Effects of Pure and Mixed Autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on Fermentation and Volatile Compounds of Narince Wines
Abstract
:1. Introduction
2. Material and Methods
2.1. Yeast Strains
2.2. Culture Media and Chemicals
2.3. Fermentations
2.4. Chemical Analysis of Wines
2.5. Volatile Compounds Analysis
2.6. GC-MS-FID Conditions and Identification of Volatile Compounds
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth of the T. delbrueckii-2014 and S. cerevisiae-1088 Populations During Fermentation
3.2. Chemical Analysis of Wines
3.3. Volatile Composition of the Narince Wines Produced in the Pure and Mixed Culture
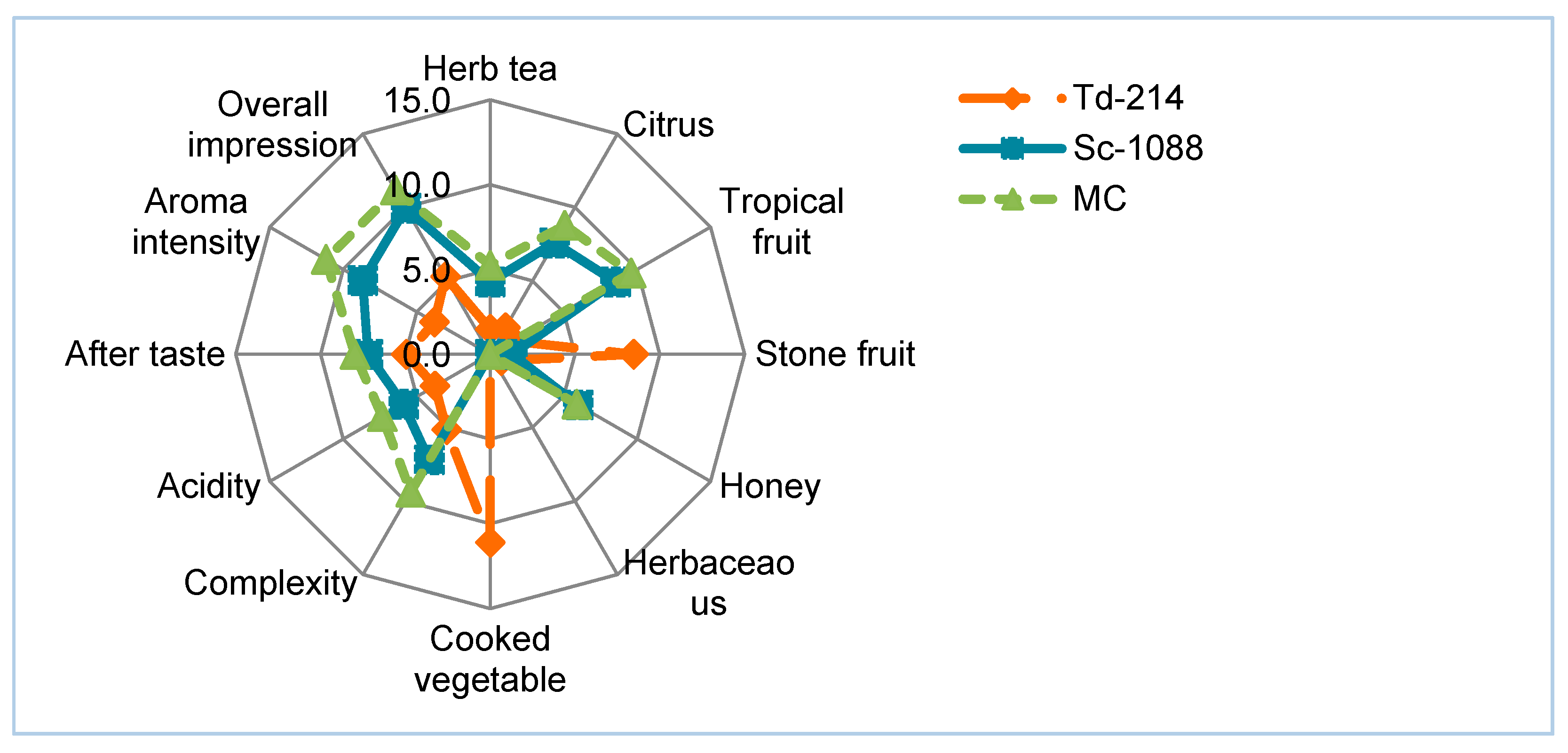
3.4. Sensory Analysis of the Narince Wines Produced in the Pure and Mixed Culture
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Andorrá, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzosa, B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, R.M.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Mircobiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 27–49. [Google Scholar]
- Jemec, K.P.; Cadez, N.; Zagorc, T.; Bubic, V.; Zupec, A.; Raspor, P. Yeast population dynamics in five spontaneous fermentations of Malvasia must. Food Microbiol. 2001, 18, 247–259. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jiménez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Heras-Vázquez, F.J.L.; Rodriguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interaction of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Barbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial resources and innovation in the wine production sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Alvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of the white table wine. Front. Microbiol. 2015, 6, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Siesto, G.; Poeta, C.; Pietrafesa, R.; Romano, P. Indigenous yeast population from Georgia aged wines produced by traditional “Kakhetian” method. Food Microbiol. 2013, 36, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Canonico, L.; Ciani, M.; Comitini, F. Fitness of selected indigenous Saccharomyces cerevisiae strains for white Piceno DOC wines production. Fermentation 2018, 4, 37. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, R.; Romano, P. Indigenous Saccharomyces cerevisiae yeasts as a source of biodiversity for the selection of starters for specific fermentations. BIO Web Conf. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Çelik, Z.D.; Erten, H.; Darıcı, M.; Cabaroğlu, T. Molecular characterization and technological properties of wine yeasts isolated during spontaneous fermentation of Vitis vinifera L. cv. ‘Narince’ grape must grow in ancient wine making area Tokat, Anatolia. BIO Web Conf. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Capece, A.; Pietrafesa, R.; Romano, P. Experimental approach for target slection of wild wine yeasts from spontaneous fermentation of ‘Inzolia’ grapes. World J. Microbiol. Biotechnol. 2011, 27, 2775–2783. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wine and Musts; International Organisation of Vine and Wine: Paris, France, 2015; Volume 1. [Google Scholar]
- Erten, H. Metabolism of fructose as an electron acceptor by Leuconostoc mesenteroides. Process Biochem. 1998, 33, 735–739. [Google Scholar] [CrossRef]
- Selli, S.; Cabaroğlu, T.; Canbaş, A.; Erten, H.; Nurgel, C.; Lepoutre, J.P.; Günata, Z. Volatile composition of red wine from cv. Kalecik karası grown in central Anatolia. Food Chem. 2004, 85, 207–213. [Google Scholar] [CrossRef]
- Schneider, R.; Baumes, R.; Bayanove, C.; Razungles, A. Volatile compounds involved in the aroma of sweet of fortified wines (Vins Doux Naturels) from Grenache Noir. J. Agric. Food Chem. 1998, 46, 3230–3237. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv. Melon B. As precursors of odorants in Muscadet wines. J. Chrom. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Cabaroğlu, T.; Yılmaztekin, M. Methanol and major volatile compounds of Turkish raki and effect distillate source. J. Inst. Brew. 2011, 117, 98–105. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food, 2nd ed.; Springer: New York, NY, USA, 2010; pp. 231–238. ISBN 978-1-4419-6487-8. [Google Scholar]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascul, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces ceresviaie in base wine for sparkling wine production. Eur. Food Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Sadineni, V.; Kondapalli, N.; Obulam, V.S.R. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann. Microbiol. 2012, 62, 1353–1360. [Google Scholar] [CrossRef]
- Berry, D.R.; Slaughter, J.C. Alcoholic Beverage Fermentations. In Fermented Beverage Production, 2nd ed.; Lea, A.G.H., Piggott, J.R., Eds.; Kluwer Academic: New York, NY, USA, 2003; pp. 25–29. ISBN 978-0-306-47705-5. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretoriu, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- De Benedictis, M.; Bleve, G.; Grieco, F.; Tristian, M.; Tufariello, M.; Grieco, F. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Anton. Leeuw. 2011, 99, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]


| General Composition | Td-214 | Sc-1088 | MC | F |
|---|---|---|---|---|
| Alcohol (%v/v) | 10.12 ± 0.0 a | 11.6 ± 0.2 b | 11.48 ± 0.0 b | * |
| Total acidity ** | 7.04 ± 0.0 b | 6.05 ± 0.0 a | 6.56 ± 0.0 b | * |
| pH | 3.68 ± 0.0 a,b | 3.66 ± 0.0 a | 3.71 ± 0.0 b | * |
| Volatile acidity (g/L) *** | 0.58 ± 0.0 c | 0.40 ± 0.0 a | 0.43 ± 0.0 b | * |
| Total SO2 | 56 ± 1 | 56 ± 0.5 | 57 ± 0.2 | ns |
| Dry matter (g/L) | 21.6 ± 0.0 | 21.9 ± 0.0 | 21.7 ± 0.0 | ns |
| Glycerol | 7.37 ± 0.3 b | 5.93 ± 0.0 a | 6.06 ± 0.1 a | * |
| Sugars (g/L) | ||||
| Glucose | 8.06 ± 0.1 c | 0.95 ± 0.0 a | 1.26 ± 0.0 b | * |
| Fructose | 14.25 ± 0.0 c | 0.87 ± 0.0 a | 1.52 ± 0.0 b | * |
| Total | 22.31 | 1.82 | 2.78 |
| AROMAC OMPOUNDS (µg/L) | ||||||
|---|---|---|---|---|---|---|
| Higher alcohols | RI | Td-214 | Sc-1088 | Td + Sc | F | ID |
| 1-Propanol | 1037 | 1702.4 ± 7 b | 1224.5 ± 64 a | 1283.0 ± 5 a | * | RI, MS, Std |
| Isobutyl alcohol | 1085 | 10,621.5 ± 62 b | 9123.2 ± 153 a | 10,649.2 ± 1012 b | * | RI, MS, Std |
| 1-Butanol | 1165 | 617.9 ± 1 c | 309.6 ± 29 a | 400.5 ± 59 b | * | RI, MS, Std |
| Isoamyl alcohol | 1210 | 120,544.6 ± 757 a | 144,208.5 ± 1146 b | 167,995.9 ± 459 c | * | RI, MS, Std |
| 2-Hexanol | 1226 | 200.0 ± 9 a | 295.0 ± 20 c | 246.4 ± 13 b | * | RI, MS, Std |
| 3-Pentanol | 1295 | 104.0 ± 2 c | 67.2 ± 0 a | 73.6 ± 1 b | * | RI, MS, Std |
| 1-Hexanol | 1370 | 1329.5 ± 4 b | 1249.5 ± 25 a | 1228.9 ± 18 a | * | RI, MS, Std |
| (E)-3-Hexen-1-ol | 1386 | 3.2 ± 0 a | 136.8 ± 4 c | 116.4 ± 2 b | * | RI, MS, Std |
| 3-Ethoxy-1-propanol | 1390 | 741.6 ± 1 c | 43.9 ± 1 a | 162.9 ± 5 b | * | RI, MS, Std |
| (Z)-3-Hexen-1-nol | 1401 | 104.4 ± 4 | 103.2 ± 5 | 93.4 ± 7 | ns | RI, MS, Std |
| 2,3-Butanediol | 1495 | 771.6 ± 9 a | 1371.7 ± 19 c | 1029.1 ± 42 b | * | RI, MS, Std |
| 1,3-Butanediol | 1566 | 296.5 ± 2 c | 239.7 ± 1 b | 215.9 ± 13 a | * | RI, MS |
| Methionole | 1737 | 1746.5 ± 8 c | 590.9 ± 5 a | 722.7 ± 11 b | * | RI, MS, Std |
| Benzyl alcohol | 1804 | 36.2 ± 2 a | 59.9 ± 4 c | 46.9 ± 0 b | * | RI, MS, Std |
| 2-Phenyl ethanol | 1916 | 48,153.0 ± 337 c | 32,738.2 ± 584 a | 35,718.7 ± 765 b | * | RI, MS, Std |
| Sum | 186,971.5 | 191,755.8 | 219,983.5 | |||
| Esters | ||||||
| Ethyl acetate ** | 895 | 38,712.4 ± 1266 b | 29,309.0 ± 499 a | 36,891.9 ± 1894 b | * | MS, RI |
| Isoamyl acetate | 1119 | 646.6 ± 0 a | 1336.7 ± 37 b | 1565.2 ± 62 c | * | RI, MS, Std |
| Ethyl hexanoate | 1241 | 826.5 ± 8 a | 1403.6 ± 6 b | 1438.5 ± 41 b | * | RI, MS, Std |
| Hexyl acetate | 1250 | 117.9 ± 0 a | 202.6 ± 7 b | 190.7 ± 12 b | * | RI, MS, Std |
| Ethyl lactate | 1353 | 978.2 ± 4 c | 609.0 ± 8 a | 775.6 ± 18 b | * | RI, MS, Std |
| Ethyl octanoate | 1430 | 173.8 ± 5 a | 1226.1 ± 8 c | 1052.6 ± 10 b | * | RI, MS, Std |
| Ethyl-3-hydroxybutyrate | 1524 | nd | 109.2 ± 1 a | 116.4 ± 2 b | * | RI, MS, Std |
| Ethyl decanoate | 1635 | 28.3 ± 2 a | 300.3 ± 1 c | 232.2 ± 7 b | * | RI, MS, Std |
| 1,3-Propanediol diacetate | 1650 | 258.1 ± 0 c | 194.5 ± 7 a | 223.8 ± 6 b | * | RI, MS |
| Diethyl succinate | 1690 | 152.2 ± 4 a | 248.9 ± 5 c | 215.9 ± 4 b | * | RI, MS, Std |
| Ethyl-4-hydroxybutyrate | 1819 | 656.6 ± 0 a | 4202.7 ± 22 c | 3945.0 ± 120 b | * | RI, MS |
| Ethyl dodecanoate | 1851 | 26.6 ± 1 a | 83.2 ± 3 c | 73.6 ± 0 b | * | RI, MS, Std |
| Diethyl-dL-malate | 2041 | 108.3 ± 10 | 115.2 ± 2 | 116.8 ± 1 | ns | RI, MS, Std |
| Ethyl hexadecanoate | 2259 | 132.9 ± 2 a | 234.1 ± 12 b | 124.4 ± 6 a | * | RI, MS, Std |
| Ethyl hydrogen succinate | 2331 | 2778.7 ± 63 c | 1884.5 ± 106 a | 2524.4 ± 169 b | * | RI, MS |
| Sum | 45,597.1 | 41,459.6 | 49,263.2 | |||
| Volatile acids | ||||||
| Propionic acid | 1538 | 53.5 ± 0 a | 71.8 ± 0 b | 71.7 ± 2 b | * | RI, MS, Std |
| Isobutanoic acid | 1584 | 1052.1 ± 6 c | 373.1 ± 5 a | 526.5 ± 23 b | * | RI, MS, Std |
| Butanoic acid | 1628 | 211.6 ± 4 a | 295.5 ± 4 b | 338.4 ± 15 c | * | RI, MS, Std |
| Isovaleric acid | 1608 | 365.1 ± 5 a | 633.0 ± 11 b | 625.8 ± 14 b | * | RI, MS, Std |
| Hexanoic acid | 1840 | 787.6 ± 3 a | 2355.7 ± 70 b | 2381.1 ± 62 b | * | RI, MS, Std |
| (E)-2-Hexenoic acid | 1962 | 150.0 ± 13 | 134.9 ± 6 | 128.3 ± 6 | ns | RI, MS, Std |
| Octanoic acid | 2060 | 624.6 ± 3 a | 3383.2 ± 112 c | 3152.5 ± 98 b | * | RI, MS, Std |
| Nonanoic acid | 2158 | 94.1 ± 1 | 101.5 ± 26 | 70.6 ± 6 | ns | RI, MS, Std |
| Decanoic acid | 2183 | 40.6 ± 1 a | 573.1 ± 36 b | 623.3 ± 16 c | * | RI, MS |
| 9-Decenoic acid | 2237 | 611.4 ± 11 b | 293.9 ± 17 a | 269.5 ± 9 a | * | RI, MS |
| Sum | 3990.6 | 8215.6 | 8187.9 | |||
| Carbonyl compounds | RI | Td-214 | Sc-1088 | MC | F | ID |
| Acetoin | 1291 | 1448.5 ± 3 b | 307.9 ± 4 a | 299.1 ± 21 a | * | RI, MS, Std |
| Acetaldehyde ** | 500 | 157,040.7 ± 3248 b | 9631.6 ± 499 a | 11,237.0 ± 726 a | * | MS, RI |
| Sum | 158,489.2 | 9939.5 | 11,536.1 | |||
| Lactones | ||||||
| γ-Butyrolactone | 1635 | 1185.2 ± 4 a | 1726.8 ± 12 b | 1770.8 ± 46 b | * | RI, MS, Std |
| Pantolactone | 2414 | 146.7 ± 3 b | 83.9 ± 22 a | 59.6 ± 1 a | * | RI, MS, Std |
| 4-Ethoxycarbonyl-γ-butyrolactone | 2673 | 85.9 ± 1 a | 262.4 ± 7 c | 229.9 ± 0 b | * | RI, MS |
| Sum | 1417.9 | 2073 | 2060.4 | |||
| Volatile phenols | ||||||
| 4-Vinylguaicol | 2091 | 215.3 ± 3 c | 198.6 ± 0 b | 172.4 ± 6 a | * | RI, MS, Std |
| Acetovanillone | 2995 | 50.3 ± 0 b | 54.6 ± 0 c | 32.2 ± 0 a | * | RI, MS, Std |
| Sum | 265.6 | 253.2 | 204.6 | |||
| Total Sum | 396,731.9 | 253,696.7 | 291,235.7 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, E.; Çelik, Z.D.; Cabaroğlu, T. Effects of Pure and Mixed Autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on Fermentation and Volatile Compounds of Narince Wines. Foods 2018, 7, 147. https://doi.org/10.3390/foods7090147
Arslan E, Çelik ZD, Cabaroğlu T. Effects of Pure and Mixed Autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on Fermentation and Volatile Compounds of Narince Wines. Foods. 2018; 7(9):147. https://doi.org/10.3390/foods7090147
Chicago/Turabian StyleArslan, Ebru, Zeynep Dilan Çelik, and Turgut Cabaroğlu. 2018. "Effects of Pure and Mixed Autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on Fermentation and Volatile Compounds of Narince Wines" Foods 7, no. 9: 147. https://doi.org/10.3390/foods7090147




