Food Safety Impacts from Post-Harvest Processing Procedures of Molluscan Shellfish
Abstract
:1. Introduction
2. Considerations Associated with Post-Harvest Processing (PHP) of Molluscan Shellfish
3. Thermal Processing
4. High Pressure Processing
5. Heat-Cool Pasteurization
6. Irradiation
7. Quick Freezing/Frosting
8. High-Salinity Treatment/Relaying
9. Depuration
10. PHP Reduction of Other Potential Food Safety Issues Associated with Raw Molluscan Shellfish Consumption
11. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CC-BY | Creative Commons by Attribution |
| CDC | United States Centers for Disease Control and Prevention |
| CF | Cryogenically Frozen |
| CFU | Colony Forming Units |
| COVIS | Cholera and Other Vibrio Illness Surveillance |
| DOAJ | Directory of open access journals |
| DOI | Digital Object Identifier |
| Epi Data | Epidemiological Data from the Centers for Disease Control and Prevention |
| FDA | United States Food and Drug Administration |
| HACCP | Hazard Analysis Critical Control Point |
| HCP | Heat-Cool Pasteurization |
| HHP | High Hydrostatic Pressure |
| IQF | Individually Quick Frozen |
| ISSC | Interstate Shellfish Sanitation Conference |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MPa | Mega Pascal |
| MPN | Most Probable Number |
| NASA | United States National Aeronautical and Space Administration |
| NSSP | National Shellfish Sanitation Program |
| PHP | Post-Harvest Processing |
| ppt | parts per thousand |
| U.S. | United States of America |
| USPTO | United States Patent and Trade Office |
| V. | Vibrio |
References
- Food and Drug Administration (FDA). National Shellfish Sanitation Program (NSSP) Guide for the Control of Molluscan Shellfish (2013 Revision). Available online: http://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.htm (accessed on 11 November 2015).
- Kural, A.G.; Chen, H. Conditions for a 5-log reduction of Vibrio vulnificus in oysters through high hydrostatic pressure treatment. Int. J. Food Microbiol. 2008, 122, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Danyluk, M.D.; Otwell, W.S. Pathogens in raw foods: What the salad bar can learn from the raw bar. Curr. Opin. Biotechnol. 2009, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kural, A.G.; Shearer, A.E.H.; Kingsley, D.H.; Chen, H. Conditions for high pressure inactivation of Vibrio parahaemolyticus in oysters. Int. J. Food Microbiol. 2008, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- National Shellfish Sanitation Program (NSSP)—Guide for the Control of Molluscan Shellfish 2013 Revision. Available online: http://www.fda.gov/downloads/Food/GuidanceRegulation/FederalStateFoodPrograms/UCM415522.pdf (accessed on 3 December 2015).
- National Shellfish Sanitation Program (NSSP). Available online: http://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.htm (accessed on 3 December 2015).
- Fang, L.; Wolmarans, B.; Kang, M.; Jeong, K.C.; Wright, A.C. Application of chitosan microparticles for reduction of Vibrio species in seawater and live oysters (Cassostrea virginica). Appl. Environ. Microbiol. 2015, 81, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Garrido, V.; Debuex, G.; Farrell-Evans, M.; Mudbidri, A.A.; Otwell, W.S. Evaluation of postharvest-processed oysters by using PCR-based most-probably-number enumeration of Vibrio vulnificus bacteria. Appl. Environ. Microbiol. 2007, 73, 7477–7481. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Grove, S.F.; Lee, A.; Lewis, T.; Stewart, C.M.; Chen, H.Q.; Hoover, D.G. Inactivation of foodborne viruses of significance by high pressure and other processes. J. Food Prot. 2006, 69, 957–961. [Google Scholar] [PubMed]
- Filip, S.; Fink, R.; Jevsnik, M. Influence of food composition on freezing time. Int. J. Sanit. Eng. Res. 2010, 4, 4–13. [Google Scholar]
- Baert, L.; Debevere, J.; Uyttendaele, M. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 2009, 131, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Boonsumrej, S.; Chaiwanichsiri, S.; Tantratian, S.; Suzuki, T.; Takai, R. Effects of freezing and thawing on the quality changes of tiger shrimp (Panaeus monodon) frozen by air-blast and cryogenic freezing. J. Food Eng. 2007, 80, 292–299. [Google Scholar] [CrossRef]
- Songsaeng, S.; Sophanodora, P.; Kaewsrithong, J.; Ohshima, T. Quality changes in oyster (Cassostrea belcheri) during frozen storage as affected by freezing and antioxidant. Food Chem. 2010, 123, 286–290. [Google Scholar] [CrossRef]
- Ama, A.A.; Hamdy, M.K.; Toledo, R.T. Effects of heating, pH and thermoradiation on inactivation of Vibrio vulnificus. Food Microbiol. 1994, 11, 215–227. [Google Scholar] [CrossRef]
- Cook, D.W.; Ruple, A.D. Cold storage and mild heat treatment as processing aids to reduce the numbers of Vibrio vulnificus in raw oysters. J. Food Prot. 1992, 55, 985–989. [Google Scholar]
- Lopez-Caballero, M.E.; Perez-Mateos, M.; Borderias, A.J. Oyster preservation by high-pressure treatment. J. Food Prot. 2000, 2, 196–201. [Google Scholar]
- DiGirolamo, R.; Liston, J.; Matches, J. Effects of irradiaiton on the survival of virus in West Coast oysters. Appl. Microbiol. 1972, 24, 105–1006. [Google Scholar]
- Harewood, P.; Rippey, S.; Montesalvo, M. Effect of gamma irradiation on shelf life and bacterial and viral loads in hard-shelled clams (Mercenaria mercenaria). Appl. Environ. Microbiol. 1994, 60, 2666–2670. [Google Scholar]
- Johnston, M.D.; Brown, M.H. An investigation into the changed physiological state of Vibrio bacteria as a survival mechanism in response to cold temperatures and studies on their sensitivity to heating and freezing. J. Appl. Microbiol. 2002, 92, 1066–1077. [Google Scholar] [CrossRef]
- Thomson, W.K.; Thacker, C.L. Effect of temperature on Vibrio parahaemolyticus in oysters at refrigerator and deep freeze temperatures. J. Inst. Can. Sci. Technol. 1973, 6, 156–158. [Google Scholar] [CrossRef]
- DePaola, A.; Lee, R.; Mahoney, D.; Rivera, I.; Tamplin, M. Case study: Vibrio vulnificus in oysters. In Background Paper for the Joint FAO/WHO Expert Consultation on Development of Practical Risk Management Strategies based on Microbiological Risk Assessment Outputs, Proceedings of the Food Quality and Standards Service, Nutrition and Consumer Protection Division, Food and Agriculture Organization of the United Nations, Kiel, Germany, April 2006; Department of Food Safety, Zoonoses and Foodborne Diseases, World Health Organization: Geneva, Switzerland, 2006; pp. 1–30. [Google Scholar]
- Kingsley, D.H. High pressure processing of bivalve shellfish and HPP’s use as a virus intervention. Foods 2014, 3, 336–350. [Google Scholar] [CrossRef]
- Leal Diego, A.G.; Dores Ramos, A.P.; Marques Souza, D.S.; Durigan, M.; Greinert-Goulart, J.A.; Moresco, V.; Amstutz, R.C.; Micoli, A.H.; Neto, R.C.; Monte Barardi, C.R.; Bueno Franco, R.M. Sanitary quality of edible bivalve mollusks in Southeastern Brazil using an UV based depuration system. Ocean Coast. Manag. 2013, 72, 93–100. [Google Scholar] [CrossRef]
- Marques Souza, D.S.; Piazza, R.S.; Pilotto, M.R.; Almeida do Nascimento, M.; Moresco, V.; Taniguchi, S.; Guiguet Leal, D.A.; Schmidt, E.C.; Cargin-Ferreira, E.; Bicego, M.C.; et al. Virus, protozoa and organic compounds decay in depurated oysters. Int. J. Food Microbiol. 2013, 167, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Phuvasate, S.; Su, Y.C. Impact of water salinity and types of oysters on depuration for reducing Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Food Control 2013, 32, 569–573. [Google Scholar] [CrossRef]
- Larsen, A.M.; Rickard, F.S.; Walton, W.C.; Arias, C.R. Temperature effect on high salinity depuration of Vibrio vulnificus and V. parahaemolyticus from the Eastern oyster (Crassostrea virginica). Int. J. Food Microbiol. 2015, 192, 66–71. [Google Scholar] [CrossRef] [PubMed]
- National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2013. Available online: http://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2013-508c.pdf (accessed on 20 December 2015).
- National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2012. Available online: http://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2012-508c.pdf (accessed on 20 December 2015).
- National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2011. Available online: http://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2011-508c.pdf (accessed on 20 December 2015).
- National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2010. Available online: http://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2010-508c.pdf (accessed on 20 December 2015).
- National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. National Enteric Disease Surveillance: COVIS Annual Summary, 2009. Available online: http://www.cdc.gov/nationalsurveillance/pdfs/cstevibrio2009.pdf (accessed on 20 December 2015).
- U.S. Centers for Disease Control and Prevention. Increase in Vibrio parahaemolyticus Illnesses Associated with Consumption of Shellfish from Several Atlantic Coast Harvest Areas, United States, 2013. Available online: http://www.cdc.gov/vibrio/investigations/vibriop-09-13/epi.html (accessed on 15 January 2015).
- Berna, F.; Goldberg, P.; Horwitz, L.K.; Brink, J.; Holt, S.; Bamford, M.; Chazan, M. Microstratigraphic evidence of in situ fire in the Acheulean stratu of Wonderwerk Cave, Northern Cape province, South Africa. PNAS 2012, 109, E1215–E1220. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A. The Oxford Companion to Food; Oxford University Press: New York, NY, USA, 1999; pp. 212–214. [Google Scholar]
- Ball, C.O. Short-time pasteurization of milk. Ind. Eng. Chem. 1943, 35, 71–84. [Google Scholar] [CrossRef]
- Pederson, C.S. Pasteurization of New York State wines. Ind. Eng. Chem. 1935, 27, 1257–1265. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. Appendix 4: Bacterial Pathogen Growth and Inactivation. In Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; Food and Drug Administration: Rockville, MD, USA, 2011; p. 420. [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. The Danger of Eating Contaminated Raw Oysters. Available online: http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm085368.html (accessed on 3 December 2015).
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. Fresh and Frozen Seafood: Selecting and Serving It Safetly. Available online: http://www.fda.gov/Food/FoodborneIllnessContaminants/BuyStoreServeSafeFood/ucm077331.html (accessed on 3 December 2015).
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. Vibrio Infections. Available online: http://www.foodsafety.gov/poisoning/causes/bacteriaviruses/vibrio_infections/index.html (accessed on 3 December 2015).
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Meujo, D.A.F.; Kevin, D.A.; Peng, J.; Bowling, J.J.; Liu, J.; Hamann, M.T. Reducing oyster-associated bacteria levels using supercritical fluid CO2 as an agent of warm pasteurization. Int. J. Food Microbiol. 2009, 138, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.W. Sensitivity of Vibrio species in phosphate-buffered saline and in oysters to high-pressure processing. J. Food Prot. 2003, 66, 2276–2282. [Google Scholar] [PubMed]
- Prapaiwong, N.; Wallace, R.K.; Arias, C.R. Bacterial loads and microbial composition in high pressure treated oysters during storage. Int. J. Food Microbiol. 2009, 131, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Su, Y.C. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Huang, Y.; Gurtler, J.B.; Niemira, B.A.; Sites, J.E.; Chen, H. Effects of pre-or post-processing storage conditions on high-hydrostatic pressure inactivation of Vibrio parahaemolyticus and V. vulnificus in oysters. Int. J. Food Microbiol. 2013, 163, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Muth, M.K.; Arsenault, J.E.; Cajka, J.C.; Cates, S.C.; Coglaiti, M.C.; Karns, S.A.; O’Neil, M.; Viator, C. Analysis of How Post-harvest Processing Technologies for Controlling Vibrio vulnificus Can Be Implimented (Final Report); RTI Project Number 0211460.008; RTI International, Research Triangle Park: Durham, NC, USA, 2011. [Google Scholar]
- Kingsley, D.H. High pressure processing and its application to the challenge of virus-contaminated foods. Food Environ. Virol. 2013, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Neetoo, H.; Chen, H.; Li, J. High Hydrostatic Pressure Processing: A promising nonthermal technology to inactivate viruses in high-risk foods. Annu. Rev. Food Sci. Technol. 2015, 6, 389–409. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Tesvich, J.; Fahey, P.; Schegan, J. Mild heat treatment of oysters in their natural shell. U.S. Patent No. 5,976,601, 2 November 1999. [Google Scholar]
- Hallman, G.J.; Blackburn, C.M. Phytosanitary irradiation. Foods 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Moosekian, S.R.; Jeong, S.; Marks, B.P.; Ryser, E.T. X-ray irradiation as a microbial intervention strategy for food. Annu. Rev. Food Sci. Technol. 2012, 3, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Barakat, S.M.M. Reduction of Vibrio vulnificus in pure culture, half shell and whole shell oysters (Crassostrea virginica) by X-ray. Int. J. Food Microbiol. 2009, 130, 135–139. [Google Scholar]
- Turner, A.D.; Lewis, A.M.; Hatfield, R.G.; Powell, A.L.; Higman, W.A. Feasibility studies into the production of gamma-irradiated oyster tissue reference materials for paralytic shellfish poisoning toxins. Toxicon 2013, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Marchesani, G.; Mangiacotti, M.; Chiaravalle, A.E. Identifying irradiated oysters by luminescence techniques (TL&PSL). Food Chem. 2012, 135, 319–324. [Google Scholar] [PubMed]
- Wu, Y.; Chang, S.; Nanapaneni, R.; Coker, R.; Haque, Z.; Mahmoud, B.S.M. The efficacy of X-ray doses on murine norovirus-1 (MNV-1) in pure culture, half-shell oyster, salmon sushi, and tuna salad. Food Control 2016, 64, 77–80. [Google Scholar] [CrossRef]
- Praveen, C.; Dancho, B.A.; Kingsley, D.H.; Calci, K.R.; Meade, G.K.; Mena, K.D.; Pillai, S.D. Susceptibility of murine norovirus and hepatitis a virus to electron beam irradiation in oysters and quantifying the reduction in potential infection risks. Appl. Environ. Microbiol. 2013, 79, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Zaragoza, E.; Marcazzo, J.; Monaca, S.D.; Boniglia, C.; Gargiulo, R.; Bortolin, E. Thermoluminescence analysis of irradiated oyster shells. Appl. Radiat. Isotopes 2012, 71, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Interstate Shellfish Sanitation Conference (ISSC). Guidelines for Primary Certified Shellfish Processors on Using Controls for Irradiation of Containers of Molluscan Shellfish Pre-labeled with Vibrio Reduction Language. In Section IV. Guidance Documents, Chapter III. Harvesting, Handling, Processing, and Distribution, NSSP Guide. 2013 ISSC Summary of Actions, Proceedings of the Interstate Shellfish Sanitation Conference, San Antonio, TX, USA, 25–31 January 2014; Interstate Shellfish Sanitation Conference: Columbia, SC, USA, 2014; pp. 210–212. [Google Scholar]
- Andrews, L.S.; Park, D.L.; Chen, Y.P. Low temperature pasteurization to reduce the risk of Vibrio infections from raw shell-stock oysters. Food Addit. Contam. 2000, 17, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Muntada-Garriga, J.M.; Rodriguez-Jerez, J.J.; Lopez-Sabater, E.I.; Mora-Ventura, M.T. Effect of chill and freezing temperatures on survival of Vibrio parahaemolyticus inoculated in homogenates of oyster meat. Lett. Appl. Microbiol. 1995, 20, 225–227. [Google Scholar] [CrossRef]
- Seminario, D.M.; Balaban, M.O.; Rodrick, G. Inactivation kinetics of Vibrio vulnificus in phosphate-buffered saline at different freezing and storage temperatures and times. J. Food Sci. 2011, 76, E232–E239. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.W.; Maurer, E.M.; Childers, B.A.; Lewis, D.H. Effect of frozen storage and vacuum-packaging on survival of Vibrio vulnificus in gulf coast oysters (Crassostrea virginica). J. Food Prot. 1994, 7, 561–564. [Google Scholar]
- Liu, C.; Jianzhang, L.; Yi-Cheng, S. Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in pacific raw oysters (Crassostrea gigas). J. Food Prot. 2009, 1, 174–177. [Google Scholar]
- DePaola, A.; Jones, J.L.; Noe, K.E.; Byars, R.H.; Bowers, J. C. Survey of postharvest-processed oysters in the United States for levels of Vibrio vulnificus and Vibrio parahaemolyticus. J. Food Prot. 2009, 72, 2110–2113. [Google Scholar] [PubMed]
- Motes, M.L.; DePaola, A. Offshore suspension relaying to reduce levels of Vibrio vulnificus in oysters (Crassostrea virginica). Appl. Environ. Microbiol. 1996, 62, 3875–3877. [Google Scholar]
- Parveen, S.; Jahncke, M.; Elmahdi, S.; Crocker, H.; Bowers, J.; White, C.; Gray, S.; Brohawn, K. High salinity relaying to reduce Vibrio parahaemolyticus and Vibrio vulnificus in Chesapeake Bay Oysters. University of Maryland Eastern Shore: Princess Anne, MD, USA; Virginia Polytechnic University: Blacksburg, VA, USA, Unpublished work. 2016. [Google Scholar]
- Larsen, A.M.; Scott Rikard, F.; Walton, W.C.; Arias, C.R. Effective reduction of Vibrio vulnificus in the eastern oyster (Crassostrea virginica) using high salinity depuration. Food Microbiol. 2013, 34, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Audemard, C.; Kator, H.I.; Rhodes, M.W.; Gallivan, T.; Erskine, A.J.; Leggett, A.T.; Reece, K.S. High salinity relay as a postharvest processing strategy to reduce Vibrio vulnificus levels in Chesapeake Bay oysters (Crassostrea virginica). J. Food Prot. 2011, 74, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Audemard, C.; Gallivan, T.; Erskine, A.J.; Leggett, T.; Kator, H.I.; Reece, K.S. Preliminary assessment of high salinity relay as a Vibrio vulnificus post-harvest processing strategy for Chesapeake Bay oysters (Crassostrea virginica). J. Shellfish Res. 2011, 30, 483–484. [Google Scholar]
- Jahncke, M. New Approaches Can Help Ensure Safety of Raw Farmed Oysters; Global Aquaculture Advocate: Portsmouth, NH, USA, 2011; pp. 48–51. [Google Scholar]
- Supan, J. Container-Relaying of Oysters: An Alternative to Depuration and Relaying. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 205–216. [Google Scholar]
- Dressel, D.M.; Snyder, M.I. Depuration—The Regulatory Perspective. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 47–70. [Google Scholar]
- Canzonier, W.J. Historical Perspective on Commercial Depuration of Shellfish. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 7–15. [Google Scholar]
- Schneider, K.R.; Steslow, F.S.; Sierra, F.S.; Rodrick, G.E.; Noss, C.I. Ozone depuration of Vibrio vulnificus from the southern quahog clam, mercenaria campechiensis. J. Invertebr. Pathol. 1991, 57, 184–190. [Google Scholar] [CrossRef]
- Roderick, G.E.; Schneider, K.R. Depuration and Relaying of Molluscan Shellfish. In Environmental Indicators and Shellfish Safety; Hackney, C.R., Pierson, M.D., Eds.; Chapman & Hall: New York, NY, USA, 1994; pp. 331–363. [Google Scholar]
- Rodrick, G.E.; Schneider, K.R. Vibrios in Depuration. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 115–125. [Google Scholar]
- Herrington, T. Use of Ultraviolet Light in Depuration. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 137–143. [Google Scholar]
- Blogoslawski, W.J. Enhancing Shellfish Depuration. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 145–150. [Google Scholar]
- Rusch, K.A.; Robin, J.E.; Malone, R.F. Design of a Bench Scale Automated Recirculating System for Use in the Development of Purging/Taste Enhancement Criteria for the Rangia Clam (Rangia cuneate). In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 195–204. [Google Scholar]
- Haq, S.M.; Dayal, H.H. Chronic liver disease and consumption of raw oysters: A potentially lethal combinaton—A review of Vibrio vulnificus septicemia. Am. J. Gastroenterol. 2005, 100, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.; Dalsgaard, A.; DePaola, A.; Karunasagar, I.; McMeekin, T.; Nishibuchi, M.; Osaka, K.; Summer, J.; Walderhaug, M. Risk assessment of Vibrio vulnificus in raw oysters—Interpretive Summary and Technical Report; World Health Organization/Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; pp. 3–109. [Google Scholar]
- Sobsey, M.D.; Jaykus, L.A. Human Enteric Viruses and Depuration of Bivalve Mollusks. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 71–114. [Google Scholar]
- Klontz, K.C.; Rippey, S.R. Epidemiology of Molluscan—Borne Illnesses. In Molluscan Shellfish Depuration; Otwell, W.S., Rodrick, G.E., Martin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 47–70. [Google Scholar]
- Brands, D.A.; Inman, A.E.; Gerba, C.P.; Mare, C.J.; Billington, S.J.; Saif, L.A.; Levine, J.F.; Joens, L.A. Prevalence of Salmonella spp. in oysters in the United States. Appl. Environ. Microbiol. 2005, 71, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Jamison, J.; Jepson, M. Vibrio Vulnificus Education and Social Marketing: Implementing Strategies Needed to Reduce Illnesses Related to the Consumption of Raw Oysters; Gulf & South Atlantic Fisheries Foundation, Inc.: Tampa, FL, USA, 2008; pp. 4–6, 17. [Google Scholar]
- Strom, M.S.; Paranjpye, R.N. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000, 2, 177–188. [Google Scholar] [CrossRef]
- Horseman, M.A.; Surani, S.A. Comprehensive review of Vibrio vulnificus: An important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 2011, 15, e157–e166. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.P. Enteric virus contamination of foods through industrial practices: A primer on intervention strategies. J. Ind. Microbiol. Biotechnol. 2001, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Aldridge, K.E.; Sanders, C.V. Infections related to the ingestion of seafood Part I: Viral and bacterial infections. Lancet Infect. Dis. 2004, 4, 201–212. [Google Scholar] [CrossRef]
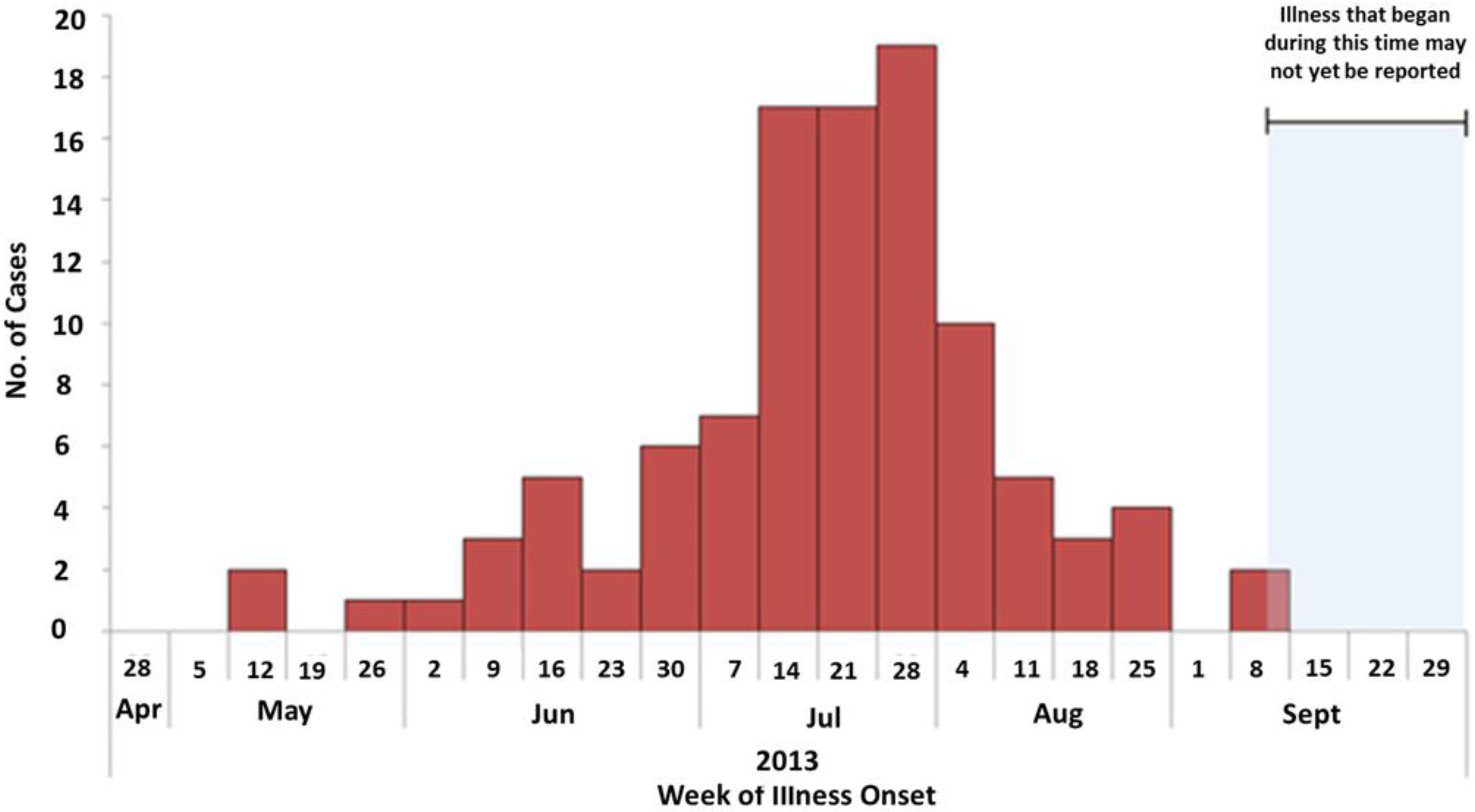
| # of patients (% of total) | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|
| V. parahaemolyticus | 386 (46.8) | 421 (45.4) | 334 (39.2) | 431 (45.7) | 594 (50.5) |
| V. vulnificus | 107 (13.0) | 133 (14.3) | 113 (13.2) | 119 (12.6) | 137 (11.6) |
| Vibrionacea Total | 825 (59.8) | 927 (59.8) | 853 (52.4) | 944 (58.3) | 1176 (62.2) |
| # of patients (% of total) | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|
| Vibriosis Patients | 825 | 927 | 853 | 944 | 1176 |
| Reported Eating a Seafood Item | 236 (28.6) | 182 (19.6) | 184 (21.6) | 211 (22.4) | 290 (24.7) |
| Consumed Raw Oysters | 106 (12.8) | 72 (7.8) | 106 (12.4) | 104 (11.0) | 149 (12.7) |
| Consumed Raw Clams | 16 (1.9) | 11 (1.2) | 9 (1.1) | 21 (2.2) | 21 (1.8) |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, G.L. Food Safety Impacts from Post-Harvest Processing Procedures of Molluscan Shellfish. Foods 2016, 5, 29. https://doi.org/10.3390/foods5020029
Baker GL. Food Safety Impacts from Post-Harvest Processing Procedures of Molluscan Shellfish. Foods. 2016; 5(2):29. https://doi.org/10.3390/foods5020029
Chicago/Turabian StyleBaker, George L. 2016. "Food Safety Impacts from Post-Harvest Processing Procedures of Molluscan Shellfish" Foods 5, no. 2: 29. https://doi.org/10.3390/foods5020029





