Affinity Sensing Strategies for the Detection of Pesticides in Food
Abstract
:1. Introduction
1.1. Pesticides
1.2. Pesticides Detection and Affinity Sensors
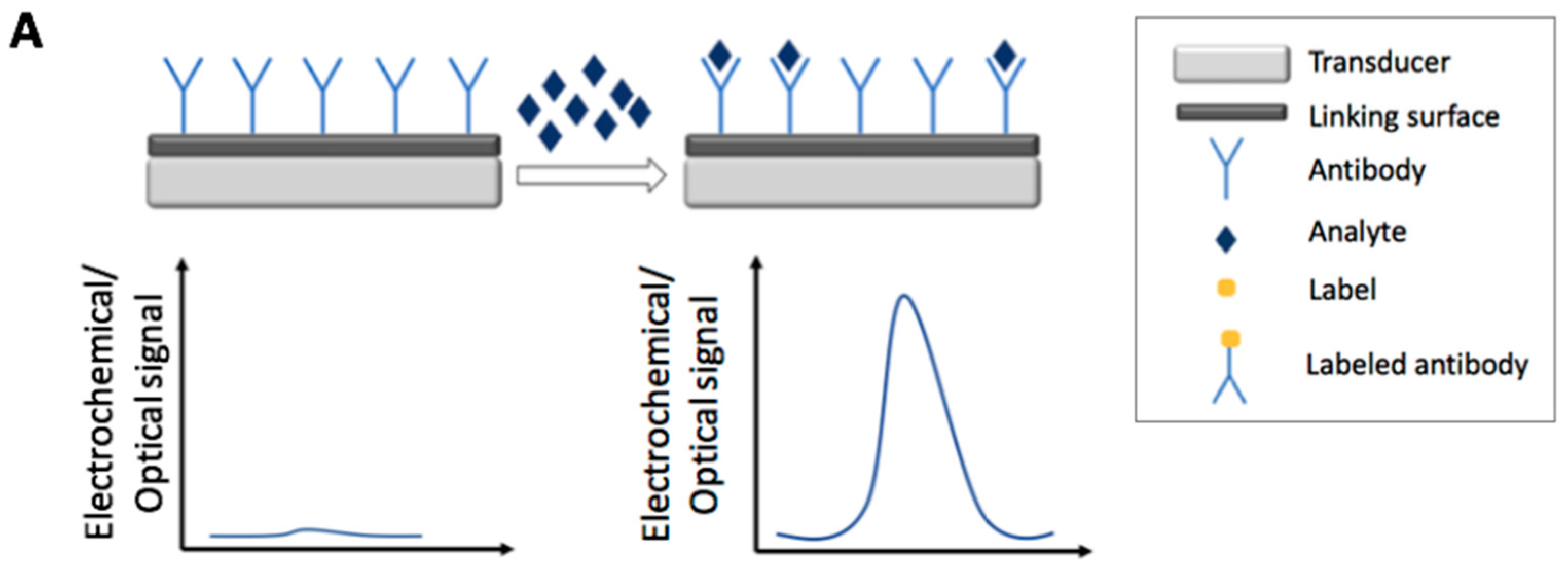
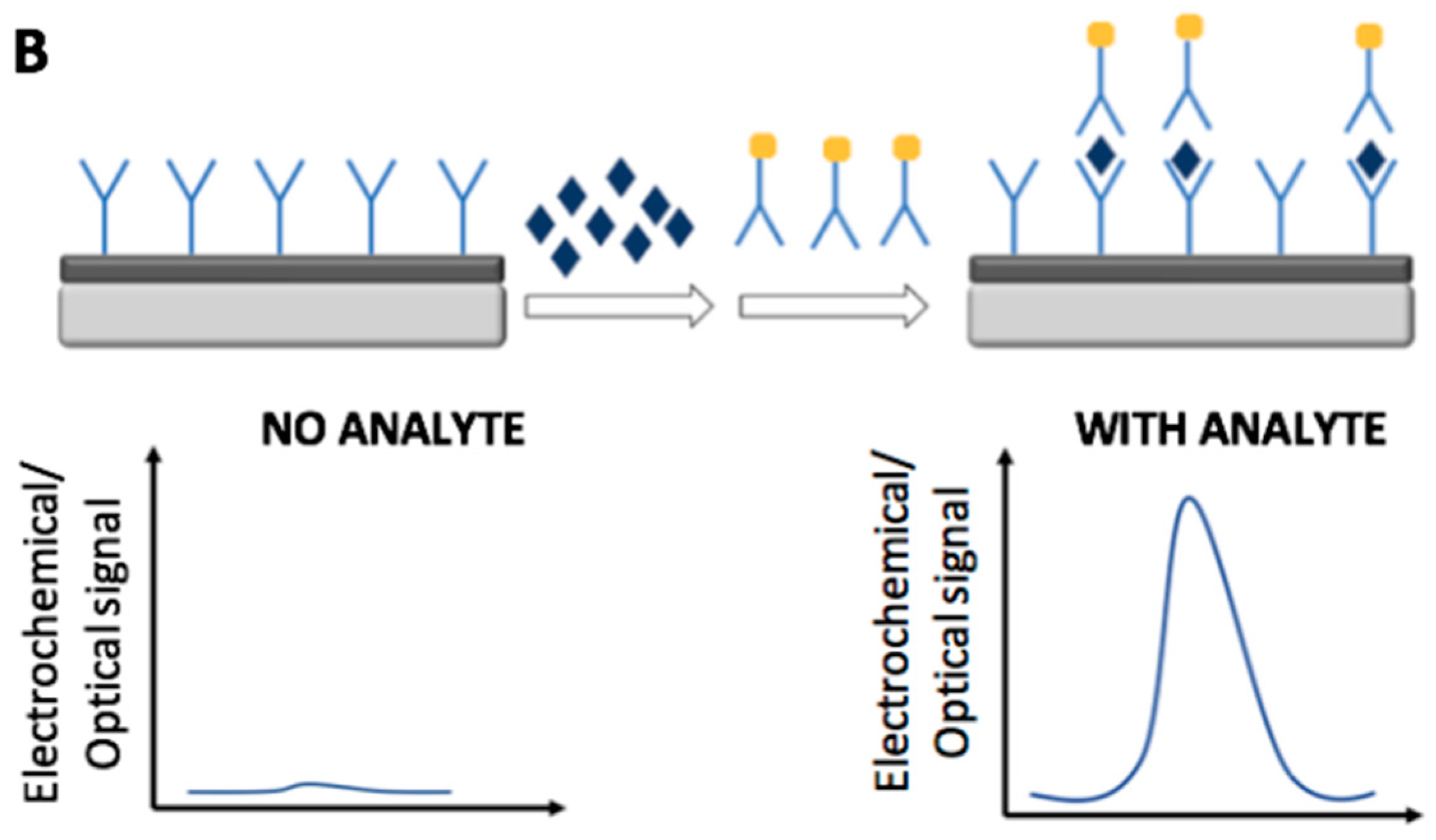
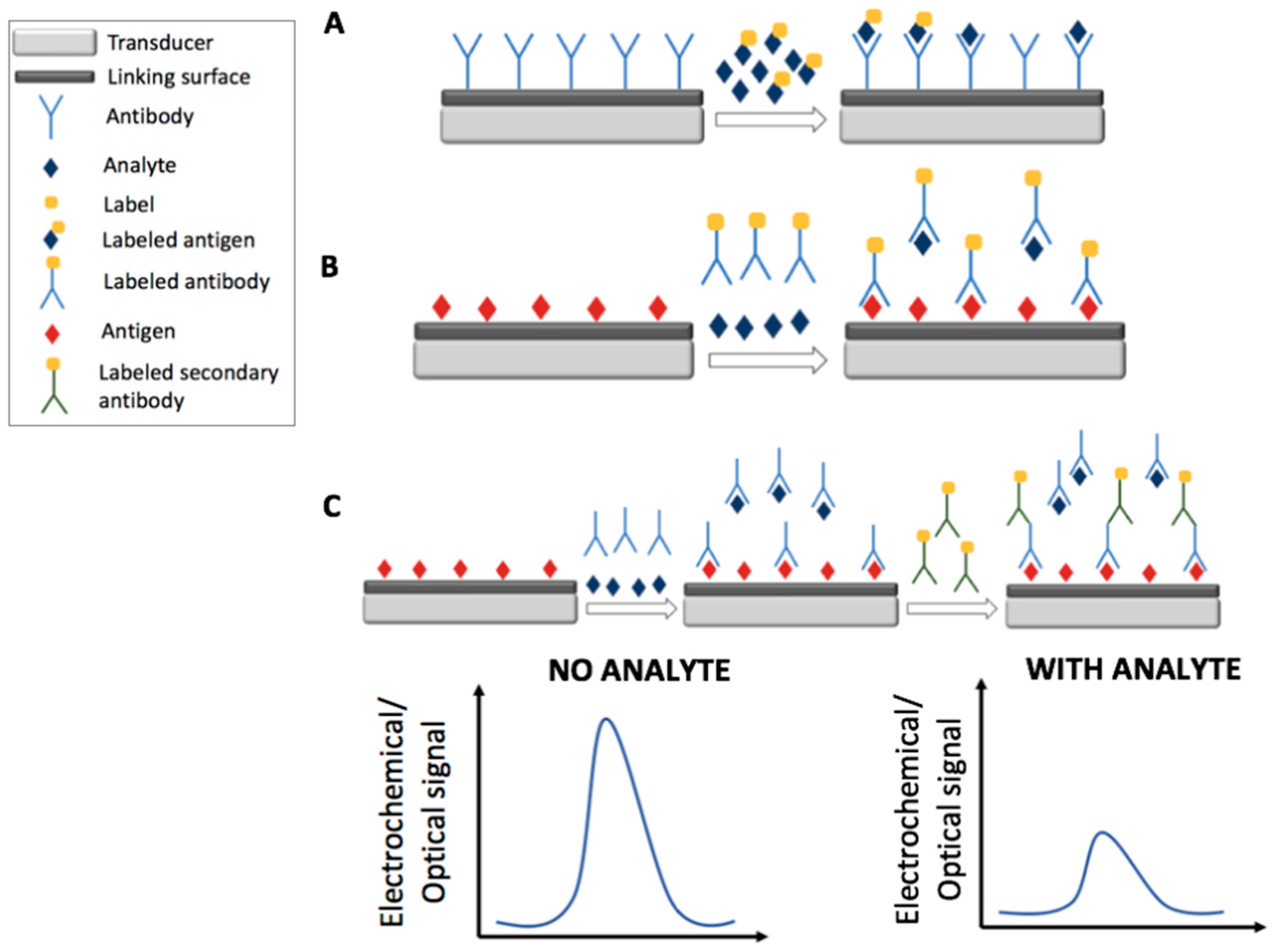
2. Immunosensors
2.1. Electrochemical Immunosensors
2.1.1. Impedimetric Immunosensors
2.1.2. Voltammetric and Amperometric Immunosensors
2.2. Optical Immunosensors
2.2.1. Surface Plasmon Resonance-Based Immunosensors
2.2.2. Fluorescence-Based and Colorimetric Immunosensors
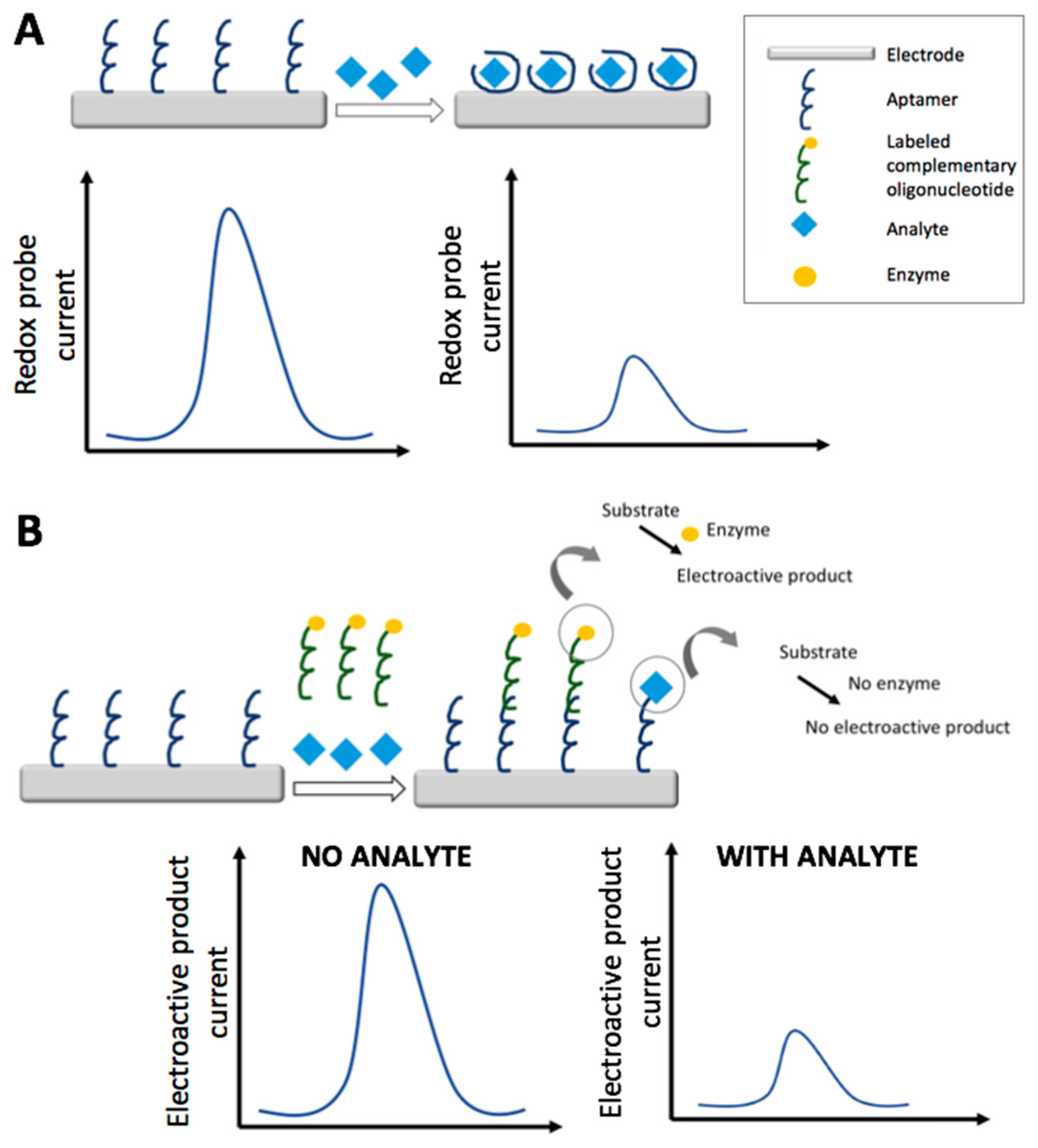
3. Aptasensors
3.1. Electrochemical Aptasensors
3.1.1. Impedimetric Aptasensors
3.1.2. Voltammetric Aptasensors
3.2. Optical Aptasensors
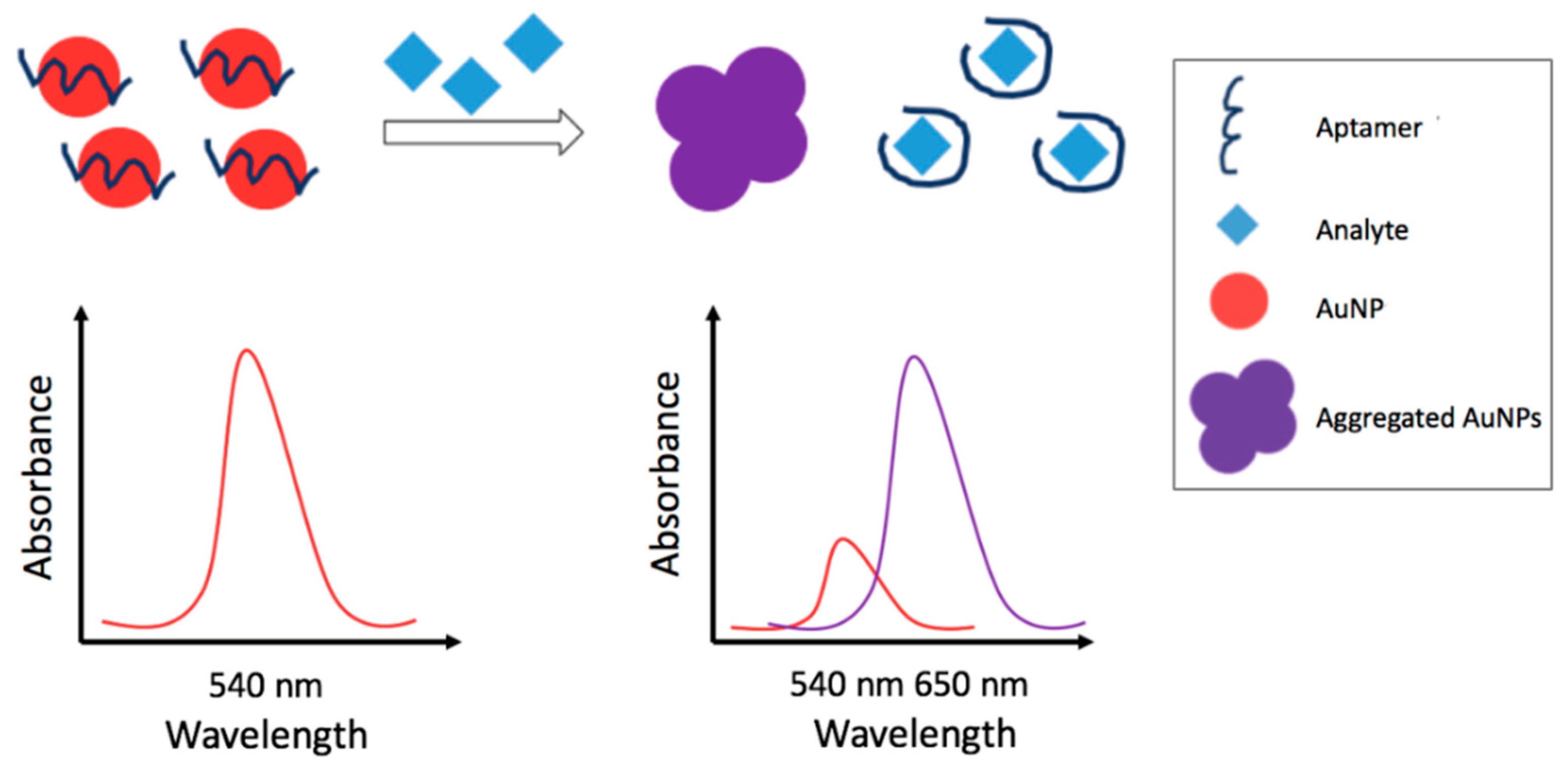
3.2.1. Colorimetric Metal Nanoparticles-Based Aptasensors
3.2.2. Surface-enhanced Raman Scattering-Based Aptasensors
3.2.3. Fluorescence Resonance Energy Transfer-Based Aptasensors
4. Molecularly Imprinted Polymer Sensors
4.1. Electrochemical MIP Sensors
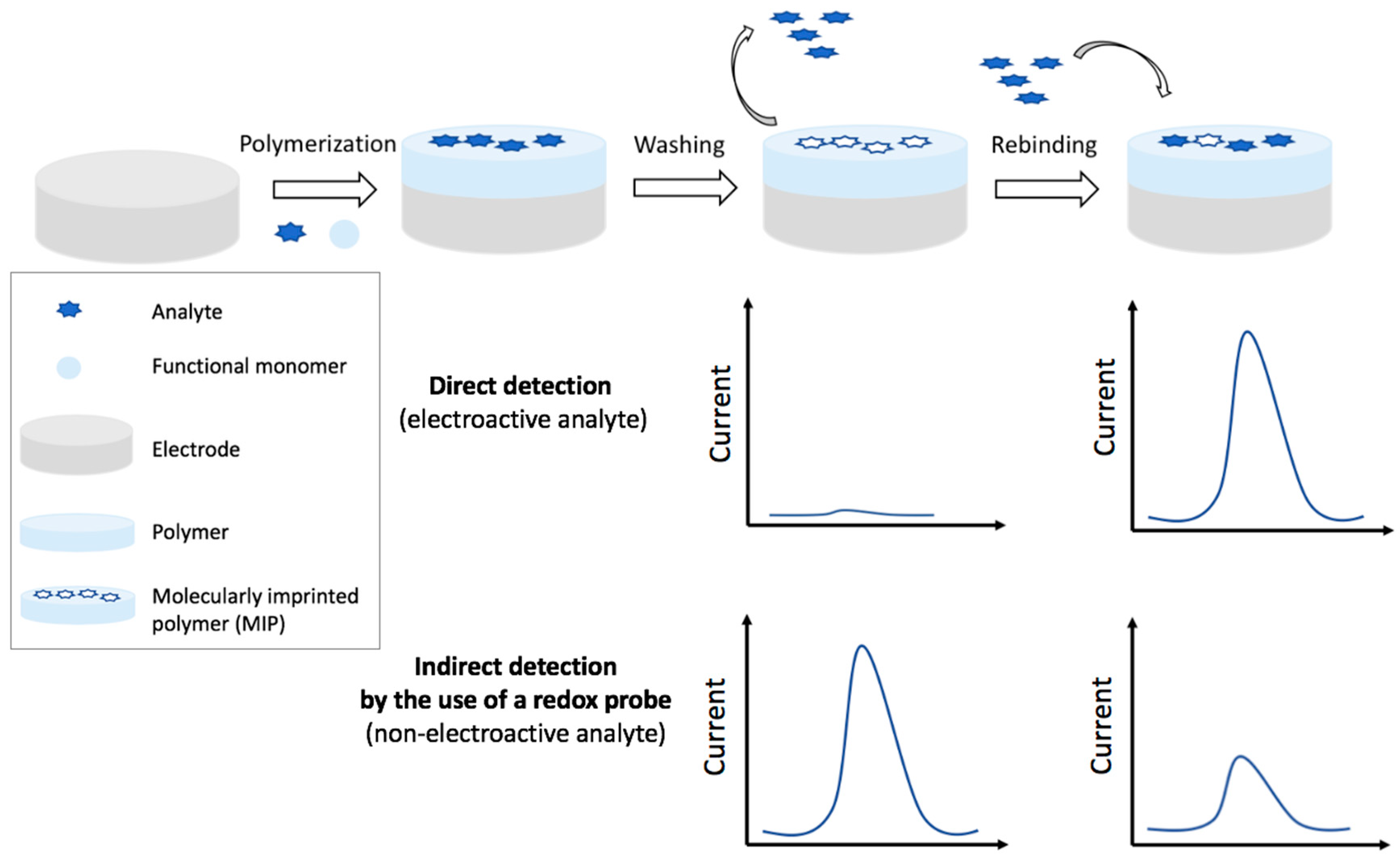
4.1.1. Voltammetric MIP Sensors
Direct Detection
Indirect Detection
4.1.2. Potentiometric and Impedimetric MIP Sensors
4.2. Optical MIP Sensors
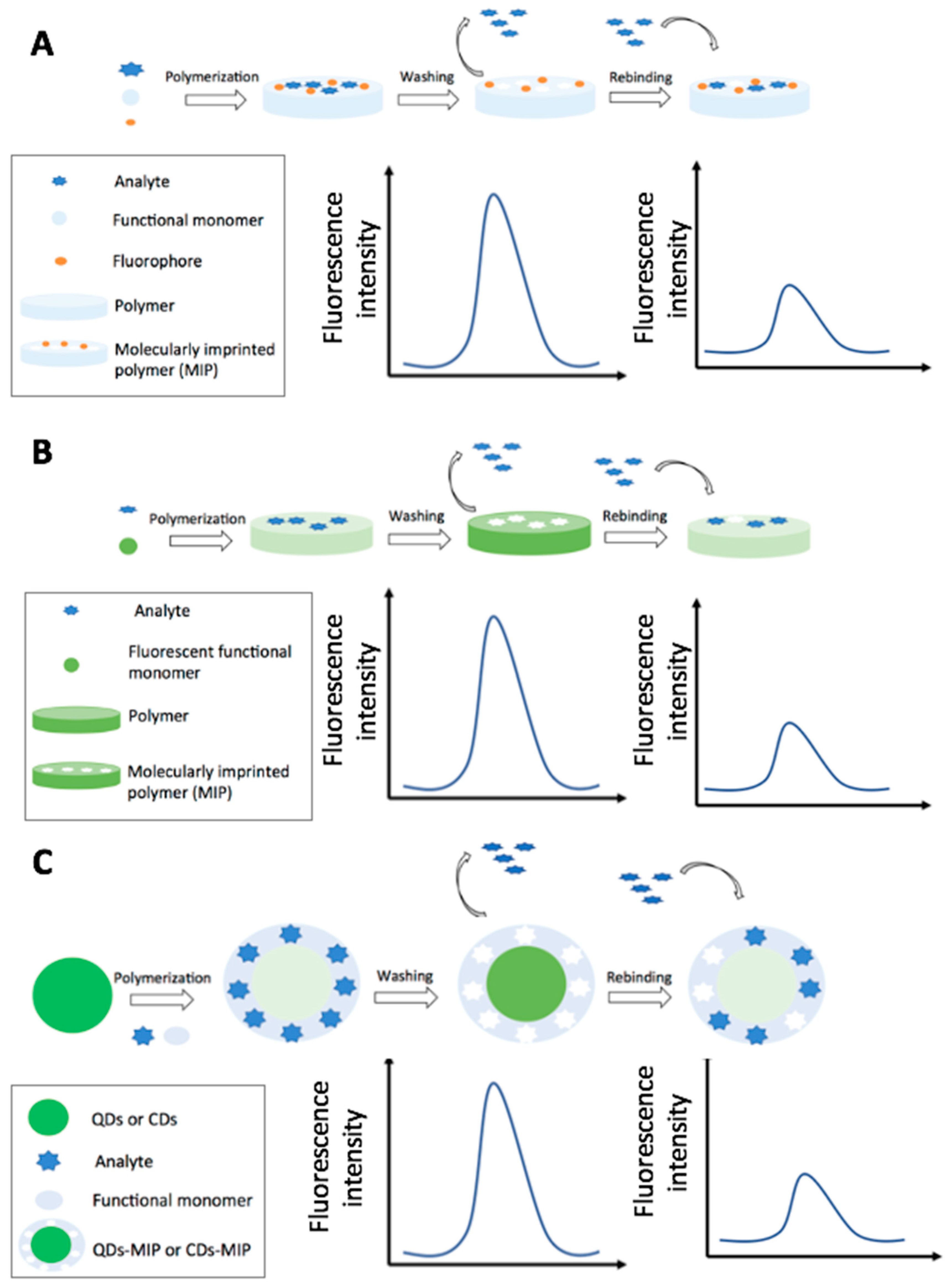
4.2.1. Fluorescence and Fluorescence Resonance Energy Transfer-Based MIP Sensors
4.2.2. Colorimetric, Dual Read-Out (Fluorescence-Based + Colorimetric) and Electrochromic MIP Sensors
4.2.3. Surface Plasmon Resonance-Based MIP Sensors
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 2-ABA | 2-aminobenzyl amine |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 2,4,6-TCP | 2,4,6-trichlorophenol |
| 3-ABA | 3-aminobenzoic acid |
| 3-PBD | 3-phenoxybenzaldehyde |
| 5-DTAF | 5-(4,6-dichlorotriazinyl) aminofluorescein |
| 6-FAM-SH-ssDNAs | 6-carboxyfluorescein labeled single-stranded thiol-oligonucleotides |
| Ab-HRP | horseradish peroxidase-labeled antibody |
| Ab | antibody |
| Ab198-cc-HRP | enzymatic labeled antibody |
| AET | 2-aminoethanethiol |
| Ag | antigens |
| Ag | silver |
| AgNPs | silver nanoparticles |
| AM | acrylamide |
| AMMB | 2-acrylamide-6-methoxybenzothiazole |
| AMP | amino-modified capture probe |
| ANI | aniline |
| Ap | aptamer |
| APTES | 3-aminopropyltriethoxysilane |
| ATR-BSA | atrazine-bovine serum albumine |
| ATR | atrazine |
| Au | gold |
| AuNPs | gold nanoparticles |
| BB | bromophenol blue |
| BiCoPc | binuclear phthalocyanine cobalt(II) sulfonate |
| BSA | bovine serum albumin |
| CB | carbon black |
| CDs | carbon dots |
| CdSNPs | CdS nanoparticles |
| CG | carboxylic graphene |
| CHIT-IO | chitosan-iron oxide nanocomposite |
| CMK-3 | ordered mesoporous carbon material |
| Co3O4/PANI | Co3O4/Polyaniline nanoparticles |
| CP | carbon paste |
| CPE | carbon paste electrode |
| CPF-BSA | Chlorpyrifos-bovine serum albumine |
| CPF | chlorpyrifos |
| CPFO | chlorpyrifos oxon |
| CS-AuPtNPs | chitosan-AuPt alloy nanoparticles |
| CS | chitosan |
| CuO NFs-SWCNTs | copper oxide nanoflowers and single-walled carbon nanotubes nanocomposite |
| CV | Cyclic Voltammetry |
| dc-ELISA | direct competitive enzyme-linked immunosorbent assay |
| DMMP | dimethyl methylphosphonate |
| DMZ | dimetridazole |
| DPV | Differential Pulse Voltammetry |
| EDI | edifenphos |
| EGDMA | ethylene glycol dimethylacrylate |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme Linked Immunosorbent Assay |
| Fc@MWCNTs-CS | ferrocene hybrid chitosan dispersed multi-walled carbon nanotubes |
| FDMA | ferrocenedimethylamine |
| Fe3O4@MWCNTs-COOH/CS | Fe3O4@carboxyl-functionalized MWCNTs/chitosan nanocomposite |
| Fe3O4@PDA NPs | Fe3O4@polydopamine nanoparticles |
| fG | graphene sheets functionalized |
| FITC | fluorescein 5(6)-isothiocyanate |
| FM | fenaminosulf |
| FRET | fluorescence resonance energy transfer |
| FTO | fluorine tin oxide |
| FuAuNP | functionalized gold nanoparticles |
| GA | glutaraldehyde |
| GCE | glassy carbon electrode |
| GECs | graphite composite electrodes |
| Gly | glyphosate |
| GN | graphene |
| GO | graphene oxide |
| GO@Fe3O4 | graphene oxide@Fe3O4 |
| GOPTS | (3-glycidyloxypropyl)triethoxysilane |
| GR-IL-Au | graphene-ionic liquid-nano Au |
| GS-MB/AuNPs | graphene sheets-methylen blue/gold nanoparticles nanocomposite |
| GS-PEI-Au | graphene sheets-ethyleneimine polymer-Au nanocomposites |
| GSPEs | graphite screen-printed electrodes |
| hapten-BSA | hapten-bovine serum albumin |
| HPSNs-NH2 | hierarchical porous dendrimer-like silica nanoparticles |
| HRP | horseradish peroxidase |
| IBF | iprobenfos |
| IDAMs | interdigitated array microelectrodes |
| IDEs | interdigitated electrodes |
| IFE | inner filter effect |
| IgG-HRP | immunoglobulin G-horseradish peroxidase |
| IL-EGN | ionic liquid-graphene composite film |
| IL | ionic liquid |
| IMI | imidacloprid |
| IPIM | imprinted polymer inclusion membrane |
| IrOx NPs | iridium oxide nanoparticles |
| ITO | indium tin oxide |
| L-Cys | L-cysteine |
| LDR | linear dynamic range |
| LOD | limit of detection |
| LSPR | localized surface plasmon resonance |
| LSV | Linear Sweep Voltammetry |
| m-GEC | magnetic graphite–epoxy composite electrode |
| MAA | methacrylic acid |
| mAb | anti-triazophos monoclonal antibody |
| MB | methylene blue |
| MCH | 6-Mercap-1-hexanol |
| MCP | micro contact printing |
| MCZ | mancozeb |
| MEPS | microextraction by packed sorbent |
| MH | 6-mercaptohexanol |
| MIECS | molecularly imprinted electrochemical sensor |
| MIM-Zn-MAA | molecularly imprinted membrane-zinc porphyrin-mathacrylate |
| MIP–IL–EGN/GCE | MIP–ionic liquid–graphene composite film coated GCE |
| MIPMs | molecularly imprinted polymer microspheres |
| MIPs | molecularly imprinted polymers |
| MISP | molecularly imprinted star polymers |
| MIT | Molecular imprinting technology |
| MMIP | magnetic molecularly imprinted polymer |
| MNPs | metal nanoparticles |
| MOFs | metal-organic frameworks |
| MPS | 3-(methacryloxyl) propyl trimethoxysilane |
| MR | methyl red |
| MRLs | maximum residue levels |
| MTMC | metolcarb |
| MUA | 1,1-mercaptoundecanoic acid |
| MWCNTs | multi-walled carbon nanotubes |
| nano-MIP | MIP nanoparticles |
| NG | nitrogen doped graphene |
| o-PD | o-phenylenediamine |
| oligo2 | complementary biotinylated oligonucleotide |
| OMC-CS | mesoporous carbon (OMC) functionalized by chitosan |
| OVA | ovalbumin |
| p-ABA | para aminobenzoic acid |
| p-ATP | p-aminothiophenol |
| P | pesticide solution |
| PAMAM | poliamidoaminic dendrimers |
| PANI | polyaniline |
| PANABA | polymer poly-(aniline-co-3-aminobenzoic acid) |
| PB-CMK-3 | ordered mesoporous carbon material prussian blue |
| PB | prussian blue |
| PDA NPs | polydopamine nanoparticles |
| PDDA | poly (diallydimethylammonium chloride) |
| PGE | pencil graphite electrode |
| PO | paraoxon |
| PoAP | Poly-o-aminophenol |
| PPy | polypyrrole |
| PQ | paraquat |
| PQ1-BSAMP | Antigen biofunctionalized magnetic micro-particles |
| PQQ | pyrroloquinoline quinone |
| PTES | phenyltrimethoxysilane |
| PtNPs microwires | platinum nanoparticles microwires |
| PtNPs | platinum nanoparticles |
| PVC | polyvinyl chloride |
| Py | pyrrole |
| QDs | quantum dots |
| Qu | quercetin |
| Re | resorcinol |
| Ret | electron transfer resistance |
| rGO | reduced graphene oxide |
| rGO@Au | reduced graphene oxide and gold nanoparticles |
| RU | resonance unit |
| SA | streptavidin |
| SAM | self-assembled monolayer |
| SELEX | (selection evolution of ligands by exponential enrichment) |
| SERS | Surface-enhanced Raman Scattering |
| SiO2@FMIP | fluorescent core-shell MIP based on the surface of SiO2 beads |
| SMIPMs | surface molecularly imprinted polymeric microsphere |
| Sp | spermine |
| SPCE | screen-printed carbon electrode |
| SPE | screen-printed electrode |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| SPR | Surface Plasmon Resonance |
| SWNTs | single wall nanotubes |
| SWSV | square wave voltammetry in stripping mode |
| SWV | Square Wave Voltammetry |
| TPD | 3,5,6-trichloro-2-pyridinol |
| TPN-BSA | Conjugates of N-(pentachlorophenoxyacetyl)glycine and bovine serum albumin |
| UCNPs | upconversion nanoparticles |
| VBA | p-vinylbenzoic acid |
References
- World Health Organization. Manual on Development and Use of FAO and WHO Specifications for Pesticides; Third Revision; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Garcia, F.P.; Ascencio, S.Y.C.; Oyarzun, J.C.G.; Hernandez, A.C.; Alavarado, P.V. Pesticides: Classification, uses and toxicity. Measures of exposure and genotoxic risks. J. Res. Environ. Sci. Toxicol. 2012, 1, 279–293. [Google Scholar]
- Díaz-González, M.; Gutiérrez-Capitán, M.; Niu, P.; Baldi, A.; Jiménez-Jorquera, C.; Fernández-Sánchez, C. Electrochemical devices for the detection of priority pollutants listed in the eu water framework directive. TrAC Trends Anal. Chem. 2016, 77, 186–202. [Google Scholar] [CrossRef]
- Wong, A.; Silva, T.A.; Caetano, F.R.; Bergamini, M.F.; Marcolino-Junior, L.H.; Fatibello-Filho, O.; Janegtiz, B.C. An overview of pesticide monitoring at environmental samples using carbon nanotubes-based electrochemical sensors. C 2017, 3, 8. [Google Scholar] [CrossRef]
- Kumar, J.; Melo, J.S. Overview on biosensors for detection of organophosphate pesticides. Curr. Trends Biomed. Eng. Biosci. 2017, 5, 555–663. [Google Scholar]
- EU Pesticides Database. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN (accessed on 4 September 2018).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Pope, J.; Skurky-Thomas, M.; Rosen, C. Toxicity. In Organochlorine Pesticides; Medscape: New York, NY, USA, 1994; pp. 259–278. [Google Scholar]
- De Jong, G. Long-term health effects of aldrin and dieldrin. A study of exposure, health effects and mortality of workers engaged in the manufacture and formulation of the insecticides aldrin and dieldrin. Toxicol. Lett. 1991, 56, 1–206. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Z.; Li, X. Advances in pesticide biosensors: Current status, challenges, and future perspectives. Anal. Bioanal. Chem. 2013, 405, 63–90. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Arduini, F.; Palleschi, G.; Rea, G. Biosensing technology for sustainable food safety. TrAC Trends Anal. Chem. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and their applications in detection of organophosphorus pesticides in the environment. Arch. Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, K.-H.; Deep, A. Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens. Bioelectron. 2015, 70, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, R.; Salunkhe, D. Toxicological implications of pesticides: Their toxic effects on seeds of food plants. Toxicology 1975, 3, 269–285. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, C.; Barot, S.; Capowiez, Y.; Hedde, M.; Vandenbulcke, F. Pesticides and earthworms. A review. Agron. Sustain. Dev. 2014, 34, 199–228. [Google Scholar] [CrossRef]
- Domínguez, I.; González, R.R.; Liébanas, F.J.A.; Vidal, J.L.M.; Frenich, A.G. Automated and semi-automated extraction methods for gc–ms determination of pesticides in environmental samples. Trends Environ. Anal. Chem. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Vieira, D.C.; Noldin, J.A.; Deschamps, F.C.; Resgalla, C. Ecological risk analysis of pesticides used on irrigated rice crops in southern brazil. Chemosphere 2016, 162, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Suddaby, L.; Beulke, S.; van Beinum, W.; Oliver, R.; Kuet, S.; Brown, C.D. Long-term experiments to investigate irreversibility in sorption of pesticides to soil. Chemosphere 2016, 162, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Smalling, K.L.; Anderson, C.; Calhoun, D.; Chestnut, T.; Muths, E. Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the united states. Sci. Total Environ. 2016, 566, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Chen, Z.; Pan, X.; Zheng, Y. Atmospheric pressure gas chromatography quadrupole-time-of-flight mass spectrometry for simultaneous determination of fifteen organochlorine pesticides in soil and water. J. Chromatogr. A 2016, 1435, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Petersen, A.; Herrmann, S.S.; Poulsen, M.E. Levels of pesticides in food and food safety aspects. In Analysis of Pesticides in Food and Environmental Samples; Tadeo, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 287–318. [Google Scholar]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plan of Action for Technical Cooperation in Food Safety, 2006–2007; PAHO/WHO: Washington, DC, USA, 2005.
- Verma, N.; Bhardwaj, A. Biosensor technology for pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Harrison, V.; Ross, S.M. Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ. Res. 2016, 151, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, É.; Freire, C. Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health 2016, 219, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Tahmasbi, A.M.; Valizadeh, R.; Naserian, A.A.; Soni, A. Organophosphate pesticides: A general review. Agric. Sci. Res. J. 2012, 2, 512–522. [Google Scholar]
- Iyaniwura, T.T. Prevention and management of human toxicosis resulting from pesticide use—A survey. Int. J. Environ. Stud. 1991, 38, 115–121. [Google Scholar] [CrossRef]
- Jaga, K.; Dharmani, C. Ocular toxicity from pesticide exposure: A recent review. Environ. Health Prev. Med. 2006, 11, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.J.; Wiberg-Larsen, P.; Baattrup-Pedersen, A.; Cedergreen, N.; McKnight, U.S.; Kreuger, J.; Jacobsen, D.; Kristensen, E.A.; Friberg, N. The legacy of pesticide pollution: An overlooked factor in current risk assessments of freshwater systems. Water Res. 2015, 84, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, G.; Sharifzadeh, M.S.; Damalas, C.A. Perceptions of the beneficial and harmful effects of pesticides among iranian rice farmers influence the adoption of biological control. Crop Prot. 2015, 75, 124–131. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; Li, J. A 1961–2010 record of fertilizer use, pesticide application and cereal yields: A review. Agron. Sustain. Dev. 2015, 35, 83–93. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef]
- Goicolea, M.A.; Gómez-Caballero, A.; Barrio, R.J. New materials in electochemical sensors for pesticides monitoring. In Pesticides-Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 333–358. ISBN 978-953-307-460-3. [Google Scholar]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. Acute organophosphorus poisoning. Clin. Chim. Acta 2014, 431, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants–trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-transferred carbon black nanoparticles on board of microfluidic chips for rapid and sensitive amperometric determination of phenyl carbamate pesticides in environmental samples. Microchim. Acta 2016, 183, 3143–3149. [Google Scholar] [CrossRef]
- Della Pelle, F.; Vázquez, L.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-printed conductive carbon black nanoparticle films for molecular detection at the microscale. Chem. Eur. J. 2016, 22, 12761–12766. [Google Scholar] [CrossRef] [PubMed]
- Di Ottavio, F.; Della Pelle, F.; Montesano, C.; Scarpone, R.; Escarpa, A.; Compagnone, D.; Sergi, M. Determination of pesticides in wheat flour using microextraction on packed sorbent coupled to ultra-high performance liquid chromatography and tandem mass spectrometry. Food Anal. Methods 2017, 10, 1699–1708. [Google Scholar] [CrossRef]
- Della Pelle, F.; Di Crescenzo, M.C.; Sergi, M.; Montesano, C.; Di Ottavio, F.; Scarpone, R.; Scortichini, G.; Compagnone, D. Micro-solid-phase extraction (µ-spe) of organophosphorous pesticides from wheat followed by lc-ms/ms determination. Food Addit. Contam. Part A 2016, 33, 291–299. [Google Scholar]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Mariani, S.; Minunni, M. Label free affinity sensing: Application to food analysis. Acta Imeko 2016, 5, 36–44. [Google Scholar] [CrossRef]
- Arugula, M.A.; Simonian, A. Novel trends in affinity biosensors: Current challenges and perspectives. Meas. Sci. Technol. 2014, 25, 032001. [Google Scholar] [CrossRef]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; De Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Holford, T.R.; Davis, F.; Higson, S.P. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.; Moore, E. Electrochemical immunosensors for food analysis: A review of recent developments. Anal. Lett. 2017, 50, 1–32. [Google Scholar] [CrossRef]
- Azam, M.S.; Rahman, M.R.T.; Lou, Z.; Tang, Y.; Raqib, S.M.; Jothi, A.S. Advancements and application of immunosensors in the analysis of food contaminants. Nusant. Biosci. 2014, 6, 186–195. [Google Scholar]
- Franek, M.; Hruska, K. Antibody based methods for environmental and food analysis: A review. Vet. Med. 2005, 50, 1–10. [Google Scholar] [CrossRef]
- Suri, C.R.; Boro, R.; Nangia, Y.; Gandhi, S.; Sharma, P.; Wangoo, N.; Rajesh, K.; Shekhawat, G. Immunoanalytical techniques for analyzing pesticides in the environment. TrAC Trends Anal. Chem. 2009, 28, 29–39. [Google Scholar]
- Wang, M.; Kang, H.; Xu, D.; Wang, C.; Liu, S.; Hu, X. Label-free impedimetric immunosensor for sensitive detection of fenvalerate in tea. Food Chem. 2013, 141, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, D.; Hu, Y.; Liu, S.; Wei, H.; Zheng, J.; Wang, G.; Hu, X.; Wang, C. Construction of an impedimetric immunosensor for label-free detecting carbofuran residual in agricultural and environmental samples. Food Control 2015, 53, 72–80. [Google Scholar] [CrossRef]
- Jia, H.; Guo, Y.; Sun, X.; Wang, X. An electrochemical immunosensor based on microfluidic chip for detection of chlorpyrifos. Int. J. Electrochem. Sci. 2015, 10, 8750–8758. [Google Scholar]
- Cao, Y.; Sun, X.; Guo, Y.; Zhao, W.; Wang, X. An electrochemical immunosensor based on interdigitated array microelectrode for the detection of chlorpyrifos. Bioprocess Biosyst. Eng. 2015, 38, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Gallo, F.; Tortolini, C.; Bollella, P.; Ietto, F.; De Mico, A.; D’Annibale, A.; Antiochia, R.; Favero, G.; Mazzei, F. Aunps-functionalized panaba-mwcnts nanocomposite-based impedimetric immunosensor for 2,4-dichlorophenoxy acetic acid detection. Biosens. Bioelectron. 2017, 93, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.; Kim, K.-H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.-J.; Li, L.; Yang, Y.; Mao, L.-G.; Peng, Z. A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sens. Actuators B 2014, 191, 408–414. [Google Scholar] [CrossRef]
- Sun, X.; Qiao, L.; Wang, X. A novel immunosensor based on au nanoparticles and polyaniline/multiwall carbon nanotubes/chitosan nanocomposite film functionalized interface. Nano-Micro Lett. 2013, 5, 191–201. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, X.; Sun, X. A novel label-free amperometric immunosensor based on graphene sheets-methylene blue nanocomposite/gold nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 1399–1414. [Google Scholar]
- Zhu, Y.; Cao, Y.; Sun, X.; Wang, X. Amperometric immunosensor for carbofuran detection based on mwcnts/gs-pei-au and aunps-antibody conjugate. Sensors 2013, 13, 5286–5301. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, M.; Umiltà, E.; Careri, M. New competitive dendrimer-based and highly selective immunosensor for determination of atrazine in environmental, feed and food samples: The importance of antibody selectivity for discrimination among related triazinic metabolites. Anal. Chim. Acta 2014, 806, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, Z.; Liang, P.; Guo, D.; Xiang, Y.; Tian, M.; Song, Z.; Zhao, H. Co3O4/pan magnetic nanoparticle-modified electrochemical immunosensor for chlorpyrifos. Dig. J. Nanomater. Biostruct. (DJNB) 2017, 12, 1–9. [Google Scholar]
- Liu, G.; Guo, W.; Song, D. A multianalyte electrochemical immunosensor based on patterned carbon nanotubes modified substrates for detection of pesticides. Biosens. Bioelectron. 2014, 52, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, D.; Chen, F. Towards the fabrication of a label-free amperometric immunosensor using swnts for direct detection of paraoxon. Talanta 2013, 104, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; García-Febrero, R.; Pividori, I.; Sánchez-Baeza, F.; Marco, M.-P. Coulombimetric immunosensor for paraquat based on electrochemical nanoprobes. Sens. Actuators B 2014, 194, 353–360. [Google Scholar] [CrossRef]
- Garcia-Febrero, R.; Valera, E.; Muriano, A.; Pividori, M.-I.; Sanchez-Baeza, F.; Marco, M.-P. An electrochemical magneto immunosensor (emis) for the determination of paraquat residues in potato samples. Anal. Bioanal. Chem. 2013, 405, 7841–7849. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Martini, E.; Campanella, L.; Favero, G.; Sanzó, G.; Mazzei, F. A new surface plasmon resonance immunosensor for triazine pesticide determination in bovine milk: A comparison with conventional amperometric and screen-printed immunodevices. Sensors 2015, 15, 10255–10270. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, R.; Liu, Y.; Xiang, D.; Liu, Y.; Gui, W.; Li, M.; Zhu, G. A non-competitive surface plasmon resonance immunosensor for rapid detection of triazophos residue in environmental and agricultural samples. Sci. Total Environ. 2018, 613, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Yamasaki, T.; Harada, A.; Ohtake, T.; Adachi, K.; Iwasa, S.; Narita, H.; Miyake, S. Analysis of the fungicide boscalid in horticultural crops using an enzyme-linked immunosorbent assay and an immunosensor based on surface plasmon resonance. J. Agric. Food Chem. 2015, 63, 8075–8082. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Yamasaki, T.; Watanabe, E.; Okazaki, F.; Murakami-Yamaguchi, Y.; Oda, M.; Iwasa, S.; Narita, H.; Miyake, S. Development of an immunosensor for determination of the fungicide chlorothalonil in vegetables, using surface plasmon resonance. J. Agric. Food Chem. 2015, 63, 6325–6330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, P.; Jiang, Z.; Jin, M.; Chen, G.; Cao, X.; Cui, X.; Zhang, Y.; Li, R.; El-Aty, A.A. A simple and sensitive competitive bio-barcode immunoassay for triazophos based on multi-modified gold nanoparticles and fluorescent signal amplification. Anal. Chim. Acta 2018, 999, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Guo, Y.; Zhao, Y.; Liu, Y.; Gui, W.; Zhu, G. Multi-residue detection of pesticides using a sensitive immunochip assay based on nanogold enhancement. Anal. Chim. Acta 2016, 938, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, H.; Liu, G. Advances in biosensor-based instruments for pesticide residues rapid detection. Int. J. Electrochem. Sci. 2015, 10, 9790–9807. [Google Scholar]
- Patel, P. (Bio) sensors for measurement of analytes implicated in food safety: A review. TrAC Trends Anal. Chem. 2002, 21, 96–115. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of rna molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; Giamberardino, A.; DeRosa, M.C. Advances in aptamer-based biosensors for food safety. In Environmental Biosensors; Somerset, V., Ed.; InTech: Rijeka, Crotia, 2011; pp. 17–42. ISBN 978-953-307-486-3. [Google Scholar]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: Rna ligands to bacteriophage t4 dna polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Marty, J.-L. Electrochemical aptasensors for the assessment of food quality and safety. TrAC Trends Anal. Chem. 2016, 79, 60–70. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Rapini, R.; Marrazza, G. Biosensor potential in pesticide monitoring. Compr. Anal. Chem. 2016, 74, 3–31. [Google Scholar]
- Tang, T.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Quantum dot-dna aptamer conjugates coupled with capillary electrophoresis: A universal strategy for ratiometric detection of organophosphorus pesticides. Talanta 2016, 146, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.X.; Guo, Z.H.; Lin, J.S. Practical application of aptamer-based biosensors in detection of low molecular weight pollutants in water sources. Molecules 2018, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Mayer, G. Selection and biosensor application of aptamers for small molecules. Front. Chem. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Sett, A.; Das, S.; Sharma, P.; Bora, U. Aptasensors in health, environment and food safety monitoring. Open J. Appl. Biosens. 2012, 1, 9–19. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X. Aptamer-based technology for food analysis. Appl. Biochem. Biotechnol. 2015, 175, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Verdian, A. Apta-nanosensors for detection and quantitative determination of Acetamiprid—A pesticide residue in food and environment. Talanta 2018, 176, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Selection and characterization of dna aptamers for electrochemical biosensing of carbendazim. Anal. Chem. 2017, 89, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Du, X.; Liu, Q.; Zhou, L.; Dai, L.; Qian, J.; Wang, K. Silver nanoparticles anchored on nitrogen-doped graphene as a novel electrochemical biosensing platform with enhanced sensitivity for aptamer-based pesticide assay. Analyst 2015, 140, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Rapini, R.; Cincinelli, A.; Marrazza, G. Acetamiprid multidetection by disposable electrochemical dna aptasensor. Talanta 2016, 161, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Thakur, H.; Bharti, A.; Kaur, N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal. Chim. Acta 2016, 939, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A regenerative and selective electrochemical aptasensor based on copper oxide nanoflowers-single walled carbon nanotubes nanocomposite for chlorpyrifos detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Jia, H.; Guo, Y.; Zhang, H.; Wang, Z.; Sun, X.; Zhao, J. An ultrasensitive aptasensor for chlorpyrifos based on ordered mesoporous carbon/ferrocene hybrid multiwalled carbon nanotubes. RSC Adv. 2016, 6, 58541–58548. [Google Scholar] [CrossRef]
- Jiao, Y.; Hou, W.; Fu, J.; Guo, Y.; Sun, X.; Wang, X.; Zhao, J. A nanostructured electrochemical aptasensor for highly sensitive detection of chlorpyrifos. Sens. Actuators B 2017, 243, 1164–1170. [Google Scholar] [CrossRef]
- Bala, R.; Sharma, R.K.; Wangoo, N. Development of gold nanoparticles-based aptasensor for the colorimetric detection of organophosphorus pesticide phorate. Anal. Bioanal. Chem. 2016, 408, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Dhingra, S.; Kumar, M.; Bansal, K.; Mittal, S.; Sharma, R.K.; Wangoo, N. Detection of organophosphorus pesticide—malathion in environmental samples using peptide and aptamer based nanoprobes. Chem. Eng. J. 2017, 311, 111–116. [Google Scholar] [CrossRef]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Nguyen, V.-T.; Park, J.G.; Gu, M.B. Detection of iprobenfos and edifenphos using a new multi-aptasensor. Anal. Chim. Acta 2015, 868, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Mittal, S.; Sharma, R.K.; Wangoo, N. A supersensitive silver nanoprobe based aptasensor for low cost detection of malathion residues in water and food samples. Spectrochim. Acta Part A 2018, 196, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Barahona, F.; Bardliving, C.L.; Phifer, A.; Bruno, J.G.; Batt, C.A. An aptasensor based on polymer-gold nanoparticle composite microspheres for the detection of malathion using surface-enhanced raman spectroscopy. Ind. Biotechnol. 2013, 9, 42–50. [Google Scholar] [CrossRef]
- Nie, Y.; Teng, Y.; Li, P.; Liu, W.; Shi, Q.; Zhang, Y. Label-free aptamer-based sensor for specific detection of malathion residues by surface-enhanced raman scattering. Spectrochim. Acta Part A 2018, 191, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Mirroshandel, A.A. Highly-sensitive aptasensor based on fluorescence resonance energy transfer between l-cysteine capped zns quantum dots and graphene oxide sheets for the determination of edifenphos fungicide. Biosens. Bioelectron. 2017, 96, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Q.; Li, H.; Ouyang, Q.; Zhao, J. Fabricating a novel label-free aptasensor for acetamiprid by fluorescence resonance energy transfer between NH2-NaYF4: Yb, Ho@ Sio2 and au nanoparticles. Biosens. Bioelectron. 2016, 80, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yu, Y.; Li, R.; Cao, Y.; Guo, M. Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sens. Actuators B 2016, 229, 100–109. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Wang, L.; Xu, J.; Huang, Y.; Luo, Y.; Shen, F.; Sun, C.; Meng, R. Aptamer-based fluorescent screening assay for acetamiprid via inner filter effect of gold nanoparticles on the fluorescence of cdte quantum dots. Anal. Bioanal. Chem. 2016, 408, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Bantz, K.C.; Lindquist, N.C.; Haynes, C.L.; Oh, S.-H. Vertically oriented sub-10-nm plasmonic nanogap arrays. Nano Lett. 2010, 10, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Sur, U.K. Surface-enhanced raman spectroscopy. Resonance 2010, 15, 154–164. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, G.; Zhang, Y. Molecularly imprinted polymers as recognition elements in sensors. In Molecularly Imprinted Sensors; Li, S., Piletsky, S.A., Ge, Y., Lunec, J., Eds.; Elsevier: New York, NY, USA, 2012; pp. 35–55. ISBN 978-0-444-56331-6. [Google Scholar]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Turner, A.P. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. Mip sensors–the electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical sensors based on magnetic molecularly imprinted polymers: A review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Truta, L.A.; Sales, M.G.F.; Moreira, F.T. Imprinting technology in electrochemical biomimetic sensors. Sensors 2017, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bondi, M.C.; Navarro-Villoslada, F.; Benito-Peña, E.; Urraca, J.L. Molecularly imprinted polymers as selective recognition elements in optical sensing. Curr. Anal. Chem. 2008, 4, 316–340. [Google Scholar] [CrossRef]
- Avila, M.; Zougagh, M.; Rios, A.; Escarpa, A. Molecularly imprinted polymers for selective piezoelectric sensing of small molecules. TrAC Trends Anal. Chem. 2008, 27, 54–65. [Google Scholar] [CrossRef]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular imprinting technology in quartz crystal microbalance (qcm) sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yang, B.; Jiang, X.; Li, J. Current progress of nanomaterials in molecularly imprinted electrochemical sensing. Crit. Rev. Anal. Chem. 2018, 48, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; D’Souza, F.; Kutner, W. Molecular imprinting for selective chemical sensing of hazardous compounds and drugs of abuse. TrAC Trends Anal. Chem. 2012, 34, 59–77. [Google Scholar] [CrossRef]
- Rao, T.P.; Prasad, K.; Kala, R.; Gladis, J.M. Biomimetic sensors for toxic pesticides and inorganics based on optoelectronic/electrochemical transducers—An overview. Crit. Rev. Anal. Chem. 2007, 37, 191–210. [Google Scholar] [CrossRef]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Luo, X.; Zhang, H.; Sun, G.; Sun, X.; Ma, H. A computational approach to design an electrochemical sensor and determination of acephate in aqueous solution based on a molecularly imprinted poly(o-phenylenediamine) film. Anal. Methods 2013, 5, 6449–6456. [Google Scholar] [CrossRef]
- Zhang, C.; She, Y.; Li, T.; Zhao, F.; Jin, M.; Guo, Y.; Zheng, L.; Wang, S.; Jin, F.; Shao, H. A highly selective electrochemical sensor based on molecularly imprinted polypyrrole-modified gold electrode for the determination of glyphosate in cucumber and tap water. Anal. Bioanal. Chem. 2017, 409, 7133–7144. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, Q.; Wu, J.; Li, X.; Li, P.; Yu, H.; Li, X.; Lei, F. Electrochemical sensor based on molecularly imprinted polymer reduced graphene oxide and gold nanoparticles modified electrode for detection of carbofuran. Sens. Actuators B 2015, 220, 216–221. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Electrochemical determination of carbaryl by using a molecularly imprinted polymer/graphene-ionic liquid-nano au/chitosan-aupt alloy nanoparticles composite film modified electrode. Int. J. Electrochem. Sci. 2014, 9, 1366–1377. [Google Scholar]
- Wang, H.; Xu, Q.; Wang, J.; Du, W.; Liu, F.; Hu, X. Dendrimer-like amino-functionalized hierarchical porous silica nanoparticle: A host material for 2,4-dichlorophenoxyacetic acid imprinting and sensing. Biosens. Bioelectron. 2018, 100, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Shi, X.; Hou, X.; Zhou, J.; Xu, Z. Development of molecularly imprinted electrochemical sensors based on Fe3O4@ mwnt-cooh/cs nanocomposite layers for detecting traces of acephate and trichlorfon. Analyst 2014, 139, 6406–6413. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Wu, K.; Zhang, L.; Ge, S.; Yu, J. A molecularly imprinted polypyrrole for ultrasensitive voltammetric determination of glyphosate. Microchim. Acta 2017, 184, 1959–1967. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Electrochemical determination of methyl parathion using a molecularly imprinted polymer–ionic liquid–graphene composite film coated electrode. Sens. Actuators B 2013, 176, 818–824. [Google Scholar] [CrossRef]
- He, B.; Mao, Y.-L.; Zhang, Y.; Yin, W.; Hou, C.-J.; Huo, D.-Q.; Fa, H.-B. A porphyrin molecularly imprinted biomimetic electrochemical sensor based on gold nanoparticles and carboxyl graphene composite for the highly efficient detection of methyl parathion. Nano 2017, 12, 1750046. [Google Scholar] [CrossRef]
- Li, S.; Yin, G.; Wu, X.; Liu, C.; Luo, J. Supramolecular imprinted sensor for carbofuran detection based on a functionalized multiwalled carbon nanotube-supported pd-ir composite and methylene blue as catalyst. Electrochim. Acta 2016, 188, 294–300. [Google Scholar] [CrossRef]
- Tan, X.; Wu, J.; Hu, Q.; Li, X.; Li, P.; Yu, H.; Li, X.; Lei, F. An electrochemical sensor for the determination of phoxim based on a graphene modified electrode and molecularly imprinted polymer. Anal. Methods 2015, 7, 4786–4792. [Google Scholar] [CrossRef]
- Hu, C.; Deng, J.; Xiao, X.; Zhan, X.; Huang, K.; Xiao, N.; Ju, S. Determination of dimetridazole using carbon paste electrode modified with aluminum doped surface molecularly imprinted siloxane. Electrochim. Acta 2015, 158, 298–305. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Yin, G.; Luo, J.; Zhang, Z.; Xie, Y. Supramolecular imprinted electrochemical sensor for the neonicotinoid insecticide imidacloprid based on double amplification by pt-in catalytic nanoparticles and a bromophenol blue doped molecularly imprinted film. Microchim. Acta 2016, 183, 3101–3109. [Google Scholar] [CrossRef]
- Li, S.; Luo, Q.; Liu, Y.; Zhang, Z.; Shen, G.; Wu, H.; Chen, A.; Liu, X.; Zhang, A. Surface molecularly imprinted polymer film with poly (p-aminothiophenol) outer layer coated on gold nanoparticles inner layer for highly sensitive and selective sensing paraoxon. Polymers 2017, 9, 359. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, M.; Chen, P.; Zhao, H.; Yang, X.; Yao, L.; Zhang, H.; Dong, A.; Wang, J.; Wang, Z. A facile molecularly imprinted electrochemical sensor based on graphene: Application to the selective determination of thiamethoxam in grain. RSC Adv. 2017, 7, 38884–38894. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.; Xie, T.; Yang, X.; Dong, A.; Zhang, H.; Wang, J.; Wang, Z. Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Sens. Actuators B 2017, 252, 991–1002. [Google Scholar] [CrossRef]
- Wu, B.; Hou, L.; Du, M.; Zhang, T.; Wang, Z.; Xue, Z.; Lu, X. A molecularly imprinted electrochemical enzymeless sensor based on functionalized gold nanoparticle decorated carbon nanotubes for methyl-parathion detection. RSC Adv. 2014, 4, 53701–53710. [Google Scholar] [CrossRef]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.-T.; Jaffrezic-Renault, N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, X.; Zeng, Y.; Zhou, T.; Shi, G. Molecularly imprinted sensor based on electropolmerized poly(o-phenylenediamine) membranes at reduced graphene oxide modified electrode for imidacloprid determination. Sens. Actuators B 2013, 185, 424–431. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y.; Wang, X.; Fang, G.; Wang, S. Prussian blue mediated amplification combined with signal enhancement of ordered mesoporous carbon for ultrasensitive and specific quantification of metolcarb by a three-dimensional molecularly imprinted electrochemical sensor. Biosens. Bioelectron. 2015, 64, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Shahtaheri, S.J. Biomimetic electrochemical sensor based on molecularly imprinted polymer for dicloran pesticide determination in biological and environmental samples. J. Iran. Chem. Soc. 2016, 13, 2077–2084. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Rahimi Foroushani, A.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Karfa, P.; Patra, S.; Madhuri, R.; Sharma, P.K. Molecularly imprinted star polymer-modified superparamagnetic iron oxide nanoparticle for trace level sensing and separation of mancozeb. RSC Adv. 2016, 6, 36751–36760. [Google Scholar] [CrossRef]
- Capoferri, D.; Del Carlo, M.; Ntshongontshi, N.; Iwuoha, E.; Sergi, M.; Di Ottavio, F.; Compagnone, D. Mip-meps based sensing strategy for the selective assay of dimethoate. Application to wheat flour samples. Talanta 2017, 174, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-R.; Lee, G.-J.; Haridharan, N.; Wu, J.J. Electrochemical sensor using molecular imprinting polymerization modified electrodes to detect methyl parathion in environmental media. Electrocatalysis 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, Y.; Gu, M.; Wang, D.; Dang, Y.; Ye, B.-C.; Li, Y. A robust electrochemical sensing platform using carbon paste electrode modified with molecularly imprinted microsphere and its application on methyl parathion detection. Biosens. Bioelectron. 2018, 106, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-J.; Pan, M.-F.; Fang, G.-Z.; He, X.-L.; Xia, Y.-Q.; Wang, S. Electrochemical sensor based on a bilayer of ppy–mwcnts–bicopc composite and molecularly imprinted p o ap for sensitive recognition and determination of metolcarb. RSC Adv. 2015, 5, 11498–11505. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. Design and fabrication of molecularly imprinted polymer-based potentiometric sensor from the surface modified multiwalled carbon nanotube for the determination of lindane (γ-hexachlorocyclohexane), an organochlorine pesticide. Biosens. Bioelectron. 2015, 64, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Alexander, S. Multiwalled carbon nanotube based molecular imprinted polymer for trace determination of 2,4-dichlorophenoxyaceticacid in natural water samples using a potentiometric method. Appl. Surf. Sci. 2014, 303, 180–186. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Hussein, L.A.; El Azab, N.F. Novel potentiometric sensors for the determination of the dinotefuran insecticide residue levels in cucumber and soil samples. Talanta 2017, 164, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Z.O.; Dilgin, Y. A novel impedimetric sensor based on molecularly imprinted polypyrrole modified pencil graphite electrode for trace level determination of chlorpyrifos. Sens. Actuators B 2013, 188, 78–84. [Google Scholar] [CrossRef]
- Prusty, A.K.; Bhand, S. A capacitive sensor for 2,4-D determination in water based on 2,4-D imprinted polypyrrole coated pencil electrode. Mater. Res. Express 2017, 4, 035306. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Kong, F.; Qiao, X.; Xu, Z. Molecularly imprinted fluorescent probe based on hydrophobic cdse/zns quantum dots for the detection of methamidophos in fruit and vegetables. Adv. Polym. Technol. 2017. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, C.; Zhang, H.; Zhao, C.; Wang, Y. Development and applications of quantum dot-based molecularly imprinted polymer composites for optosensing of carbofuran in water. Anal. Sci. 2017, 33, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, H.; Cai, J.; Duan, Y.; Liu, Y. Development of fluorescence sensing material based on cdse/zns quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and chinese cabbage. J. Agric. Food Chem. 2015, 63, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, Z.; Li, J.; Shao, H.; Chen, L.; Yang, X. A molecular imprinting fluorescence sensor based on quantum dots and a mesoporous structure for selective and sensitive detection of 2,4-dichlorophenoxyacetic acid. Sens. Actuators B 2017, 252, 934–943. [Google Scholar] [CrossRef]
- Tang, J.; Xiang, L. Development of a probe based on quantum dots embedded with molecularly imprinted polymers to detect parathion. Pol. J. Environ. Stud. 2016, 25. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Sui, W.; Lin, X.; Liu, B. Sensitive molecularly imprinted fluorescence determination of pyrethroids using green zinc oxide quantum dots. Anal. Lett. 2017, 50, 1139–1149. [Google Scholar] [CrossRef]
- Li, X.; Jiao, H.-F.; Shi, X.-Z.; Sun, A.; Wang, X.; Chai, J.; Li, D.-X.; Chen, J. Development and application of a novel fluorescent nanosensor based on fese quantum dots embedded silica molecularly imprinted polymer for the rapid optosensing of cyfluthrin. Biosens. Bioelectron. 2018, 99, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Zhang, Q.; Dai, J.; Wei, X.; Song, Z.; Yan, Y.; Li, C. Molecularly imprinted polymer microspheres for optical measurement of ultra trace nonfluorescent cyhalothrin in honey. Food Chem. 2014, 156, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Li, C.; Yan, Y. Detection of nonfluorescent cyhalothrin in honey by a spheral SiO2-based particle coating with thin fluorescent molecularly imprinted polymers film. RSC Adv. 2015, 5, 96158–96164. [Google Scholar] [CrossRef]
- Gao, L.; Han, W.; Li, X.; Wang, J.; Yan, Y.; Li, C.; Dai, J. Detection of λ-cyhalothrin by a core-shell spherical sio2-based surface thin fluorescent molecularly imprinted polymer film. Anal. Bioanal. Chem. 2015, 407, 9177–9184. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Li, X.; Yan, Y.; Li, C.; Pan, J. A core-shell surface magnetic molecularly imprinted polymers with fluorescence for λ-cyhalothrin selective recognition. Anal. Bioanal. Chem. 2014, 406, 7213–7220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, L.; Han, D.; Pan, J.; Qiu, H.; Li, H.; Wei, X.; Dai, J.; Yang, J.; Yao, H. Optical detection of λ-cyhalothrin by core–shell fluorescent molecularly imprinted polymers in chinese spirits. J. Agric. Food Chem. 2015, 63, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, G.; Zhang, Q.; Li, C.; Luo, J.; Xu, Z.; Qin, A. Selective detection of fenaminosulf via a molecularly imprinted fluorescence switch and silver nano-film amplification. Biosens. Bioelectron. 2015, 71, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shen, F.; Zhang, Z.; Ren, X. A novel 2-acrylamide-6-methoxybenzothiazole fabricated molecularly imprinted polymers for direct fluorescent sensing of alachlor. Chromatographia 2016, 79, 71–78. [Google Scholar] [CrossRef]
- Ren, B.; Qi, H.; Li, X.; Liu, L.; Gao, L.; Che, G.; Hu, B.; Wang, L.; Lin, X. A novel fluorescent functional monomer as the recognition element in core–shell imprinted sensors responding to concentration of 2,4,6-trichlorophenol. RSC Adv. 2018, 8, 6083–6089. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.; Jin, F.; Jin, M. Competitive fluorescence assay for specific recognition of atrazine by magnetic molecularly imprinted polymer based on Fe3O4-chitosan. Carbohydr. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, J.; Yin, G.; Xu, Z.; Le, Y.; Wu, X.; Wu, N.; Zhang, Q. Selective determination of dimethoate via fluorescence resonance energy transfer between carbon dots and a dye-doped molecularly imprinted polymer. Sens. Actuators B 2015, 206, 14–21. [Google Scholar] [CrossRef]
- Ye, T.; Yin, W.; Zhu, N.; Yuan, M.; Cao, H.; Yu, J.; Gou, Z.; Wang, X.; Zhu, H.; Reyihanguli, A. Colorimetric detection of pyrethroid metabolite by using surface molecularly imprinted polymer. Sens. Actuators B 2018, 254, 417–423. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Yin, W.; He, B.; Liu, F.; Hou, C.; Huo, D.; Fa, H. A dual read-out molecularly imprinted composite membrane sensor based on zinc porphyrin for the detection of dimethyl methylphosphonate. Chem. Res. Chin. Univ. 2016, 32, 725–730. [Google Scholar] [CrossRef]
- Capoferri, D.; Álvarez-Diduk, R.; Del Carlo, M.; Compagnone, D.; Merkoçi, A. Electrochromic molecular imprinting sensor for visual and smartphone-based detections. Anal. Chem. 2018, 90, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-H.; Liang, R.-P.; Huang, C.-F.; Wang, Y.; Qiu, J.-D. Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Sensing Technique and Redox Probe * | Analyte | Matrix | Linear Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| OVA/Ab/GA/CS/GCE | EIS | Fenvalerate | Tea | 2.38 × 10−9–2.38 × 10−7 | 1.91 × 10−9 | 103.0 | [58] |
| gelatin/Ab/GA/L-Cys/Au electrode | EIS | Carbofuran | Tomato, cabbage, and lettuce | 4.52 × 10−10–4.52 × 10−6 | 4.52 × 10−10 | 90.0–106 | [59] |
| Ab/protein A/AuNPs/PDDA/gold IDAMs | EIS | Chlorpyrifos | Cucumber, lettuce, and pakchoi | 1.43 × 10−9–1.43 × 10−6 | 1.43 × 10−9 | 75.2–96.5 | [60] |
| BSA/Ab/protein A/gold IDAMs | EIS | Chlorpyrifos | Chinese chives, lettuce, and cabbage | 2.85 × 10−9–2.85 × 10−4 | 3.99 × 10−11 | 87.6–96.5 | [61] |
| Ab/AuNPs-PANABA-MWCNTs/SPE | EIS | 2,4-D | Tap water | 4.52 × 10−9–4.52 × 10−7 | 1.36 × 10−9 | 82.0–120 | [62] |
| Ab/fG/SPE | EIS | Parathion | Tomato and carrot | 3.43 × 10−13–3.43 × 10−9 | 1.79 × 10−13 | [63] | |
| BSA/Ab/AuNPs/Au | DPV | Atrazina | Maize | 2.32 × 10−10–2.32 × 10−9 | 7.42 × 10−11 | 95.5–120 | [64] |
| BSA/Ab/AuNPs/PANI/MWCNTs-CS/GCE | CV | Chlorpyrifos | Cabbage, pakchoi, lettuce, and leek | 2.85 × 10−10–1.14 × 10−7 1.14 × 10−7–1.43 × 10−6 | 1.71 × 10−10 | 80.6–109 | [65] |
| BSA/Ab/GS-MB/AuNPs/GCE | CV | Chlorpyrifos | Cabbage, pakchoi, lettuce, and leek | 2.85 × 10−9–1.43 × 10−6 | 1.60 × 10−10 | 86.0–105 | [66] |
| BSA/AuNPs-Ab/GS-PEI-Au/MWCNTs/GCE | CV | Carbofuran | Lettuce, cabbage, green peppers, tomatoes, Chinese chives, and peaches | 2.26 × 10−9–2.26 × 10−6 | 1.36 × 10−10 | 86.0–103 | [67] |
| ATR-BSA/PAMAM/AET/Au/GCE | CV | Atrazine | Corn flakes | 4.64 × 10−11–4.64 × 10−6 | 5.56 × 10−9 | 109–114 | [68] |
| BSA/Antigen/Co3O4/PANI/ITO | CV | Chlorpyrifos | Green vegetables and apples | 0–2.85 × 10−5 | 2.85 × 10−8 | 82.8–107 | [69] |
| SWNTs modified GCE | SWV (FDMA) | Paraoxon | Tap water Purified water | 7.27 × 10−9–9.08 × 10−6 | 7.27 × 10−9 | 92.0 (tap water) 95.0 (purified water) | [71] |
| SWNTs/GCE | SWV (FDMA and PQQ) | Endosulfan and paraoxon simultaneous detection | Tap water Purified water | 1.23 × 10−10–2.46 × 10−7 (endosulfan) 7.27 × 10−9–9.08 × 10−6 (paraoxon) | 1.23 × 10−10 (endosulfan) 7.27 × 10−9 (paraoxon) | 95.0–96.0 97.0–98.0 | [70] |
| GEC | SWV (No redox probe) | Paraquat | Potato | 1.20 × 10−8–2.63 × 10−7 | 3.11 × 10−11 | 76.0–97.0 | [72] |
| m-GEC | Amperometry (No redox probe) | Paraquat | Potato | 7.00 × 10−10 | 91.8–144 | [73] |
| Sensing Technique | Analyte | Matrix | Linear Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|
| SPR | Triazine | Bovine milk | 1.00 × 10−7–1.50 × 10−6 (atrazine) | 5.30 × 10−8 (atrazine) | 94.9–103 (atrazine) | [74] |
| 92.6–95.8 (triazine) | ||||||
| SPR | Triazophos | Chinese cabbage, cucumber, and apple | 3.13 × 10−9–2.65 × 10−8 | 3.06 × 10−10 | 84.4–109 | [75] |
| SPR | Fungicide Boscalid | Cucumber, tomato, green sweet pepper, cabbage, spinach, and orange | 4.95 × 10−8–2.33 × 10−7 | 85.0–109 | [76] | |
| SPR | Fungicide Chlorothalonil | Lettuce, cabbage, and long green onion | 3.01 × 10−8–1.65 × 10−7 | 90.0–118 | [77] | |
| Fluorescence | Triazophos | Tap water, rice, cucumber, cabbage, and apple | 3.19 × 10−11–6.38 × 10−8 | 1.92 × 10−11 | 85.0–110 | [78] |
| Colorimetry | 7 pesticides simultaneously (triazophos, methyl-parathion, fenpropathrin, carbofuran, thiacloprid, chlorothalonil, and carbendazim) | Cucumber, Chinese cabbage, tomato, apple, and pear | 1.21 × 10−10–1.46 × 10−8 (triazophos) | 6.38 × 10−11 (triazophos) | 73.9–116 | [79] |
| 1.00 × 10−8–4.12 × 10−7 (methyl–parathion) | 3.12 × 10−9 (methyl-parathion) | |||||
| 6.87 × 10−10–3.70 × 10−8 (fenpropathrin) | 3.72 × 10−10 (fenpropathrin) | |||||
| 3.53 × 10−8–2.96 × 10−7 (carbofuran) | 2.01 × 10−8 (carbofuran) | |||||
| 4.32 × 10−8–7.95 × 10−7 (thiacloprid) | 2.55 × 10−8 (thiacloprid) | |||||
| 2.78 × 10−9–2.55 × 10−8 (chlorothalonil) | 1.54 × 10−9 (chlorothalonil) | |||||
| 4.08 × 10−10–1.47 × 10−8 (carbendazim) | 2.09 × 10−10 (carbendazim) |
| Electrode | Sensing Technique and Redox Probe * | Analyte | Matrix | Linear Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| MCH/aptamer/Au electrode | EIS | Carbendazim | Soya milk, mango juice, tomato, and plum fruit | 5.23 × 10−11–5.23 × 10−8 | 4.29 × 10−11 | 89.0–95.0 | [96] |
| MCH/aptamer/AuNPs/Au electrode | EIS | Acetamiprid | Tomato | 5.00 × 10−9–6.00 × 10−7 | 1.00 × 10−9 | 85.8–105 | [97] |
| MCH/aptamer/GOPTS/PtNPs microwires modified Au IDEs | EIS | Acetamiprid and atrazine | Tap and mineral water | 1.00 × 10−11–1.00 × 10−7 (acetamiprid) 1.00 × 10−10–1.00 × 10−6 (atrazine) | 1.00 × 10−12 (acetamiprid) 1.00 × 10−11 (atrazine) | 86.0–112 (acetam.) 79.0–113 (atrazine) | [98] |
| MCH/aptamer/Ag-NG/GCE | EIS | Acetamiprid | Cucumber and tomato | 1.00 × 10−13–5.00 × 10−9 | 3.30 × 10−14 | 86.4–109 | [99] |
| MCH/aptamer (oligo 1)/AuNPs/PANI/GSPEs | DPV (No redox probe) | Acetamiprid | Blackberry juice, peach juice, apricot juice, and apricot juice | 2.50 × 10−7–2.00 × 10−6 (NO LINEAR RANGE) | 8.60 × 10−8 | 72.5–110 | [100] |
| Aptamer/SA/CHIT-IO/FTO | DPV | Malathion | Lettuce leave | 3.03 × 10−12–3.03 × 10−8 | 3.03 × 10−12 | 80.0–88.0 | [101] |
| Aptamer/AMP/CuO NFs-SWCNTs/Nafion/GCE | DPV (Methylene blue) | Chlorpyrifos | Apple and celery cabbage | 2.85 × 10−10–4.28 × 10−7 | 2.00 × 10−10 | 96.0–107 | [102] |
| BSA/aptamer/Fc@MWCNTs/OMC/GCE | CV | Chlorpyrifos | Leek lettuce and pakchoi | 2.85 × 10−9–2.85 × 10−4 | 9.41 × 10−10 | 98.5–107 | [103] |
| BSA/aptamer/GO@Fe3O4/CB-CS/GCE | CV | Chlorpyrifos | Cabbage, lettuce, leek, and pakchoi | 2.85 × 10−10–2.85 × 10−4 | 9.41 × 10−11 | 96.0–106 | [104] |
| Sensing Technique | Analyte | Matrix | Liner Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|
| Colorimetry | Phorate | Apple | 1.00 × 10−11–1.30 × 10−6 | 1.00 × 10−11 | 93.0–105 | [105] |
| Colorimetry | Malathion | Mineral water and apple | 1.00 × 10−11–7.5 × 10−10 | 1.94 × 10−12 | [106] | |
| Colorimetry | Malathion | Apple | 5.00 × 10−13–1.00 × 10−9 | 6.00 × 10−14 | [107] | |
| Colorimetry | Iprobenfos (IBF) and edifenphos (EDI) | unwashed and washed rice | 1.00 × 10−8–1.00 × 10−7 (IBF) 5.00 × 10−9–2.5 × 10−8 (EDI) | 1.00 × 10−8 (IBF) 5.00 × 10−9 (EDI) | 81.1–104 (IBF) (unwashed rice) 22.3–48.4 (IBF) (washed rice) 80.5–117 (EDI) (unwashed rice) 24.3–54.8 (EDI) (washed rice) | [108] |
| Colorimetry | Malathion | Tap water and apple | 1.00 × 10−11–7.50 × 10−10 | 5.00 × 10−13 | 89.0–110 | [109] |
| SERS | Malathion | Tap water | 9.99 × 10−6–1.01 × 10−4 | 9.99 × 10−6 | 93.9–109 | [110] |
| SERS | Malathion | Tap water | 5.00 × 10−7–1.00 × 10−5 | 5.00 × 10−7 | 87.4–111 | [111] |
| SERS | Isocarbophos, omethoate, phorate and profenofos | Apple juice | 0–3.80 × 10−6 (phorate) | 3.40 × 10−6 (isocarbophos) 2.4 × 10−5 (omethoate) 4.00 × 10−7 (phorate) 1.40 × 10−5 (profenofos) | [112] | |
| FRET | Edifenphos | Rice | 1.61 × 10−9–1.93 × 10−8 | 4.19 × 10−10 | [113] | |
| FRET | Acetamiprid | Adulterated tea | 5.00 × 10−8–1.00 × 10−6 | 3.20 × 10−9 | 97.6–102 | [114] |
| FRET | Acetamiprid | Cabbage leaves | 0–1.5 × 10−7 | 7.00 × 10−10 | 90.0–95.0 | [115] |
| Fluorescence | Acetamiprid | Chinese cabbage | 5.00 × 10−8–1.00 × 10−6 | 7.29 × 10−9 | 85.7–90.9 | [116] |
| Functional Monomer | Polymerization | Electrode | Sensing Technique and Redox Probe * | Analyte | Matrix | Linear Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| o-PD | Electrochemical | MIP/GCE | DPV (K3[Fe(CN)6]) | Acephate | Tea soup | 5.00 × 10−7–1.00 × 10−4 | 1.30 × 10−7 | 96.8–104 | [138] |
| Py | Electrochemical | MIP/Au electrode | DPV (K3[Fe(CN)6]) | Glyphosate | Cucumber and tapwater | 2.96 × 10−8–4.73 × 10−6 | 1.60 × 10−9 | 72.7–99.0 | [139] |
| MAA | Free radical | MIP/rGO@Au/GCE | DPV (K3[Fe(CN)6]) | Carbofuran | Cabbage and cucumber | 5 × 10−8–2.00 × 10−5 | 2 × 10−8 | 97.7–111 | [140] |
| p-ATP | Electrochemical | MIP/GR-IL-Au/CS-AuPt-NPs/GCE | DPV | Carbaryl | Apple peel and cabbage | 3.00 × 10−8–6.00 × 10−6 | 8.00 × 10−9 | 96.0–105 | [141] |
| o-PD | Electrochemical | MIP/HPSNs-NH2/GCE | DPV | 2,4-D | Bean sprouts | 1.00 × 10−10–2.50 × 10−8 | 1.17 × 10−11 | 94.4–108 | [142] |
| APTES | Sol-gel | MIP/Fe3O4@MWCNTs-COOH/CS/GCE | DPV | Acephate and trichlorfon | Kidney bean and cucumber | 1.00 × 10−10–1.00 × 10−4 (acephate) 1.00 × 10−11–1.00 × 10−5 (trichlorfon) | 6.81 × 10−11 (aceph.) 8.94 × 10−12 (trich.) | 85.7–94.9 | [143] |
| Py | Electrochemical | AuNP-PB-MIP/ITO | DPV (PB) | Glyphosate | Corn | 2.37 × 10−6–7.10 × 10−6 | 5.44 × 10−7 | 97.5–101 | [144] |
| MAA | Precipitation (Free radical) | MIP-IL-EGN/GCE | DPV (no redox probe) | Methyl Parathion | Cabbage and apple peel | 1.00 × 10−8–7.00 × 10−6 | 6.00 × 10−9 | 97.0–110 | [145] |
| Zinc porphyrin | MIP microspheres by free radical (precipitation polymerization) | MIPMs/AuNPs/CG/GCE | DPV (no redox probe) | Methyl parathion | Apple | 8.00 × 10−9–1.00 × 10−6 | 3.16 × 10−10 | 96.0–100 | [146] |
| MB-doped o-phenylene-diamine | Electrochemical | MIP/MWCNT/Pd-Ir nanocomposite/GCE | DPV (no redox probe) | Carbofuran | Cowpea, Chinese cabbage, tomato, and apple | 4.00 × 10−11–4.00 × 10−9 | 1.70 × 10−12 | 87.5–107 | [147] |
| AM | Free radical | MIP/Graphene/GCE | DPV (no redox probe) | Phoxim | Cucumber | 8.00 × 10−7–1.40 × 10−4 | 2.00 × 10−8 | 98.1–101 | [148] |
| APTES | Sol-gel | MIS (molecularly imprinted siloxane)/CPE | DPSV (no redox probe) | Dimetridazole | Egg and milk powder | 1 × 10−8–1.00 × 10−6 1.00 × 10−6–1.00 × 10−4 | 3.60 × 10−9 | 93.0–108 | [149] |
| Bromophenol blue doped o-aminophenol | Electrochemical | MIP/Pt-In/GCE | DPV (no redox probe) | Imidacloprid | Tomato, cabbage, chili, and lettuce | 2.00 × 10−10–5.00 × 10−8 | 1.20 × 10−11 | 93.6–106 | [150] |
| p-ATP | Electrochemical | MIP/AuNPs/SPCE | DPV (no redox probe) | Paraoxon | Apple and cabbage | 1.00 × 10−8–1.00 × 10−4 | 1.00 × 10−9 | 95.2–103 | [151] |
| VBA | MIP/GN/GCE | LSV (no redox probe) | Thiamethoxam | Brown rice | 5.00 × 10−7–2.00 × 10−5 | 4.00 × 10−8 | 88.7–94.0 | [152] | |
| VBA | MIP/GN/GCE | LSV (no redox probe) | Imidacloprid | Brown rice | 5.00 × 10−7–1.5 × 10−5 | 1.00 × 10−7 | 75.0–78.0 | [153] | |
| AuNPs + p-ATP | Electrochemical | FuAuNPs/ATP/MIP/AuNP-MWCNTs/GCE | LSV (no redox probe) | Methyl parathion | Tap water, apple, and cucumber | 3.80 × 10−10–4.18 × 10−9 4.18 × 10−9–4.18 × 10−8 | 3.04 × 10−10 | 95.2–106 | [154] |
| p-ATP-FuAuNPs | Electrochemical | MIP/MOF film/Au electrode | LSV | Glyphosate | Tap water | 5.90 × 10−15–5.90 × 10−9 | 5.00 × 10−15 | 98.7–103 | [155] |
| o-PD | Electrochemical | MIP-rGO/GCE | LSV (no redox probe) | Imidacloprid | Pear | 7.50 × 10−7–7.00 × 10−5 | 4.00 × 10−7 | 91.3–96.6 | [156] |
| p-ABA | Electrochemical | MIP/PB-CMK-3/GCE | LSV (PB) | Metolcarb | Cucumber, cabbage, and apple juice | 5.00 × 10−10–1.00 × 10−4 | 9.30 × 10−11 | 92.4–98.6 | [157] |
| MAA | MIP/CPE | SWV (no redox probe) | Dicloran | Tap water | 1.00 × 10−9–1.00 × 10−6 | 4.80 × 10−10 | 94.2–96.5 | [158] | |
| MAA | MIP/CPE | SWV (no redox probe) | Diazinon | Tap water | 5.00 × 10−10–1.00 × 10−6 | 4.10 × 10−10 | 94.0–96.5 | [159] | |
| MAA | MIP NPs by suspension polymerization | Nano-MIP/CPE | SWV (no redox probe) | Diazinon | Apple fruit | 2.50 × 10−9–1.00 × 10−7 1.00 × 10−7–2.00 × 10−6 | 7.90 × 10−10 | 92.5–94.7 | [160] |
| Itaconic acid | Surface imprinting via controlled radical polymerization | MISP-modified SPIONs/PGE | SWSV (no redox probe) | Mancozeb | Vegetables | 1.10 × 10−8–4.75 × 10−7 | 1.77 × 10−9 | 99.0–100 | [161] |
| Py | Electrochemical | MIP/GCE | SWV (K3[Fe(CN)6]) | Dimethoate | Wheat flour | 1.00 × 10−10–1.00 × 10−9 | [162] | ||
| Quercetin (Qu) and Resorcinol (Re) | Electrochemical | MIP/AuNPs/GCE | CV (no redox probe) | Methyl Parathion | Water and fruit Juice (tangerine), and vegetable juice (sweet potato leaves) | 5.00 × 10−8–1.50 × 10−5 | 1.00 × 10−8 | 87.7–125 | [163] |
| MAA | Distillation precipitation | SMIPMs/CPE | CV | Methyl Parathion | Romaine and spinach | 1.00 × 10−12–8.00 × 10−9 | 3.40 × 10−13 | 97.2–101 | [164] |
| PoAP | Electrochemical | MIP/PPy-MWCNTs-BiCo Pc/GCE | CV (K3[Fe(CN)6]) | Metolcarb | Cucumber and cabbage | 1.00 × 10−8–6.00 × 10−7 | 7.88 × 10−9 | 88.8–93.3 | [165] |
| MAA | MIP/MWCNTs/Cu electrode | Potentiometry (no redox probe) | Lindane | Tap water, grape, orange, tomato, and cabbage | 1.00 × 10−9–1.00 × 10−3 | 1.00 × 10−10 | [166] | ||
| MAA | MIP/MWCNTs/IPIM | Potentiometry (no redox probe) | 2,4-D | Tap water | 1.00 × 10−9–1.00 × 10−5 | 1.20 × 10−9 | 97.6–99.2 | [167] | |
| AM or MAA | Sensor 1 MIP/AM/EGDMA washed Sensor 2 MIP/AM/EGDMA unwashed Sensor 3 MIP/MAA/EGDMA washed Sensor 4 MIP/MAA/EGDMA unwashed Sensor 5 Carboxylated-PVC | Potentiometry (no redox probe) | Dinotefuran | Cucumber | Sensor 1 1.00 × 10−7–1.00 × 10−2 Sensor 2 1.00 × 10−7–1.00 × 10−3 Sensor 3 1.00 × 10−7–1.00 × 10−2 Sensor 4 1.00 × 10−7–1.00 × 10−3 Sensor 5 1.00 × 10−7–1.00 × 10−3 | Sensors 1 and 3 1.73 × 10−9 Sensor 2 4.98 × 10−8 Sensor 4 3.41 × 10−8 Sensor 5 2.13 × 10−8 | 87.9–106 | [168] | |
| Py | Electrochemical | MIP/PGE | EIS | Chlorpyrifos | Tap water and corn leaves | 5.70 × 10−8–8.56 × 10−7 | 1.28 × 10−8 | 101–103 | [169] |
| Py | Electrochemical | MIP/PGE | EIS (capacitance) (no redox probe) | 2,4-D | Packaged drinking water and tap water | 2.71 × 10−10–5.66 × 10−8 | 9.05 × 10−11 | 92.0–110 | [170] |
| Functional Monomer | Polymerization | Sensing Technique | Analyte | Matrix | Linear Dynamic Range LDR (M) | Limit of Detection LOD (M) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MAA | Bulk polymerization | Fluorescence | Methamidophos | Apple and pear | 3.5 × 10−7–7.10 × 10−4 | 9.16 × 10−8 | 89.7–94.9 | [171] |
| MAA | Multi-step swelling + polymerization | Fluorescence | Carbofuran | Tap water | 4.52 × 10−9–9.04 × 10−8 | 9.04 × 10−10 | 94.1–98.4 | [172] |
| MAA | Fluorescence | Carbaryl | Rice and Chinese cabbage | 4.97 × 10−7–3.98 × 10−4 | 1.47 × 10−7 | 74.0–88.0 | [173] | |
| APTES | Sol-gel polymerization | Fluorescence | 2,4-D | Bean sprout (Soybean sprout and Mung bean sprout juice) | 6.60 × 10−7–8.00 × 10−5 | 2.10 × 10−9 | 95.0–110 | [174] |
| APTES | Reverse microemulsion polymerization | Fluorescence | Parathion | Tap water | 5.00 × 10−8–1.00 × 10−3 (NO LINEAR RANGE) | 2.18 × 10−7 | 99.3–100 | [175] |
| AM | Precipitation polymerization | Fluorescence | Cyhalothrin | Milk | 0–1.00 × 10−4 | 99.6–103 | [176] | |
| APTES + MAA | Modified reverse micro-emulsion | Fluorescence | Cyfluthrin | Fish | 2.30 × 10−8–4.61 × 10−7 | 2.30 × 10−9 | 88.0–90.7 | [177] |
| AM | Precipitation | Fluorescence | Cyhalothrin | Honey | 0–1.00 × 10−9 | 4.00 × 10−12 | 97.0–104 | [178] |
| AM | Surface imprinting fluorescent MIP spheres | Fluorescence | Cyhalothrin | Honey | 0–2.50 × 10−9 | 4.00 × 10−12 | 29.0–114 (0–10nM) 94.0–114 (0–2.5nM) | [179] |
| AM | Surface imprinting technology (Free radical) | Fluorescence | Gamma-cyhalothrin | Soda water | 0–5.00 × 10−9 | 5.00 × 10−12 | 96.0–111 (0–5nM) 31.0 (10nM) | [180] |
| AM | Surface molecular imprinting | Fluorescence | Gamma-cyhalothrin | Honey | 0–5.00 × 10−8 | 5.11 × 10−9 | 98.0–107 | [181] |
| AM | Precipitation polymerization | Fluorescence | Gamma-cyhalothrin | Tap water and Chinese spirits | 0–6.00 × 10−8 | 9.17 × 10−9 | 102–106 (0–60nM) 61.4–74.2 (500nM) | [182] |
| Hydroquinone doped with neutral red | Electrochemical | Fluorescence | Fungicide fenaminosulf | Vegetables | 2.00 × 10−10–4.00 × 10−8 | 1.60 × 10−11 | 92.0–110 | [183] |
| AMMB | Precipitation polymerization (free radical) | Fluorescence | Alachlor | Corn seed | 1.00 × 10−6–1.50 × 10−4 | 5.00 × 10−7 | 95.6–104 | [184] |
| 7-allyloxycoumarin | Surface molecular imprinting technique | Fluorescence | 2,4,6-trichlorophenol (2,4,6-TCP) | Soda water | 0–1.00 × 10−7 | 5.34 × 10−11 | 98.0–108 | [185] |
| MAA | Surface molecular imprinted method (free radical) | Fluorescence | Atrazine | Tap water | 2.32 × 10−6–1.85 × 10−4 | 8.60 × 10−7 | 77.6–115 | [186] |
| MR-doped o-phenylenediamine | Electrochemical | FRET | Dimethoate | Chinese cabbage, broccoli and cucumber | 6.00 × 10−10–3.40 × 10−8 | 1.83 × 10−11 | 95.0–106 | [187] |
| APTES + PTES | Sol-Gel | Colorimetry | 3-phenoxybenzaldehyde (3-PBD) | Fruit juice and beverage | 5.04 × 10−7–5.04 × 10−6 | 2.62 × 10−7 | 90.0–97.8 | [188] |
| Zinc porphyrin and methacrylate | Thermal polymerization | Fluorescence photometry + Colorimetry | Dimethyl methylphosphonate | Tap water | 1.00 × 10−7–1.00 × 10−2 (fluorescence) 1.00 × 10−7–1.00 × 10−2 (colorimetric) | 1.00 × 10−7 (fluor–) 1.00 × 10−7 (colorim.) | 96.5–106 | [189] |
| Py | Thermal | Electrochromism | Chlorpyrifos | Drinking water | 1.00 × 10−13–1.00 × 10−3 | 1.00 × 10−13 | 81.0–107 | [190] |
| Dopamine | Self polymerization | SPR | Chlorpyrifos | Apple | 1.00 × 10−9–1.00 × 10−5 | 7.60 × 10−10 | 93.0–104 | [191] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capoferri, D.; Della Pelle, F.; Del Carlo, M.; Compagnone, D. Affinity Sensing Strategies for the Detection of Pesticides in Food. Foods 2018, 7, 148. https://doi.org/10.3390/foods7090148
Capoferri D, Della Pelle F, Del Carlo M, Compagnone D. Affinity Sensing Strategies for the Detection of Pesticides in Food. Foods. 2018; 7(9):148. https://doi.org/10.3390/foods7090148
Chicago/Turabian StyleCapoferri, Denise, Flavio Della Pelle, Michele Del Carlo, and Dario Compagnone. 2018. "Affinity Sensing Strategies for the Detection of Pesticides in Food" Foods 7, no. 9: 148. https://doi.org/10.3390/foods7090148
APA StyleCapoferri, D., Della Pelle, F., Del Carlo, M., & Compagnone, D. (2018). Affinity Sensing Strategies for the Detection of Pesticides in Food. Foods, 7(9), 148. https://doi.org/10.3390/foods7090148








