Evaluation of Antioxidant Properties in Cereals: Study of Some Traditional Italian Wheats
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling
2.2. Chemicals
2.3. General Chemical Analysis
2.4. Total Polyphenol Content (TPC) and Antioxidant Properties
2.5. Statistical Analysis
3. Results and Discussion
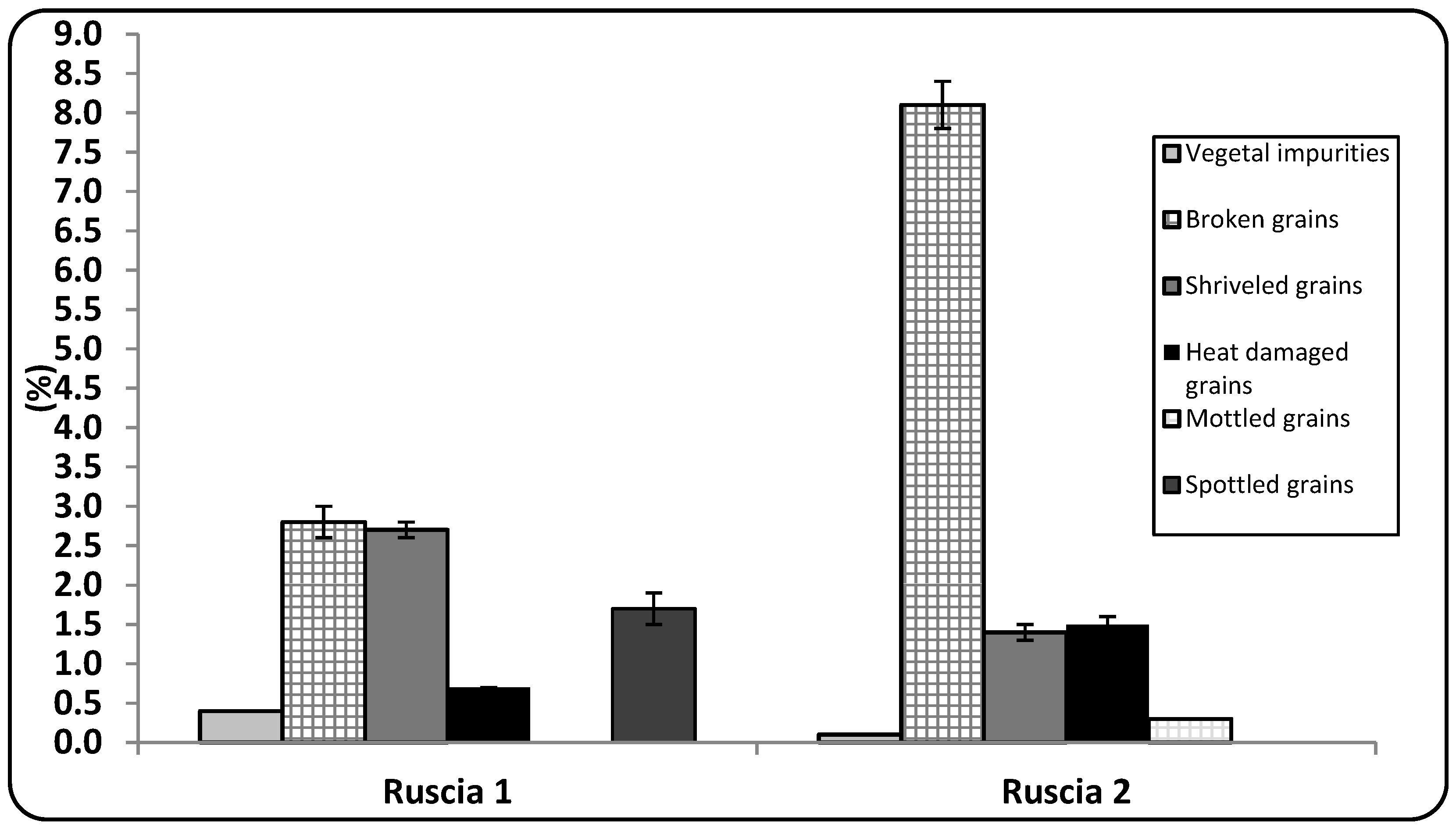
3.1. General Chemical Analysis
| Sample | TW (kg/hL) | 1000 KW (g) | Moisture (g/100 g) | Protein (g/100 g dm) | Ash (g/100 g dm) |
|---|---|---|---|---|---|
| RUSCIA 1 | 74.6 ± 0.1a | 41.4 ± 0.1a | 12.4 | 14.1 ± 0.0a | 1.77 ± 0.01a |
| RUSCIA 2 | 78.2 ± 0.1b | 49.8 ± 0.1b | 11.6 | 14.8 ± 0.0b | 1.99 ± 0.00b |
3.2. Antioxidant Properties and Total Polyphenol Content
| Sample | TPC (mg/100 g dm) | FRAP (µmol/g dm) | |||||
|---|---|---|---|---|---|---|---|
| Grain | Flour | p valueΩ | Grain | Flour | p valueΩ | ||
| Aqueous-Organic Extract | |||||||
| RUSCIA 1 | 175.85 ± 32.58b | 145.60 ± 8.86b | n.s. | 5.28 ± 0.15b | 4.44 ± 0.26b | 0.0001 | |
| RUSCIA 2 | 149.46 ± 23.73a | 102.07 ± 5.55a | 0.0001 | 4.46 ± 0.41a | 2.18 ± 0.10a | 0.0001 | |
| Residue | |||||||
| RUSCIA 1 | 1036.19 ± 57.56b | 1161.39 ± 17.37 | 0.05 | 96.79 ± 8.29b | 170.71 ± 1.54b | 0.0001 | |
| RUSCIA 2 | 952.54 ± 16.14a | 1142.19 ± 46.07 | 0.0001 | 85.45 ± 4.36a | 133.09 ± 11.53a | 0.0001 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schatzkin, A.; Mouw, T.; Park, Y.; Subar, A.F.; Kipnis, V.; Hollenbeck, A.; Leitzmann, M.F.; Thompson, F.E. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2007, 85, 1353–1360. [Google Scholar] [PubMed]
- Mellen, P.; Walsh, T.; Herrington, D. Whole grain intake and cardiovascular disease. A meta-analysis. Nutr. Met. Card. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Grafenauer, S.J.; O’Shea, J.E. Cereal grains, legumes and weight management: A comprehensive review of the scientific evidence. Nutr. Rev. 2008, 66, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.L.; Tuohy, K.M.; Lovegrove, J.A. Wholegrain oat-based cereals have prebiotic potential and low glycaemic index. Br. J. Nutr. 2012, 108, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Okarter, N.; Liu, C.S.; Sorrels, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Kubilay Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidants activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowka, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bhanger, M.I.; Anwar, F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005, 93, 265–272. [Google Scholar] [CrossRef]
- Delgrado-Andrade, C.; Conde-Aguilera, J.A.; Haro, A.; de la Cueva, S.P.; Rufian-Henares, J.A. A combined procedure to evaluate the global antioxidant response of bread. J. Cereal Sci. 2010, 52, 239–246. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.; Liu, R. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Diaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Perez-Jimenéz, J.; Goni, I. Contribution of cereals to dietary fibre and antioxidant intakes: Toward more reliable methodology. J. Cereal Sci. 2009, 50, 291–294. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Casale, G.; Melini, V.; Maiani, G.; Acquistucci, R. Total polyphenol content and antioxidant properties of Solina (Triticum aestivum L.) and their derived products. Ital. J. Food Sci. in press.
- American Association of Cereal Chemists. Approved Methods of the AACC, 10th ed.; The Association: St. Paul, MN, USA, 2003; Methods 55–31. [Google Scholar]
- International Association for Cereal Science and Technology. Standard Methods of ICC; The Association: Vienna, Austria, 2003; Methods No. 104/1, 105/2, 110/1. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The assay. Anal. Biochem. 1993, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin- Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Troccoli, A.; Di Fonzo, N. Relationship between kernel size features and test weight in Triticum durum. Cereal Chem. 1999, 76, 45–49. [Google Scholar] [CrossRef]
- Acquistucci, R.; Carcea, M.; Cardarilli, D.; Salvatorelli, S. Esperienza di Ricerca sulla qualità del frumento coltivato con tecniche di agricoltura biologica. Tecnica Molit. 2001, 798–805. [Google Scholar]
- Troccoli, A.; Borrelli, G.M.; De Vita, P.; Fares, C.; Di Fonzo, N. Mini review: Durum wheat quality: A multidisciplinary concept. J. Cereal Sci. 2000, 32, 99–113. [Google Scholar] [CrossRef]
- Durazzo, A.; Raguzzini, A.; Azzini, E.; Foddai, M.S.; Narducci, V.; Maiani, G.; Carcea, M. Bioactive molecules in cereals. Tecnica Molit 2009, 60, 130–141. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Foods Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fibre as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.G. Effects of Processing on Antioxidant Phenolics of Cereal and Legume Grains. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011; Chapter 3; pp. 31–54. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durazzo, A.; Casale, G.; Melini, V.; Maiani, G.; Acquistucci, R. Evaluation of Antioxidant Properties in Cereals: Study of Some Traditional Italian Wheats. Foods 2015, 4, 391-399. https://doi.org/10.3390/foods4030391
Durazzo A, Casale G, Melini V, Maiani G, Acquistucci R. Evaluation of Antioxidant Properties in Cereals: Study of Some Traditional Italian Wheats. Foods. 2015; 4(3):391-399. https://doi.org/10.3390/foods4030391
Chicago/Turabian StyleDurazzo, Alessandra, Gaetana Casale, Valentina Melini, Giuseppe Maiani, and Rita Acquistucci. 2015. "Evaluation of Antioxidant Properties in Cereals: Study of Some Traditional Italian Wheats" Foods 4, no. 3: 391-399. https://doi.org/10.3390/foods4030391






