Biocatalytic Synthesis of Fluorescent Conjugated Indole Oligomers
Abstract
:1. Introduction
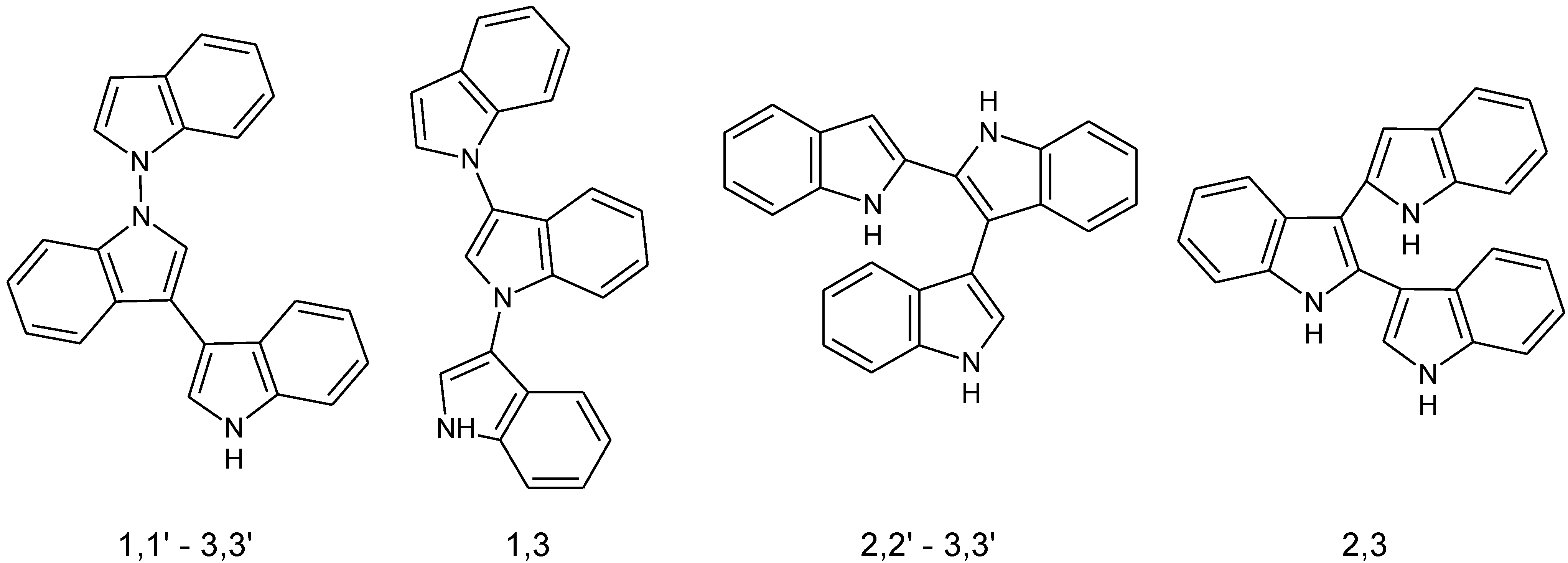
2. Experimental Section
2.1. Chemical Reagents
2.2. Procedure
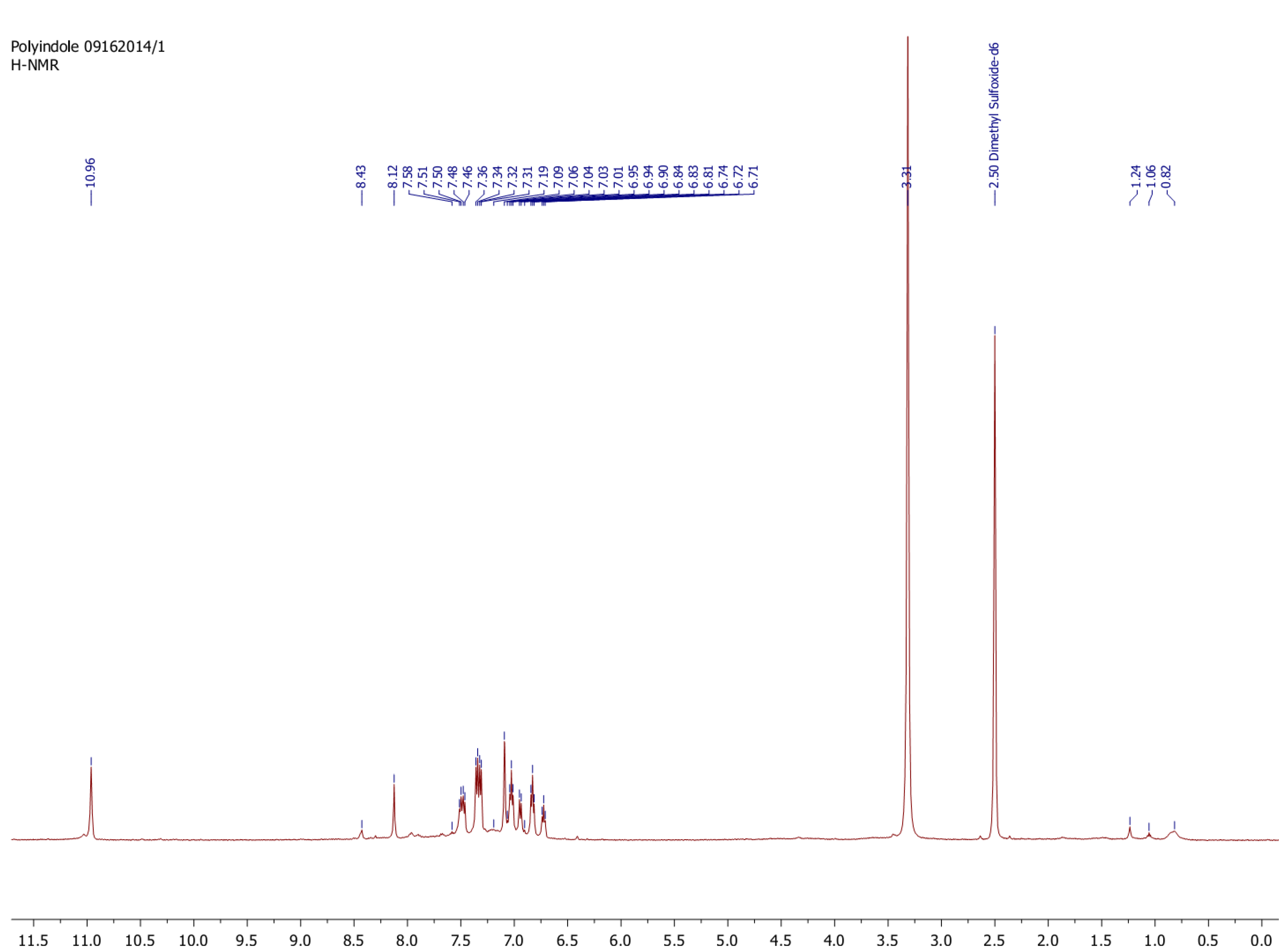
2.3. Characterization
3. Results and Discussion
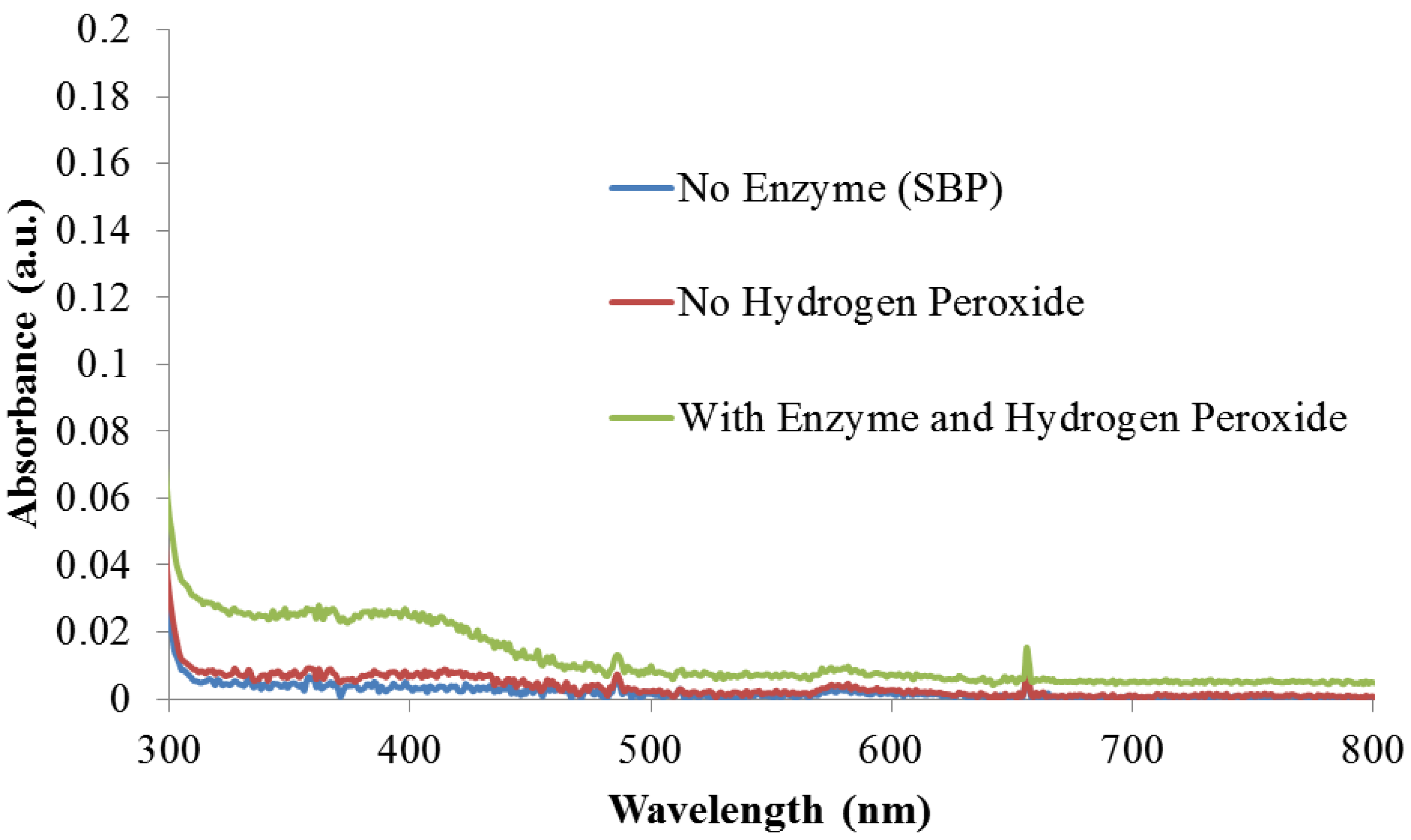
3.1. Enzyme Catalysis
3.2. Enzymatic Polymerization of Indole
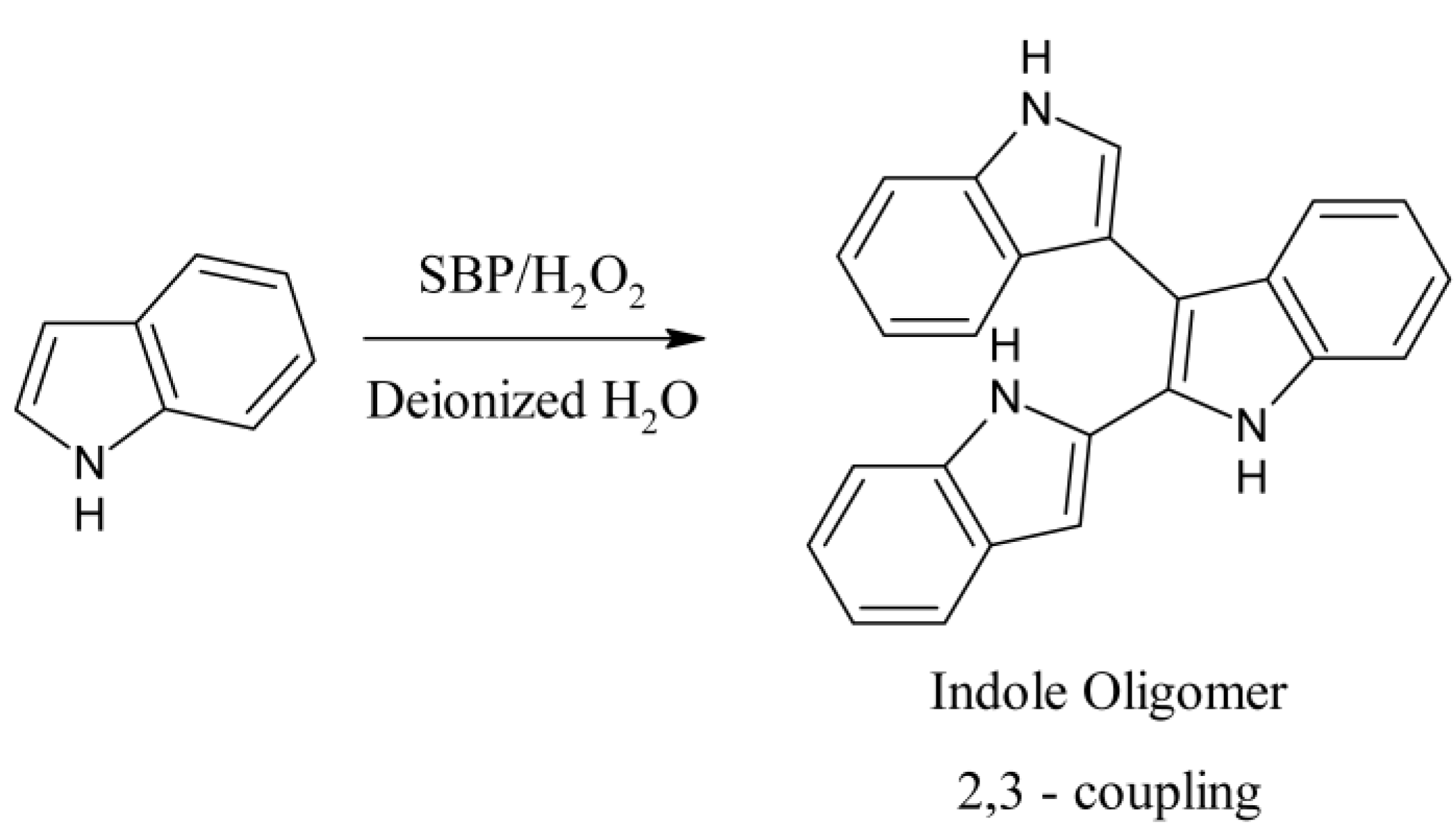
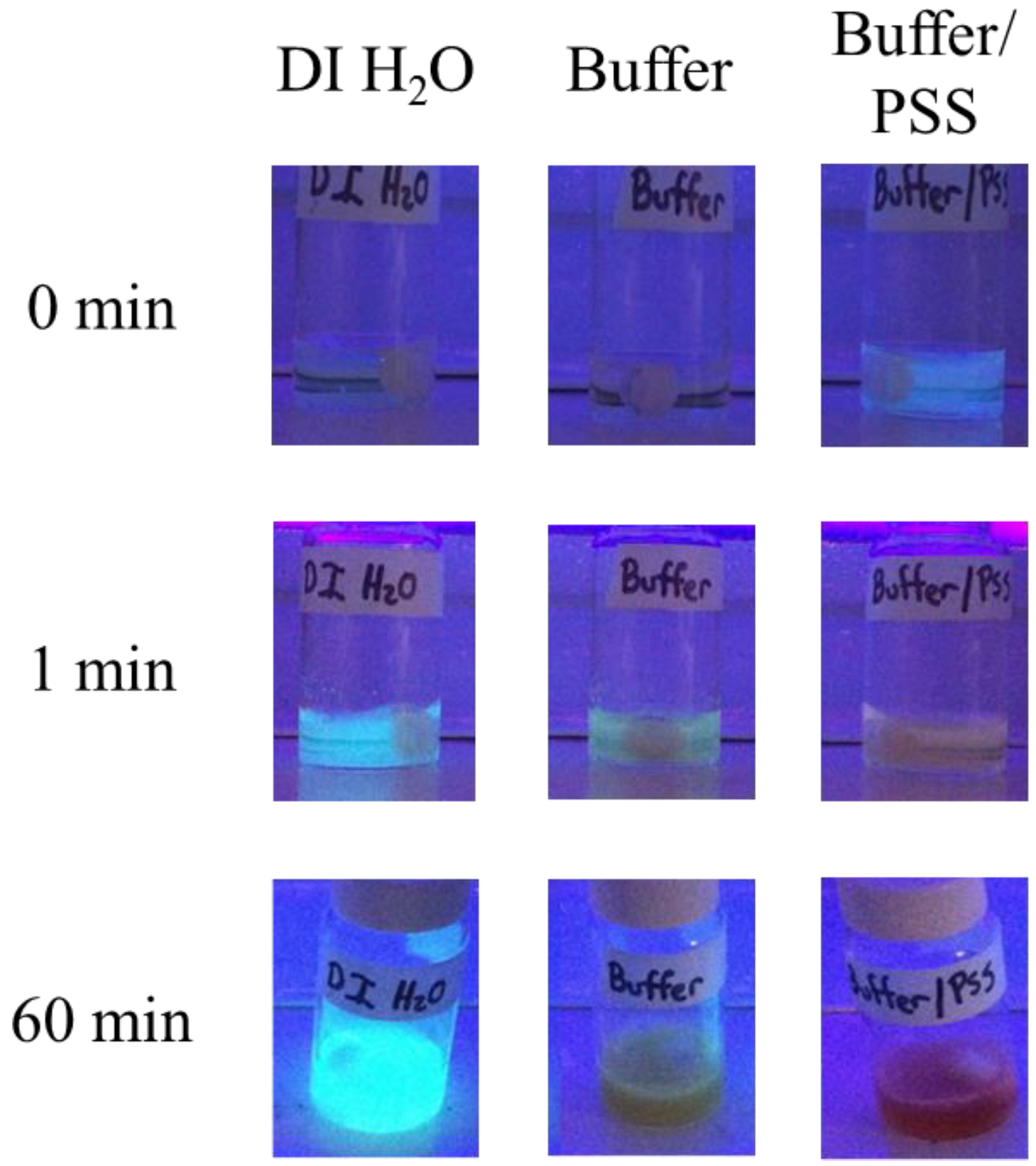
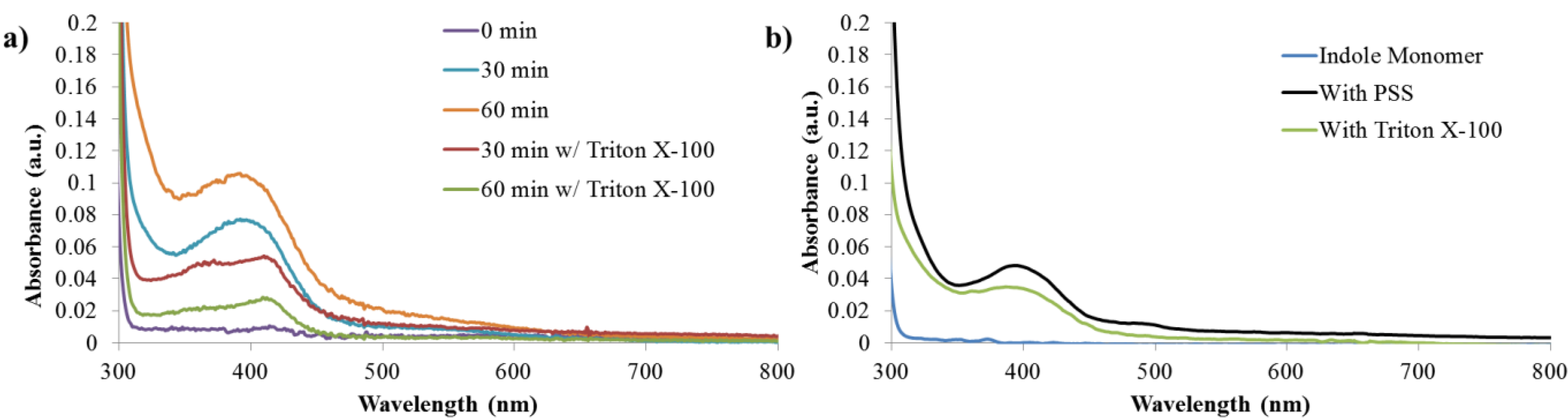
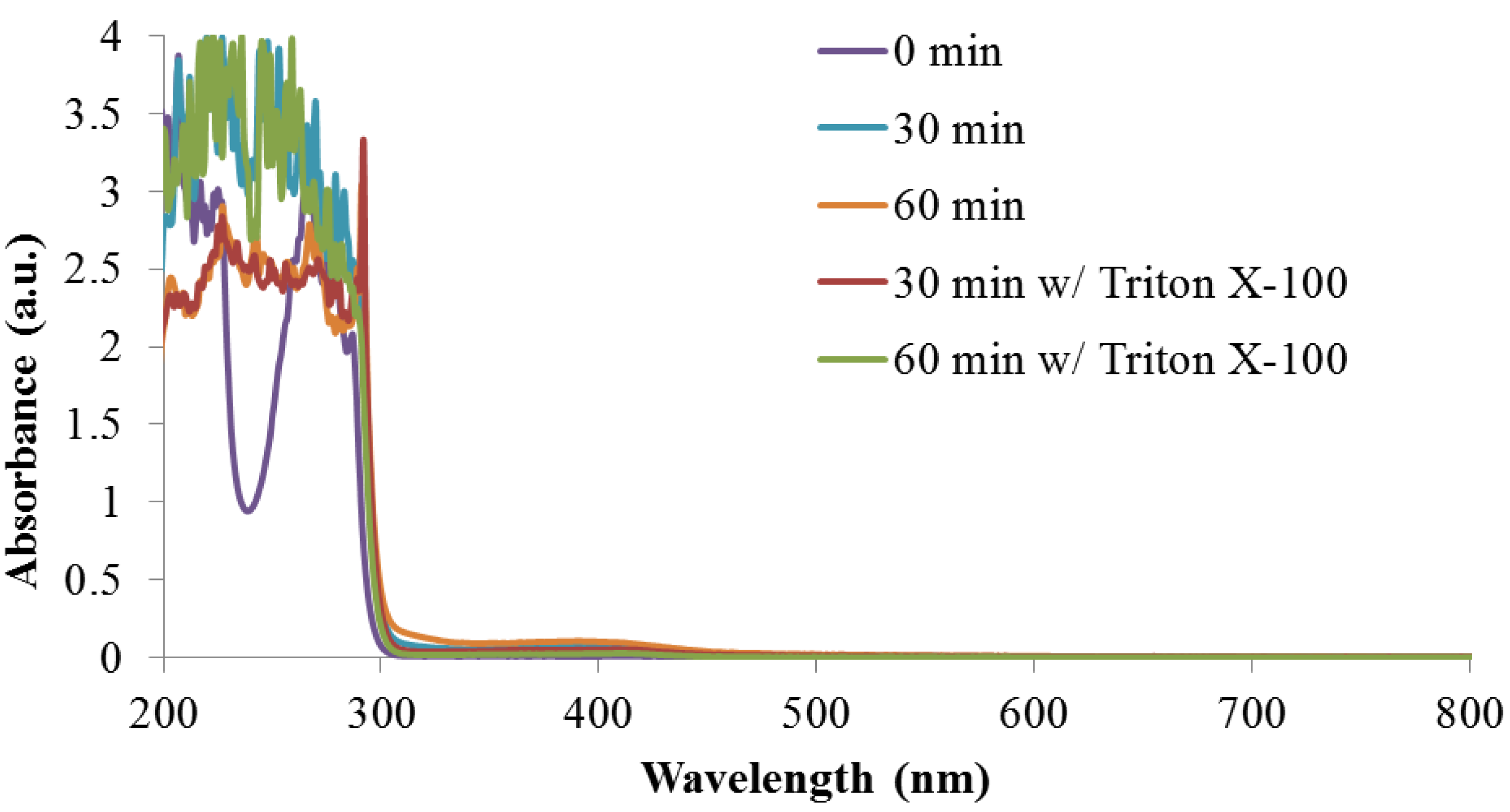
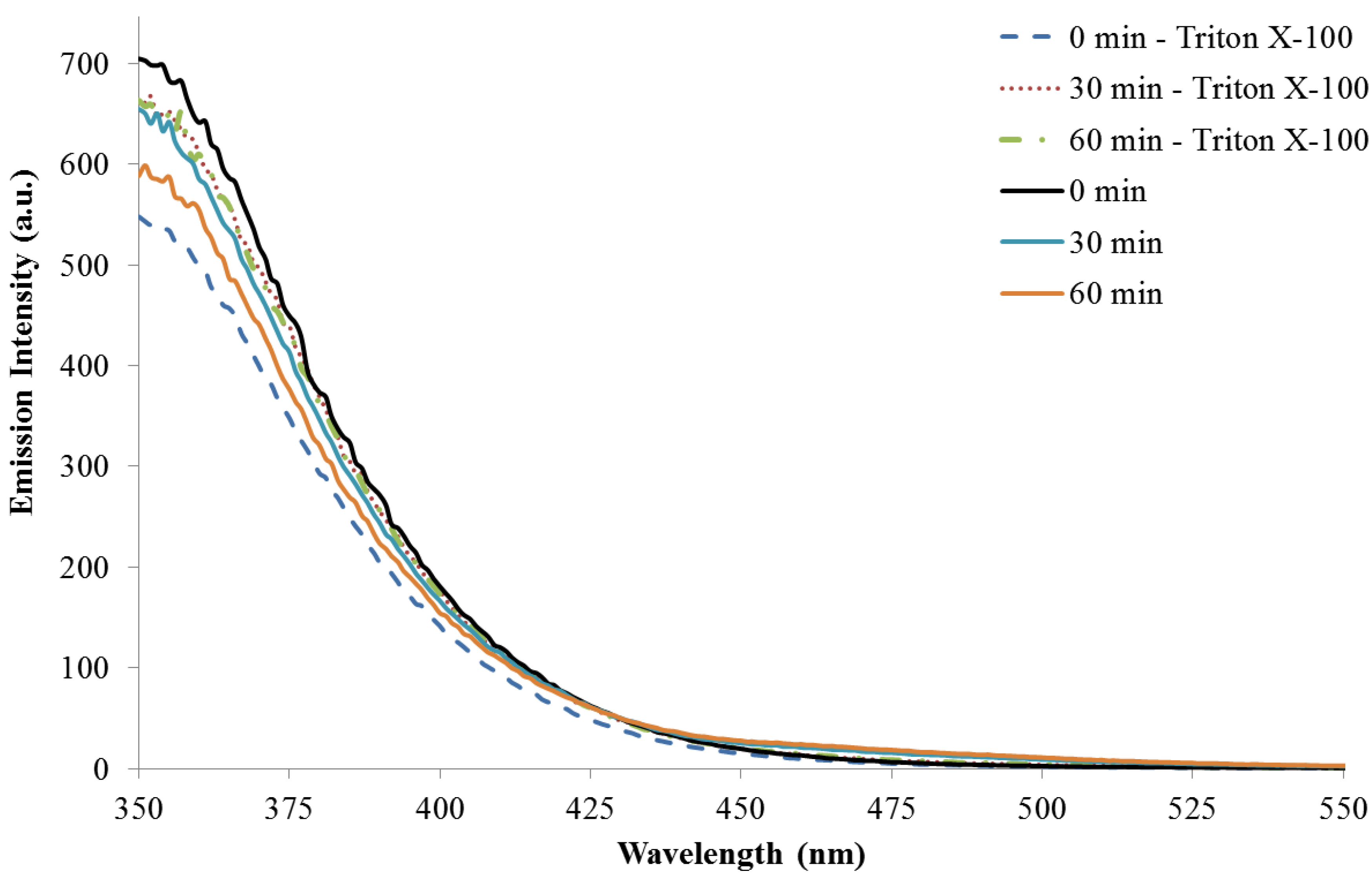
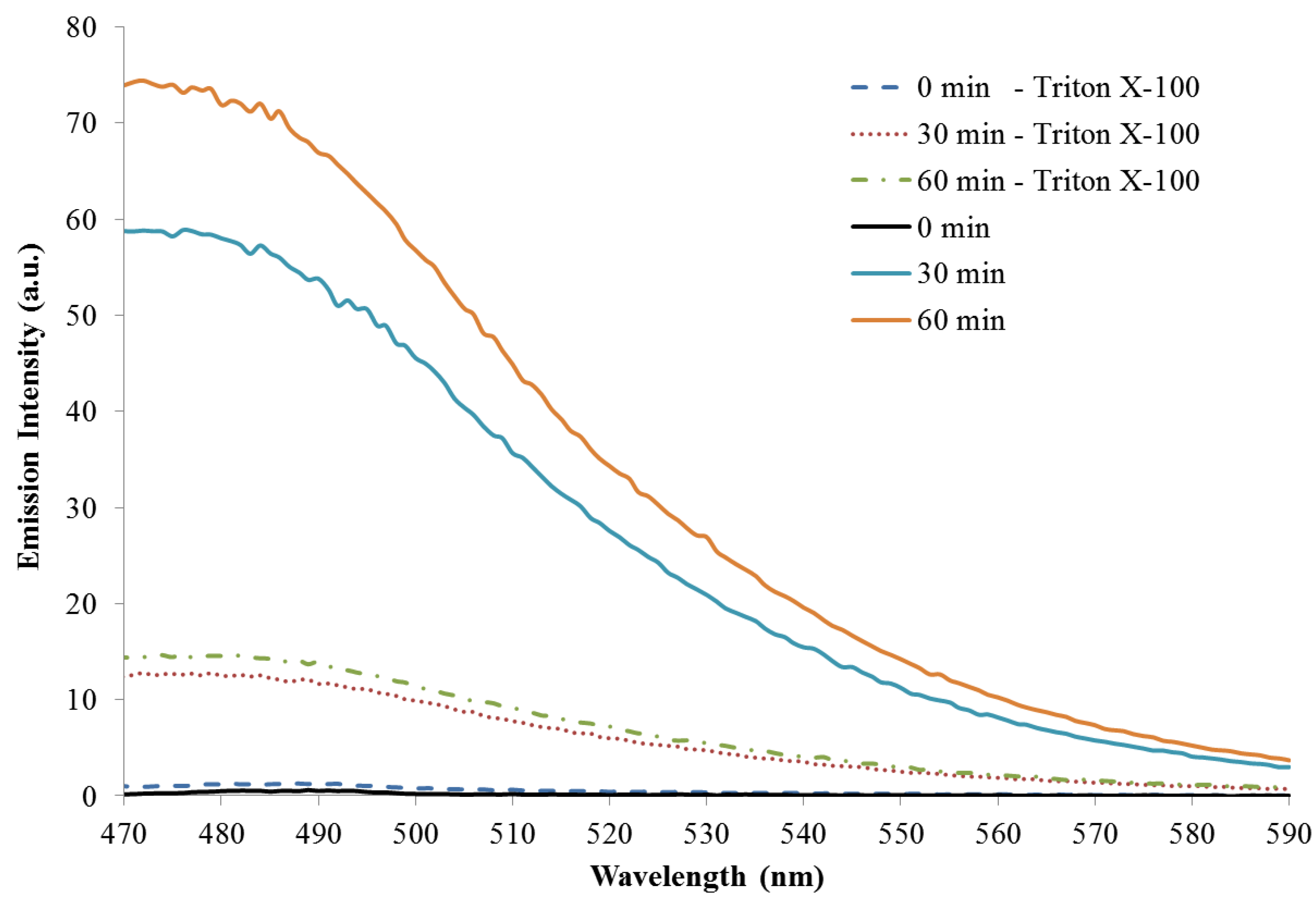
3.3. Reaction Monitoring with Absorption and Fluorescence Spectroscopy


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guiseppi-Elie, A.; Wallace, G.G.; Matsue, T. Chemical and Biological Sensors Based on Electrically Conducting Polymers. In Handbook of Conducting Polymers, 2nd ed.; Skotheim, T.A., Elsenbaumer, R.L., Reynolds, J.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 963–988. [Google Scholar]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Wu, P.-J. Semiconducting polymer nanoparticles as fluorescent probes for biological imaging and sensing. Part. Part. Syst. Char. 2014. [Google Scholar] [CrossRef]
- Faïd, K.; Leclerc, M. Responsive supramolecular polythiophene assemblies. J. Am. Chem. Soc. 1998, 120, 5274–5278. [Google Scholar] [CrossRef]
- Hussain, M.; Wackerlig, J.; Lieberzeit, P.A. Biomimetic strategies for sensing biological species. Biosensors 2013, 3, 89–107. [Google Scholar] [CrossRef]
- Chen, L.; McBrach, G.C.; Wang, H.-L.; Helgeson, R.; Wudl, R.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1996, 96, 12287–12292. [Google Scholar] [CrossRef]
- Bridges, J.W.; Williams, R.T. The fluorescence of indoles and aniline derivatives. Biochem. J. 1968, 107, 225–237. [Google Scholar] [PubMed]
- John, A.; Palaniappan, S. Synthesis and characterization of soluble poly(N-heptyl indole). Polymer 2005, 46, 12037–12039. [Google Scholar] [CrossRef]
- Zotti, G.; Zecchin, S.; Schiavon, G.; Seraglia, R.; Berlin, A.; Canavesi, A. Structure of polyindoles from anodic coupling of indoles: an electrochemical approach. Chem. Mater. 1994, 6, 1742–1748. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, R.; Shi, X. Preparation and characterization of polyindole nanofibers by electrospinning method. Synthetic Met. 2012, 162, 2069–2074. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically synthesized conducting polyaniline. J. Am. Chem. Soc. 1999, 1, 71–78. [Google Scholar] [CrossRef]
- Liu, W.; Cholli, A.L.; Nagarajan, R.; Kumar, J.; Tripathy, S.; Bruno, F.F.; Samuelson, L. The role of template in the enzymatic synthesis of conducting polyaniline. J. Am. Chem. Soc. 1999, 121, 11345–11355. [Google Scholar] [CrossRef]
- Bruno, F.F.; Fossey, S.A.; Nagarajan, S.; Nagarajan, R.; Kumar, J.; Samuelson, L.A. Biomimetic synthesis of water-soluble conducting copolymers/homopolymers of pyrrole and 3,4-ethylenedioxythiophene. Biomacromolecules 2006, 7, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S; Nagarajan, S.; Kokil, A.; Ponrathnam, T.; Bouldin, R.M.; Bruno, F.F.; Samuelson, L.; Kumar, J.; Nagarajan, R. Micellar nanoreactors for hematin catalyzed synthesis of electrically conducting polypyrrole. Langmuir 2012, 28, 13380–13386. [Google Scholar]
- Sessa, D.J.; Anderson, R.L. Soybean peroxidases: Purification and some properties. J. Agric. Food Chem. 1981, 29, 960–965. [Google Scholar] [CrossRef]
- Billaud, D.; Maarouf, E.B.; Hannecart, E. Chemical oxidation and polymerization of indole. Synthetic Met. 1995, 69, 571–572. [Google Scholar] [CrossRef]
- Billaud, D.; Maarouf, E.B.; Hannecart, E. Electrochemical polymerization of indole. Polymer 1994, 35, 2010–2011. [Google Scholar] [CrossRef]
- Tewari, A.I.; Kokil, A.; Ravichandran, S.; Nagarajan, S.; Bouldin, R.; Bruno, F.F.; Samuelson, L.A.; Nagarajan, R.; Kumar, J. Soybean peroxidase catalyzed enzymatic synthesis of pyrrole/EDOT copolymers. Macromol. Chem. Phys. 2010, 211, 1610–1617. [Google Scholar] [CrossRef]
- Bouldin, R.; Ravichandran, S.; Kokil, A.; Garhwal, R.; Nagarajan, S.; Kumar, J.; Bruno, F.F.; Samuelson, L.A.; Nagarajan, R. Synthesis of polypyrrole with fewer structural defects using enzyme catalysis. Synthetic Met. 2011, 161, 1611–1617. [Google Scholar] [CrossRef]
- Nagarajan, S.; Jayaraman, S.; Ravichandran, S.; Bouldin, R.; Nagarajan, R.; Kumar, J. Enzyme Mediated Synthesis as an Eco-Friendly Tool for Chemical Education. In Proceedings of the American Chemical Society National Meetings, San Diego, CA, USA, 25–29 March 2012.
- An, S.; Abdiryim, T.; Ding, Y.; Nurulla, I. A comparative study of the microemulsion and interfacial polymerization for polyindole. Mater. Lett. 2008, 62, 935–938. [Google Scholar] [CrossRef]
- Talbi, H.; Ghanbaja, J.; Billaud, D.; Humbert, B. Vibrational properties and structural studies of doped and dedoped polyindole by FTIR, Raman and EEL spectroscopies. Polymer 1997, 38, 2099–2106. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Campa, A.; Catalani, L.H. The oxidation of indole derivatives catalyzed by horseradish peroxidase is highly chemiluminescent. Arch Biochem Biophys. 2001, 387, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bouldin, R.M.; Kokil, A.; Ponrathnam, T.; Urban, N.; Kumar, J.; Samuelson, L.A.; Nagarajan, R. Biocatalyic synthesis of unusually photoluminescent oligomers and electrically conducting polymers of 4-(3-pyrrolyl)butyric acid. J. Appl. Poly. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Kim, Y.H.; An, E.S.; Song, B.K.; Kim, D.S.; Chelikani, R. Polymerization of cardanol using soybean peroxidase and its potential application as anti-biofilm coating material. Biotechnol. Lett. 2003, 25, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, F.I.; Alemzadeh, I.; Motamed, S. Seed coating soybean peroxidase: Extraction and biocatalytic properties determination. Iranian J. Chem. Eng. 2010, 7, 28–38. [Google Scholar] [CrossRef]
- Steevensz, A.; Madur, S.; Feng, W.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Crude soybean hull peroxidase treatment of phenol in synthetic and real wastewater: Enzyme economy enhanced by Triton X-100. Enzym. Microb. Tech. 2014, 55, 65–71. [Google Scholar] [CrossRef]
- Hassanien, R.; Al-Hinai, M.; Al-Said, S.A.F.; Little, R.; Siller, L.; Wright, N.G.; Houlton, A.; Horrocks, B.R. Preparation and characterization of conductive and photoluminescent DNA-templated polyindole nanowires. ACS Nano 2010, 4, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Nie, G.; Zhang, S.; Han, X.; Hou, J.; Pu, S. Electrosynthesis of free-standing polyindole films in borontrifluoride diethyl etherate. J. Polymer Sci. Polymer Chem. 2005, 43, 1444–1453. [Google Scholar] [CrossRef]
Appendix

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouldin, R.M.; Singh, A.; Magaletta, M.; Connor, S.; Kumar, J.; Nagarajan, R. Biocatalytic Synthesis of Fluorescent Conjugated Indole Oligomers. Bioengineering 2014, 1, 246-259. https://doi.org/10.3390/bioengineering1040246
Bouldin RM, Singh A, Magaletta M, Connor S, Kumar J, Nagarajan R. Biocatalytic Synthesis of Fluorescent Conjugated Indole Oligomers. Bioengineering. 2014; 1(4):246-259. https://doi.org/10.3390/bioengineering1040246
Chicago/Turabian StyleBouldin, Ryan M., Ankita Singh, Michael Magaletta, Sean Connor, Jayant Kumar, and Ramaswamy Nagarajan. 2014. "Biocatalytic Synthesis of Fluorescent Conjugated Indole Oligomers" Bioengineering 1, no. 4: 246-259. https://doi.org/10.3390/bioengineering1040246
APA StyleBouldin, R. M., Singh, A., Magaletta, M., Connor, S., Kumar, J., & Nagarajan, R. (2014). Biocatalytic Synthesis of Fluorescent Conjugated Indole Oligomers. Bioengineering, 1(4), 246-259. https://doi.org/10.3390/bioengineering1040246



