Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality
Abstract
:1. Introduction
2. Gluten-Related Disorders and the Gluten-Free Market
3. Role of Gluten in Baked Products
4. Microbial Proteases for Gluten Degradation
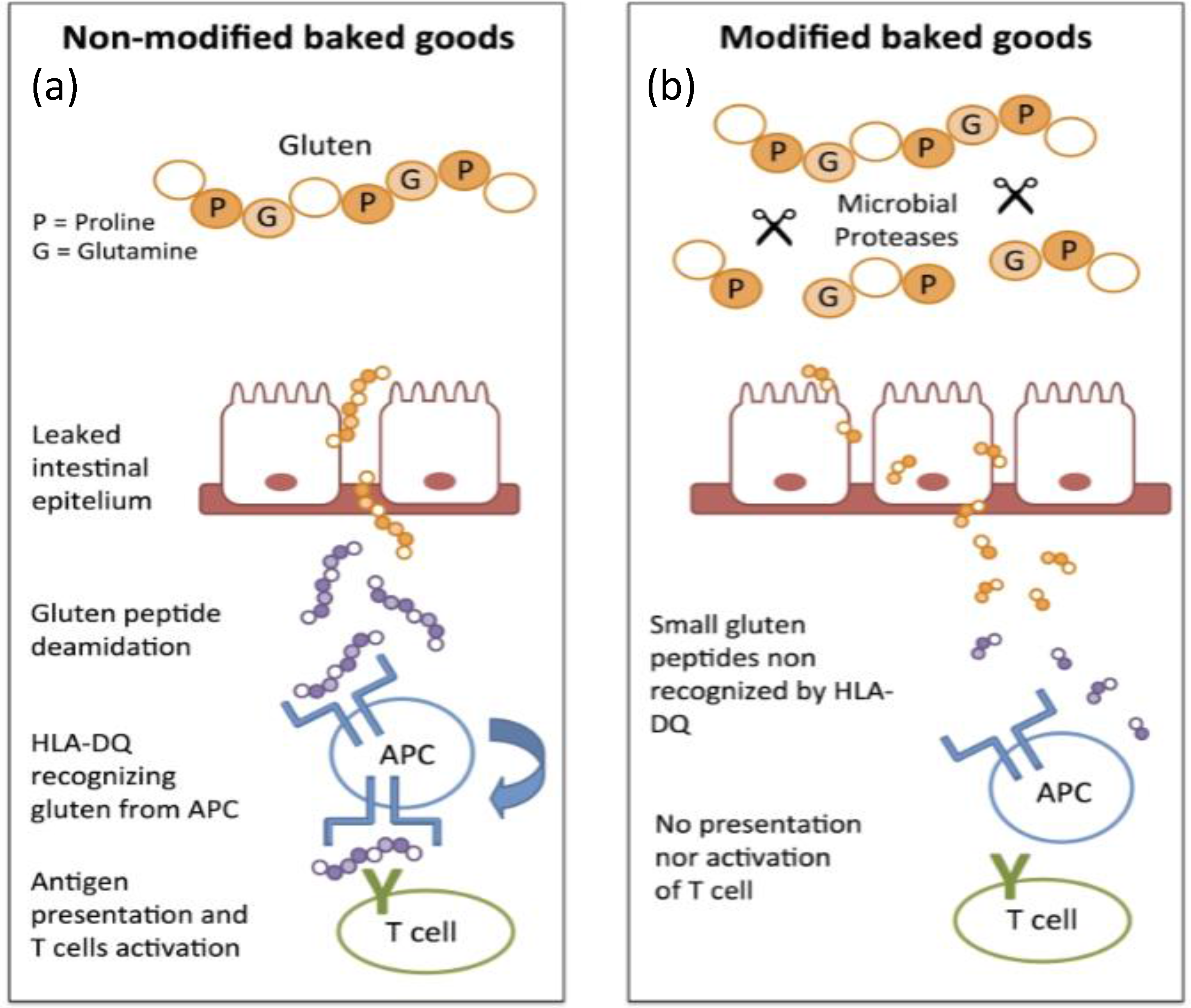
4.1. Implications on Immunogenicity
4.2. Implications on Product Quality
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AnPEP | Aspergillus niger Prolyl-Endopeptidase |
| LAB | Lactic Acid Bacteria |
| APC | Antigen Presenting Cell |
| GRAS | Generally Recognized As Safe |
References
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [PubMed]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical report on the diagnosis and treatment of gluten related disorders. J. Pediatr. Gastroenterol. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Capriles, V.D.; Arêas, J.A.G. Novel approaches in gluten-free breadmaking: Interface between food science, nutrition, and health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- De Angelis, M.; Cassone, A.; Rizzello, C.G.; Gagliardi, F.; Minervini, F.; Calasso, M.; Di Cagno, R.; Francavilla, R.; Gobbetti, M. Mechanism of degradation of immunogenic gluten epitopes from Triticum turgidum L. var. durum by sourdough lactobacilli and fungal proteases. Appl. Environ. Microbiol. 2010, 76, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fassano, A.; Catassi, C. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.L.; Atwell, W.A. Gluten-Free Baked Products; Cargill, Inc.: Wayzata, MN, USA, 2014. [Google Scholar]
- Mintel. Available online: http://www.mintel.com/press-centre/food-and-drink/gluten-free-foods-surge-63-percent (accessed on 22 April 2016).
- Parco, S.; Città, A.; Vascotto, F.; Tamaro, G. Celiac disease and immigration in Northeastern Italy: The “drawn double nostalgia” of “cozonac” and “panettone” slices. Clin. Exp. Gastroenterol. 2011, 4, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Available online: www.fao.org/input/download/standards/291/CXS_118e_2015.pdf (accessed on 20 May 2016).
- Villafuerte-Galvez, J.; Vanga, R.R.; Dennis, M.; Hansen, J.; Leffler, D.A.; Kelly, C.P.; Mukherjee, R. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2015, 42, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Weiten, D.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Living gluten-free: Adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J. Hum. Nutr. Diet. 2016, 29, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sluimer, P. Principles of Breadmaking: Functionality of Raw Materials and Process Steps; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2005. [Google Scholar]
- Cabrera-Chávez, F.; Calderón de la Barca, A.M. Trends in wheat technology and modification of gluten proteins for dietary treatment of coeliac disease patient. J. Cereal Sci. 2010, 52, 337–341. [Google Scholar] [CrossRef]
- Melim-Miguel, A.S.; Martins-Meyer, T.S.; da Costa Figueiredo, E.V.; Paulo-Lobo, P.W.; Dellamora-Ortiz, G.M. Enzymes in Bakery: Current and Future Trends. In Food Industry; Muzzalupo, I., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Frazer, A.C.; Fletcher, R.F.; Ross, C.A.C.; Shaw, B.; Sammons, H.G.; Schneider, R. Gluten-induced enteropathy the effect of partially digested gluten. Lancet 1959, 2, 252–255. [Google Scholar] [CrossRef]
- Messer, M.; Anderson, C.M.; Hubbard, L. Studies on the mechanism of destruction of toxic action of wheat gluten in celiac disease by crude papain. Gut 1964, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Wieser, H.; Koehler, P. Degradation of gluten in rye sourdough products by means of a proline-specific peptidase. Eur. Food Res. Technol. 2015, 240, 517–524. [Google Scholar] [CrossRef]
- Stepniak, D.; Spaenij-Dekking, L.; Mitea, C.; Moester, M.; de Ru, A.; Baak-Pablo, R.; Van Velen, P.; Edens, L.; Koning, F. Highly efficient gluten degradation with a newly identified prolyl endoprotease: Implications for celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G621–G629. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Barbato, M.; Di Camillo, C.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; De Vincenzi, M.; Gobbetti, M.; Cucchiara, S. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: A pilot study. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfo, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A.; et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin. Gastroenterol. Hepatol. 2011, 9, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Candalh, C.; Cellier, C.; Dugave, C.; Namane, A.; Vidal-Martinez, T.; Cerf-Bensussan, N.; Heyman, M. Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology 2005, 129, 786–796. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Effect of AN-PEP Enzyme on Gluten Digestion. Number: NCT01335503. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01335503 (accessed on 26 April 2016).
- ClinicalTrials.gov. Effect of Aspergillus Niger Prolyl Endoprotease (AN-PEP) Enzyme on the Effects of Gluten Ingestion in Patients with Coeliac Disease. Number: NCT00810654. Available online: https://clinicaltrials.gov/ct2/show/NCT00810654 (accessed on 26 April 2016).
- ClinicalTrials.gov. Effect of AN-PEP Enzyme on Gluten Digestion in Guten Sensitive Individuals (AN-PEP-03). Available online: https://clinicaltrials.gov/ct2/show/NCT02060864 (accessed on 26 April 2016).
- M’hir, S.; Rizzello, C.G.; Di Cagno, R.; Cassone, A.; Hamdi, M. Use of selected enterococci and Rhizopus oryzae proteases to hydrolyse wheat proteins responsible for celiac disease. J. Appl. Microbiol. 2009, 106, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.V.; Dal Bello, F.; Arendt, E.K. Sourdough in gluten-free bread-making: An ancient technology to solve a novel issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Curiel, J.A.; Codaa, R.; Limitonec, A.; Katina, K.; Raulio, M.; Giuliani, G.; Rizzello, C.G.; Gobbetti, M. Manufacture and characterization of pasta made with wheat flour rendered gluten-free using fungal proteases and selected sourdough lactic acid bacteria. J. Cereal Sci. 2014, 59, 79–87. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; Minervini, F.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Qiao, S.W.; Arentz-Hansen, H.; Molberg, Ø.; Gray, G.M.; Sollid, L.M.; Khosla, C. Identification and analysis of multivalent proteolytically resistant peptides from gluten: Implications for celiac sprue. J. Proteom. Res. 2005, 4, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- M’hir, S.; Ziadi, M.; Chammem, N.; Hamdi, M. Gluten proteolysis as alternative therapy for celiac patients: A mini-review. Afr J. Biotechnol. 2012, 11, 7323–7330. [Google Scholar]
- Cauvain, S. Bread: The Product. In Technology of Breadmaking, 3rd ed.; Cauvain, S., Ed.; Springer: Basel, Switzerland, 2015; pp. 1–21. [Google Scholar]
- Venturi, M.; Guerrini, S.; Vincenzini, M. Stable and non-competitive association of Saccharomyces cerevisiae, Candida milleri and Lactobacillus sanfranciscensis during manufacture of two traditional sourdough baked goods. Food Microbiol. 2012, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Wieser, H.; Koehler, P. Degradation of gluten in wheat bran and bread drink by means of a proline-specific peptidase. J. Nutr. Food Sci. 2014, 4, 293. [Google Scholar] [CrossRef]
- Nightingale, M.J.; Marchylo, B.A.; Clear, R.M.; Dexter, J.E.; Preston, K.R. Fusarium Head Blight: Effect of Fungal Proteases on Wheat Storage Proteins. Cereal Chem. 1999, 76, 150. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Angioloni, A.; Piga, A.; Collar, C. Impact of sourdough, yeast and gluten on small and large deformation rheological profiles of durum wheat bread doughs. Eur. Food Res. Technol. 2010, 231, 431–440. [Google Scholar] [CrossRef]
| Target of Modification | Microbial Enzyme Source | Gluten Content after Modification | Reference |
|---|---|---|---|
| Wheat flour | Lactobacillus alimentarius, 15M, L. brevis 14G, L. sanfranciscensis 7A, L. hilgardii | ND 1 | [32] |
| Wheat flour | Lactobacillus alimentarius, 15M, L. brevis 14G, L. hilgardii, L sanfranciscensis (7A, LS3, LS10, LS19, LS23, LS38, LS47); Aspergillus oryzae and A. niger | <12 mg/kg (in sourdough). | [4] |
| Wheat flour | Enterococcus faecalis G32, ND3 and HM3C; Rhizopus oryzae CH4 | 1106 mg/kg (in treated flour) | [28] |
| Wheat flour | Lactobacillus sanfranciscensis (7A, LS3, LS10, LS19, LS23, LS38, and LS47), L. alimentarius, L. brevis 14G, L. hilgardii 51B; Aspergillus oryzae and A. niger. | <10 mg/kg (in sweet baked goods). | [22] |
| Wheat flour | Pool 1: Lactobacillus alimentarius 15M, L. brevis 14G, L sanfranciscensis 7A, L. hilgardii; Pool 2: L. sanfranciscensis (LS3, LS10, LS19, LS23, LS38, LS47); Fungal proteases: Aspergillus oryzae and A. niger. | 2480 mg/kg (Pool 1) and <10 mg/kg; (Fungal proteases, Pool 1 and 2) (in biscuits and cakes). | [23] |
| Wheat flour | Lactobacillus sanfranciscensis (7A, LS3, LS10, LS19, LS23, LS38 and LS47), L. alimentarius 15 M, L. brevis 14G, and L. hilgardii 51B; Aspergillus oryzae and A. niger. | 20000–76431 mg/kg (in sourdough bread). | [7] |
| Rye flour | Lactobacillus brevis and A. niger. | 8–532 mg/kg (in rye treated flour). | [20] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Sandoval, N.G.; Valencia-Tapia, M.Y.; Calderón de la Barca, A.M.; Islas-Rubio, A.R. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality. Foods 2016, 5, 59. https://doi.org/10.3390/foods5030059
Heredia-Sandoval NG, Valencia-Tapia MY, Calderón de la Barca AM, Islas-Rubio AR. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality. Foods. 2016; 5(3):59. https://doi.org/10.3390/foods5030059
Chicago/Turabian StyleHeredia-Sandoval, Nina G., Maribel Y. Valencia-Tapia, Ana M. Calderón de la Barca, and Alma R. Islas-Rubio. 2016. "Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality" Foods 5, no. 3: 59. https://doi.org/10.3390/foods5030059





