular arrhythmias |
| SVT | Sustained Ventricular Tachycardia |
| SCD | Sudden Cardiac Death |
| TOPCAT | Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist |
| Vas | Ventricular Arrhythmias |
| VT | Ventricular Tachycardia |
| VF | Ventricular Fibrillation |
References
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation 2009, 119, 391–479. [Google Scholar]
- Ehrlich, J.R.; Nattel, S.; Hohnloser, S. Atrial fibrillation and congestive heart failure: Specific considerations at the intersection of two common and important cardiac disease sets. J. Cardiovasc. Electrophysiol. 2009, 13, 399–405. [Google Scholar] [CrossRef]
- Olsson, L.G.; Swedberg, K.; Ducharme, A.; Granger, C.B.; Michelson, E.L.; McMurray, J.J.V.; Puu, M.; Yusuf, S.; Pfeffer, M.A. Atrial Fibrillation and Risk of Clinical Events in Chronic Heart Failure with and Without Left Ventricular Systolic Dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) Program. J. Am. Coll. Cardiol. 2006, 47, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, M.; Cioffi, G.; Traversi, E.; Pinna, G.D.; Cobelli, F.; Tavazzi, L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: A prospective study in 344 patients with baseline sinus rhythm. J. Am. Coll. Cardiol. 1998, 32, 197–204. [Google Scholar] [CrossRef]
- Luu, M.; Stevenson, W.G.; Stevenson, L.W.; Baron, K.; Walden, J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989, 80, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar]
- Stevenson, W.G.; Ellison, K.E.; Sweeney, M.O.; Epstein, L.M.; Maisel, W.H. Management of arrhythmias in heart failure. Cardiol. Rev. 2002, 10, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Schuger, C.D.; Goldstein, S. Sudden death in heart failure: Underlying electrophysiologic mechanisms. Heart Fail. Rev. 2002, 7, 255–260. [Google Scholar] [CrossRef] [PubMed]
- De Bakker, J.M.; Van Capelle, F.J.; Janse, M.J. Myocardial infarction: Slow conduction in the infarcted human heart: “Zigzag” course of activation. Circulation 1998, 88, 915–992. [Google Scholar] [CrossRef]
- Stevenson, W.G.; Khan, H.; Sager, P.; Saxon, L.A.; Middlekauff, H.R.; Natterson, P.D.; Wiener, I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation 1993, 88, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Pye, M.P.; Cobbe, S.M. Arrhythmogenesis in experimental models of heart failure: The role of increased load. Cardiovasc. Res. 1996, 32, 248–257. [Google Scholar] [CrossRef]
- Zipes, D.P.; Jalife, J. Cardiac Electrophysiology: From Cell to Bedside, 6th ed.; Saunders Company: Philadelphia, PA, USA, 2013. [Google Scholar]
- Zabel, M.; Koller, B.; Sachs, F.; Franz, M. Stretch-induced voltage changes in the isolated beating heart: Importance of the timing of stretch and implication for stretch-activated ion channels. Cardiovasc. Res. 1996, 32, 120–130. [Google Scholar] [CrossRef]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Interventional Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar]
- Schwartz, P.J.; La Rovere, M.T.; Vanoli, E. Autonomic nervous system and sudden cardiac death: Experimental basis and clinical observations for post-myocardial risk stratification. Circulation 1992, 85, 77–91. [Google Scholar]
- Meredith, I.T.; Eisenhofer, G.; Lambert, G.W.; Dewar, E.M.; Jennings, G.L.; Esler, M.D. Cardiac sympathetic nervous activity in congestive heart failure: Evidence for increased neuronal norepinephrine release. Circulation 1993, 88, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.M.; Fishbein, M.C.; Han, J.B.; Lai, W.W.; Lai, A.C.; Wu, T.J.; Czer, L.; Wolf, P.L.; Denton, T.A.; Shintaku, I.P.; et al. Relationship between regional cardiac hyper innervation and ventricular arrhythmias. Circulation 2000, 101, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Gettes, L.S. Electrolyte abnormalities underlying lethal ventricular arrhythmias. Circulation 1992, 85, 70–76. [Google Scholar]
- Hoss, S.; Elizur, Y.; Luria, D.; Keren, A.; Lotan, C.; Gotsman, I. Serum Potassium Levels and Outcome in Patients With Chronic Heart Failure. Am. J. Cardiol. 2016, 118, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, G.F.; Zipes, D.P. What causes sudden death in heart failure? Circ. Res. 2004, 95, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Urso, C.; Brucculeri, S.; Caimi, G. Acid-base and electrolyte abnormalities in heart failure: Pathophysiology and implications. Heart Fail. Rev. 2015, 20, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Heist, E.K.; Ruskin, J.N. Drug induced arrhythmia. Circulation 2010, 122, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, M.W.; Krishnan, S. Mechanisms of Ventricular Arrhythmias in Heart Failure. Curr. Heart Fail. Rep. 2005, 2, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Janse, M.J.; Vermeulen, J.T.; Opthof, T. Arrhythmogenesis in heart failure. J. Cardiovasc. Electrophysiol. 2001, 12, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Wakili, R.; Qi, X.Y.; Chartier, D.; Boknik, P.; Kääb, S.; Ravens, U.; Coutu, P.; Dobrev, D.; Nattel, S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ. Arrhythm. Electrophysiol. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Prestle, J.; Quinn, F.R.; Smith, G.L. Ca2+-handling proteins and heart failure: Novel molecular targets? Curr. Med. Chem. 2003, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Janardhan, A.; Efimov, I.R. Remodeling of calcium handling in human heart failure. Adv. Exp. Med. Biol. 2012, 740, 1145–1174. [Google Scholar] [PubMed]
- Luo, M.; Anderson, M.E. Mechanisms of altered Ca2+ handling in heart Failure. Circ. Res. 2013, 113, 690–708. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Tan, H.L.; Baartscheer, T.; Ravesloot, J.H. Limited role of Ca2+-activated Cl− current in early afterdepolarisations. Neth. Heart J. 2002, 10, 506–511. [Google Scholar] [PubMed]
- DeSantiago, J.; Ai, X.; Islam, M.; Acuna, G.; Ziolo, M.T.; Bers, D.M.; Pogwizd, S.M. Arrhythmogenic effects of β2-adrenergic stimulation in the failing heart are due to enhanced SR Ca load. Circ. Res. 2008, 102, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, K.S.; Weber, C.R.; Bers, D.M. Cardiac Na+–Ca2+ exchanger: Dynamics of Ca2+-dependent activation and deactivation in intact myocytes. J. Physiol. 2013, 591, 2067–2086. [Google Scholar] [CrossRef] [PubMed]
- Kho, C.; Lee, A.; Hajjar, R.J. Altered sarcoplasmic reticulum calcium cycling—Targets for heart failure therapy. Nat. Rev. Cardiol. 2012, 9, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hill, J.A. Electrophysiological remodeling in heart failure. J. Mol. Cell. Cardiol. 2010, 48, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Beuckelmann, D.J.; Näbauer, M.; Erdmann, E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993, 73, 379–385. [Google Scholar] [CrossRef] [PubMed]
- De Marneffe, M.; Gregoire, J.M.; Waterschoot, P.; Kestemont, M.P. The sinus node function: Normal and pathological. Eur. Heart J. 1993, 1993, 649–654. [Google Scholar] [CrossRef]
- Deal, N. Evaluation and management of bradydysrhythmias in the emergency department. Emerg. Med. Pract. 2013, 15, 1–15. [Google Scholar] [PubMed]
- McVay, M.R. Atrioventricular block a review. S D J. Med. 1984, 37, 21–26. [Google Scholar] [PubMed]
- Barold, S.S.; Hayes, D.L. Second-degree atrioventricular block: A reappraisal. Mayo Clin. Proc. 2001, 76, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Rosen, K.M.; Dhingra, R.C.; Loeb, H.S.; Rahimtoola, S.H. Chronic heart block in adults. Clinical and electrophysiological observations. Arch. Intern. Med. 1973, 131, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Puech, P.; Wainwright, R.J. Clinical electrophysiology of atrioventricular block. Cardiol. Clin. 1983, 1, 209–215. [Google Scholar] [PubMed]
- Kearney, K.; Ellingson, S.; Stout, K.; Patton, K.K. From Bradycardia to Tachycardia: Complete Heart Block. Am. J. Med. 2015, 128, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Maisel, W.H.; Stevenson, L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003, 91, 2–8. [Google Scholar] [CrossRef]
- Hugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar]
- Van Deursen, V.M.; Urso, R.; Laroche, C.; Damman, K.; Dahlstrom, U.; Tavazzi, L.; Maggioni, A.P.; Voors, A.A. Co-morbidities in patients with heart failure: An analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014, 16, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Figueredo, V.M. Tachycardia mediated cardiomyopathy: Pathophysiology, mechanisms, clinical features and management. Int. J. Cardiol. 2014, 172, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Apostolakis, S.; Lane, D.A.; Lip, G.Y. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: A systematic review. Clin. Ther. 2014, 36, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, J. Arrhythmias and mortality in congestive heart failure. Am. J. Cardiol. 1990, 65, 42–48. [Google Scholar] [CrossRef]
- Lo, R.; Hsia, H. Ventricular arrhythmias in heart failure patients. Cardiol. Clin. 2008, 26, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Hsia, H.H.; Jessup, M.L.; Marchlinski, F.E. Debate: Do all patients with heart failure require implantable defibrillators to prevent sudden death? Curr. Control Trials Cardiovasc. Med. 2000, 65, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.J.; Stevenson, L.W. Sudden death in heart failure: Bradyarrhythmias and secondary causes. Cardiol. Rev. 1994, 33, 546–555. [Google Scholar] [CrossRef]
- Lu, H.T.; Kam, J.; Nordin, R.B.; Khelae, S.K.; Wang, J.M.; Choy, C.N.; Lee, C.Y. Beta-blocker use and risk of symptomatic bradyarrhythmias: A hospital-based case-control study. J. Geriatr. Cardiol. 2016, 13, 749–759. [Google Scholar] [PubMed]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [PubMed]
- Kotecha, D.; Holmes, J.; Krum, H.; Altman, D.G.; Manzano, L.; Cleland, J.G.; Lip, G.Y.; Coats, A.J.; Andersson, B.; Kirchhof, P.; et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: An individual-patient data meta-analysis. Lancet 2014, 384, 2235–2243. [Google Scholar] [CrossRef]
- Bhuriya, R.; Singh, M.; Sethi, A.; Molnar, J.; Bahekar, A.; Singh, P.P.; Khosla, S.; Arora, R. Prevention of recurrent atrial fibrillation with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: A systematic review and meta-analysis of randomized trials. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.M.; Connolly, S.J. Atrial fibrillation in heart failure: Stroke risk stratification and anticoagulation. Heart Fail. Rev. 2014, 19, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Piccini, J.P. Atrial fibrillation in heart failure: What should we do? Eur. Heart J. 2015, 36, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Groenveld, H.F.; Crijns, H.J.; Tuininga, Y.S.; Tijssen, J.G.; Alings, A.M.; Hillege, H.L.; Bergsma-Kadijk, J.A.; Cornel, J.H.; Kamp, O.; et al. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010, 362, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Heinzel, F.R.; Gaita, F.; Juanatey, J.R.; Le Heuzey, J.Y.; Potpara, T.; Svendsen, J.H.; Vos, M.A.; Anker, S.D.; Coats, A.J.; et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2016, 18, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Crijns, H.J.; Weijs, B.; Fairley, A.M.; Lewalter, T.; Maggioni, A.P.; Martín, A.; Ponikowski, P.; Rosenqvist, M.; Sanders, P.; Scanavacca, M.; et al. Contemporary real life cardioversion of atrial fibrillation: Results from the multinational RHYTHM-AF study. Int. J. Cardiol. 2014, 172, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; George, L.K.; Koshy, S.K. Safety and efficacy of ibutilide in cardioversion of atrial flutter and fibrillation. J. Am. Board Fam. Med. 2011, 24, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P.C.; Singh, B.N.; Ellenbogen, K.; Fisher, S.; Fletcher, R.; Singh, S.N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: Observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation 1998, 98, 2574–2579. [Google Scholar] [PubMed]
- Zimetbaum, P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012, 125, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [PubMed]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.; Buxton, A.E.; Camm, A.J.; et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008, 25, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, K.G.; Laufs, U.; Endres, M. Chronic heart failure and ischemic stroke. Stroke 2011, 42, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Melgaard, L.; Gorst-Rasmussen, A.; Lane, D.A.; Rasmussen, L.H.; Larsen, T.B.; Lip, G.Y. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015, 314, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; Brown, L. Anticoagulation in atrial fibrillation and chronic heart failure: The risk and drug of choice. Curr. Opin. Cardiol. 2016, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.F.; Jaïs, P.; Sanders, P.; Garrigue, S.; Hocini, M.; Sacher, F.; Takahashi, Y.; Rotter, M.; Pasquié, J.L.; Scavée, C.; et al. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004, 351, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Jaïs, P.; Cummings, J.; Di Biase, L.; Sanders, P.; Martin, D.O.; Kautzner, J.; Hao, S.; Themistoclakis, S.; Fanelli, R.; et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008, 359, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Daoud, E.G. Atrioventricular junction ablation for atrial fibrillation. Heart Fail. Clin. 2016, 12, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Alboni, P.; Scarfò, S.; Fucà, G.; Paparella, N.; Mele, D. Atrial and ventricular pressures in atrial flutter. Pacing Clin. Electrophysiol. 1999, 22, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Peyrol, M.; Sbragia, P.; Bonello, L.; Lévy, S.; Paganelli, F. Characteristics of isolated atrial flutter versus atrial flutter combined with atrial fibrillation. Arch. Cardiovasc. Dis. 2011, 104, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Glover, B.M.; Chen, J.; Hong, K.L.; Boveda, S.; Baranchuk, A.; Haugaa, K.H.; Dorian, P.; Potpara, T.S.; Crystal, E.; Mitchell, B.; et al. Catheter ablation for atrial flutter: A survey by the European Heart Rhythm Association and Canadian Heart Rhythm Society. Europace 2017. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, K.C.; Kistler, P.M.; Kalman, J.M. Atrial tachycardia: Mechanisms, diagnosis, and management. Curr. Probl. Cardiol. 2005, 30, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Rubart, M.; Zipes, D.P. Arrhythmias, sudden death and syncope. In Braunwald’s Heart Disease, 10th ed.; Libby, P., Bonow, R.O., Mann, D.L., Zipes, D., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2014; pp. 909–921. [Google Scholar]
- Rai, V.; Agrawal, D.K. Role of risk stratification and genetics in sudden cardiac death. Can. J. Physiol. Pharmacol. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Link, M.S.; Atkins, D.L.; Passman, R.S.; Halperin, H.R.; Samson, R.A.; White, R.D.; Cudnik, M.T.; Berg, M.D.; Kudenchuk, P.J.; Kerber, R.E. Part 6: Electrical therapies: Automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Nolan, J.P.; Böttiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, K.C.; Lau, D.H.; Sanders, P. The diagnosis and management of ventricular arrhythmias. Nat. Rev. Cardiol. 2011, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Srivathsan, K.; Ng, D.W.; Mookadam, F. Ventricular tachycardia and ventricular fibrillation. Expert Rev. Cardiovasc. Ther. 2009, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Aliot, E.M.; Stevenson, W.G.; Almendral-Garrote, J.M.; Bogun, F.; Calkins, C.H.; Delacretaz, E.; Della Bella, P.; Hindricks, G.; Jaïs, P.; Josephson, M.E.; et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009, 6, 886–933. [Google Scholar] [PubMed]
- Lee, G.K.; Klarich, K.W.; Grogan, M.; Cha, Y.M. Premature ventricular contraction-induced cardiomyopathy: A treatable condition. Circ. Arrhythm. Electrophysiol. 2012, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Daly, M.; Sacher, F.; Cochet, H.; Denis, A.; Derval, N.; Jesel, L.; Zellerhoff, S.; Lim, H.S.; Jadidi, A.; et al. Endocardial ablation to eliminate epicardial arrhythmia substrate in scar-related ventricular tachycardia. J. Am. Coll. Cardiol. 2014, 63, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kay, G.N. Optimal ablation strategies for different types of ventricular tachycardias. Nat. Rev. Cardiol. 2012, 9, 512–525. [Google Scholar] [CrossRef] [PubMed]
- John, R.M.; Stevenson, W.G. Catheter-based ablation for ventricular arrhythmias. Curr. Cardiol. Rep. 2011, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mathuria, N.; Tung, R.; Shivkumar, K. Advances in ablation of ventricular tachycardia in nonischemic cardiomyopathy. Curr. Cardiol. Rep. 2012, 14, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Shivkumar, K. Epicardial Ablation of Ventricular Tachycardia. Methodist. Debakey Cardiovasc. J. 2015, 11, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Gillespie, J.; Zareba, W.; Brown, M.W.; Goldenberg, I.; Klein, H.; Mcnitt, S.; Polonsky, S.; Andrews, M.; Dwyer, E.M.; et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverterdefibrillators. Heart Rhythm. 2009, 6, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Zelenkofske, S.; Mcmurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.; Verrier, R.L. Usefulness of T-wave alternans in sudden death risk stratification and guiding medical therapy. Ann. Noninvasive Electrocardiol. 2010, 15, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Verrier, R.L.; Klingenheben, T.; Malik, M.; El-Sherif, N.; Exner, D.V.; Hohnloser, S.H.; Ikeda, T.; Martínez, J.P.; Narayan, S.M.; Nieminen, T.; et al. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility—Consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J. Am. Coll. Cardiol. 2011, 58, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Verrier, R.L.; Klingenheben, T.; Malik, M.; El-Sherif, N.; Exner, D.V.; Hohnloser, S.H.; Ikeda, T.; Martínez, J.P.; Narayan, S.M.; Nieminen, T.; et al. Microvolt T-wave alternans testing has a role in arrhythmia risk stratification. J. Am. Coll. Cardiol. 2012, 59, 1572–1573. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.A.; Handelsman, H. Signal-averaged electrocardiography. Health Technol. Assess. 1998, 11, 1–15. [Google Scholar]
- Stein, K.M. Noninvasive risk stratification for sudden death: Signal-averaged electrocardiography, non sustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog. Cardiovasc. Dis. 2008, 51, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Wong, K.L.; Simson, M.B. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation 1993, 87, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Moss, A.J.; Myerburg, R.J.; et al. American College of Cardiology, American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J. Am. Coll. Cardiol. 2006, 48, 247–288. [Google Scholar]
- Singer, D.H.; Martin, G.J.; Magid, N.; Weiss, J.S.; Schaad, J.W.; Kehoe, R.; Zheutlin, T.; Fintel, D.J.; Hsieh, A.M.; Lesch, M. Low heart rate variability and sudden cardiac death. J. Electrocardiol. 1988, 21, 46–55. [Google Scholar] [CrossRef]
- Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Thomas, K.E.; Josephson, M.E. The role of electrophysiology study in risk stratification of sudden cardiac death. Prog. Cardiovasc. Dis. 2008, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, K.A.; Vouliotis, A.I.; Tsiachris, D.; Salourou, M.; Archontakis, S.; Dilaveris, P.; Gialernios, T.; Arsenos, P.; Karystinos, G.; Sideris, S.; et al. Primary prevention of sudden cardiac death in a non ischemic dilated cardiomyopathy population: Reappraisal of the role of programmed ventricular stimulation. Circ. Arrhythm. Electrophysiol. 2013, 6, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Patel, R.B.; Shah, S.J.; Butler, J. Sudden cardiac death in heart failure with preserved ejection fraction: A target for therapy? Heart Fail. Rev. 2016, 21, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Nahlawi, M.; Waligora, M.; Spies, S.M.; Bonow, R.O.; Kadish, A.H.; Goldberger, J.J. Left ventricular function during and after right ventricular pacing. J. Am. Coll. Cardiol. 2004, 44, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Dilaveris, P.; Pantazis, A.; Giannopoulos, G.; Synetos, A.; Gialafos, J.; Stefanadis, C. Upgrade to biventricular pacing in patients with pacing-induced heart failure: Can resynchronization do the trick? Europace 2006, 8, 352–357. [Google Scholar] [CrossRef] [PubMed]

| Structural and Hemodynamic Abnormalities | Myocardial scar |
| Left ventricular hypertrophy | |
| Left ventricular stretch | |
| Metabolic Abnormalities | Neurohormonal activation |
| Electrolyte abnormalities | |
| Electrophysiologic Changes | Prolongation of action potential |
| Changes of calcium homeostasis | |
| Changes of potassium current | |
| Others | Pharmacologic agents |
| Myocardial ischemia |
| 2012 ACC/AHA/HRS Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities | COR-LOE |
| Ischemic DCM (at least 40 days post-MI), LVEF less than or equal to 35%, NYHA functional Classes II or III | I-A |
| Non-ischemic DCM, LVEF less than or equal to 35%, NYHA functional Classes II or III | I-B |
| Ischemic DCM (at least 40 days post-MI) LVEF less than or equal to 35%, NYHA functional Class I | I-A |
| 2013 ACCF/AHA Guideline for the Management of Heart Failure | |
| HFrEF (irrespective of etiology, but in case of ischemic etiology at least 40 days post-MI), LVEF less or equal to 35%, NYHA Classes II or III | I-A |
| HFrEF (irrespective of etiology, but in case of ischemic etiology at least 40 days post-MI), LVEF less or equal to 30%, NYHA Class I | I-B |
| 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death | |
| Ischemic HFrEF (at least 6 weeks after myocardial infarction), LVEF less or equal to 35%, NYHA Classes II or III | I-A |
| Non ischemic HFrEF, LVEF less or equal to 35%, NYHA Classes II or III | I-B |
| Patients who are listed for heart transplant | IIa-C |
| 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | |
| Ischemic HFrEF, LVEF less or equal to 35%, NYHA Classes II or III | I-A |
| Non-ischemic HFrEF, LVEF less or equal to 35%, NYHA Classes II or III | I-B |
| Epidemiology of SCD in HFpEF | 39.4% of total cardiovascular death in CHARM-Preserved trial |
| 43.4% of total cardiovascular death in I-PRESERVE trial | |
| 38.1% of total cardiovascular death in TOPCAT trial | |
| Factors associated to SCD risk in HFpEF | Age * |
| Male sex * | |
| History of diabetes mellitus * | |
| History of prior myocardial infarction * | |
| Left bundle branch block * | |
| Natriuretic peptides * | |
| Other Biomarkers (Galectin 3, soluble ST-2) ** | |
| Strategy to prevent | Clinical trials evaluating established therapies for patients with HFrEF in patients with HFpEF have not resulted in improvements in clinical outcomes. Trial with ARNI is ongoing (PARAGON-HF). Identification of specific phenotype (e.g., hypertrophic cardiomyopathy) is mandatory for tailored treatment |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masarone, D.; Limongelli, G.; Rubino, M.; Valente, F.; Vastarella, R.; Ammendola, E.; Gravino, R.; Verrengia, M.; Salerno, G.; Pacileo, G. Management of Arrhythmias in Heart Failure. J. Cardiovasc. Dev. Dis. 2017, 4, 3. https://doi.org/10.3390/jcdd4010003
Masarone D, Limongelli G, Rubino M, Valente F, Vastarella R, Ammendola E, Gravino R, Verrengia M, Salerno G, Pacileo G. Management of Arrhythmias in Heart Failure. Journal of Cardiovascular Development and Disease. 2017; 4(1):3. https://doi.org/10.3390/jcdd4010003
Chicago/Turabian StyleMasarone, Daniele, Giuseppe Limongelli, Marta Rubino, Fabio Valente, Rossella Vastarella, Ernesto Ammendola, Rita Gravino, Marina Verrengia, Gemma Salerno, and Giuseppe Pacileo. 2017. "Management of Arrhythmias in Heart Failure" Journal of Cardiovascular Development and Disease 4, no. 1: 3. https://doi.org/10.3390/jcdd4010003







