Ag/SiO2- and Ag/Co3O4-Based Monolithic Flow Microreactors for Hydrogenation of Dyes: Their Activity and Stability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the SiO2 Monolith Template
2.3. Synthesis of the Co3O4 Replica by Nanocasting
2.4. Synthesis of Ag/SiO2 and Ag/Co3O4 Composite Monoliths by Solution Infiltration
2.5. Characterization
2.6. Catalytic Reduction of EO Using Supported Ag Nanoparticles as Catalyst
2.7. Washing Studies
3. Results and Discussion
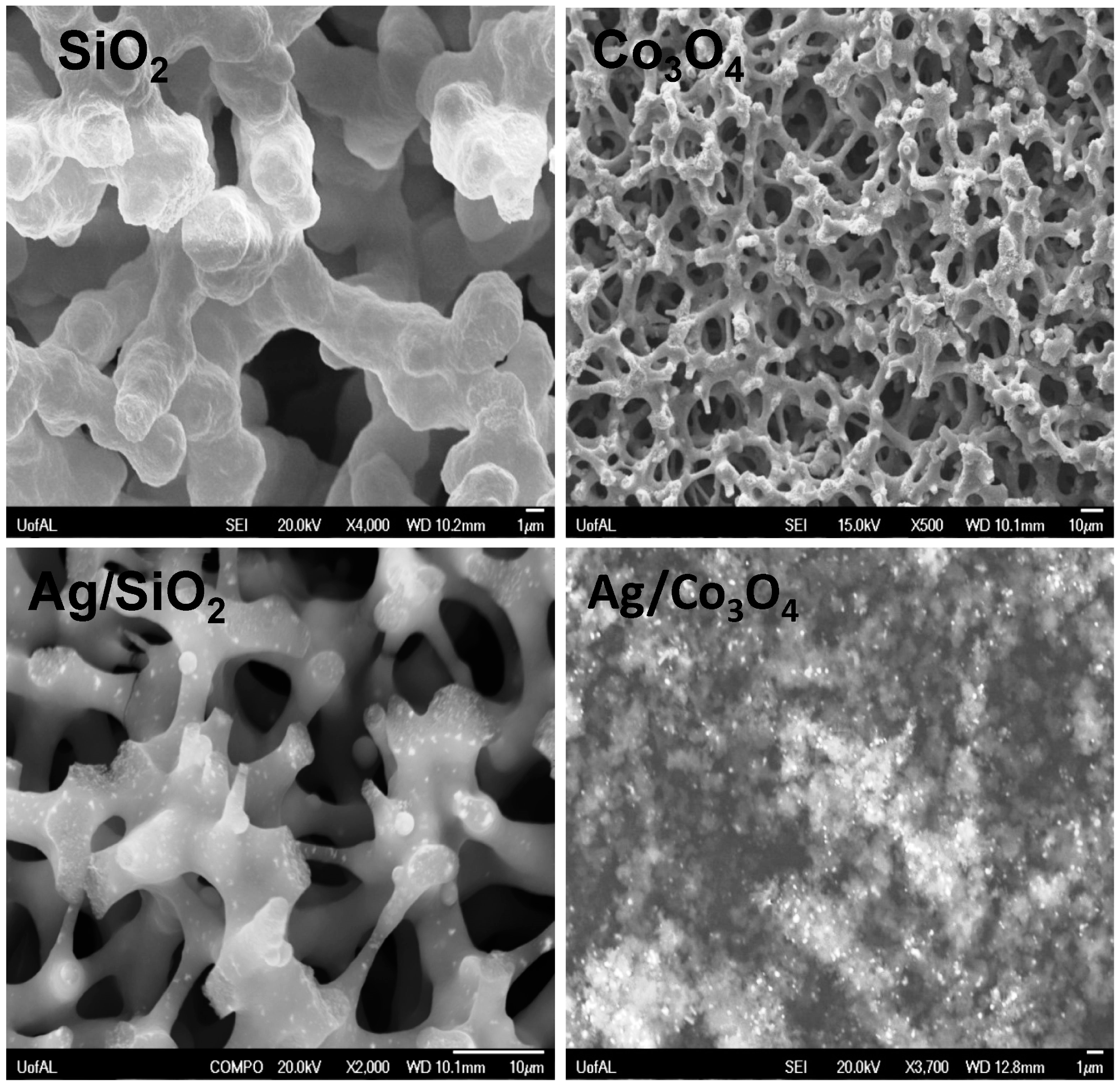
3.1. Catalyst Characterization
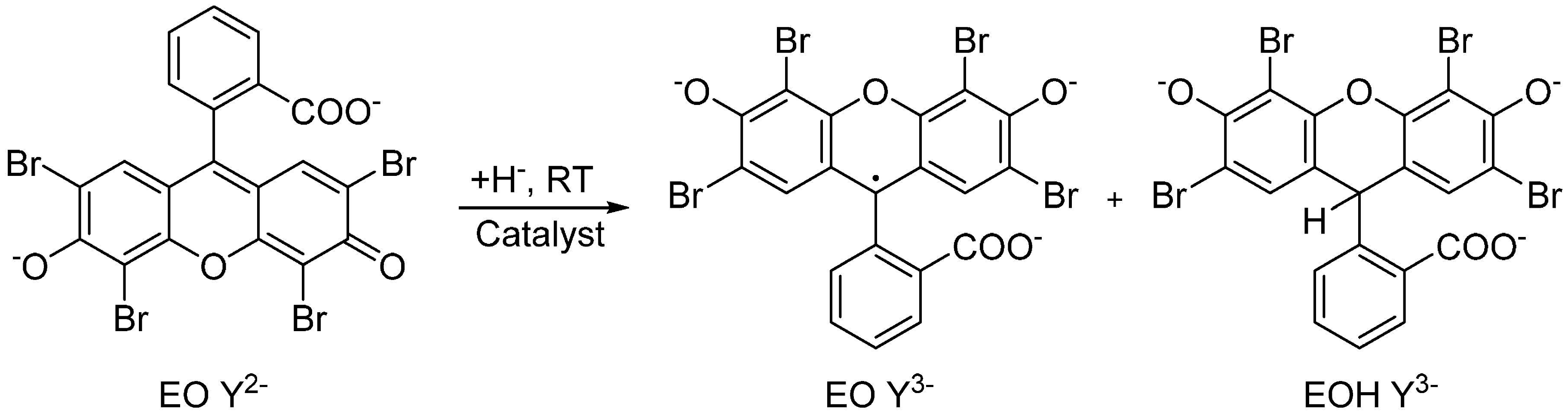
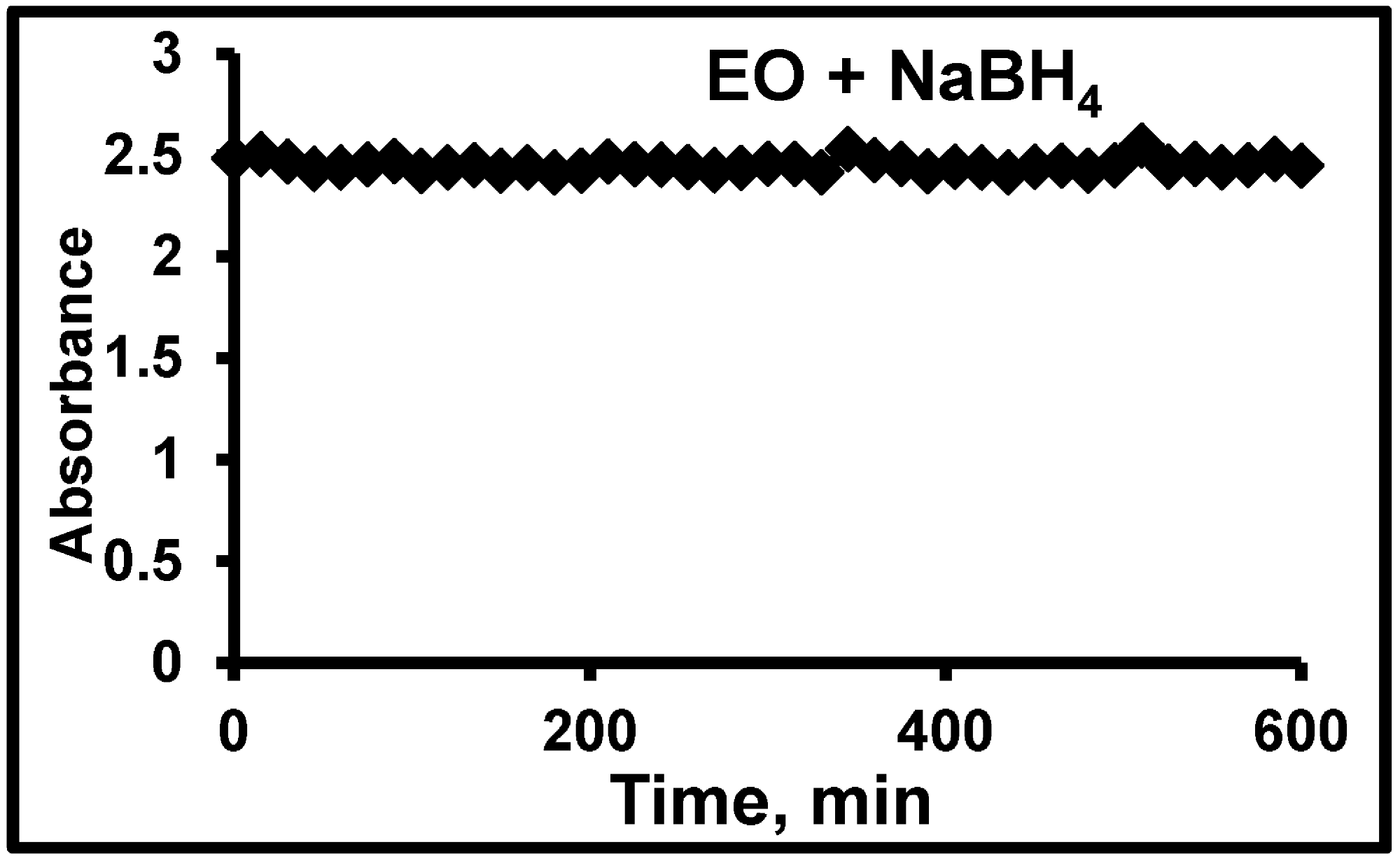
3.2. Hydrogenation of EO in Continuous Monolithic Microreactors


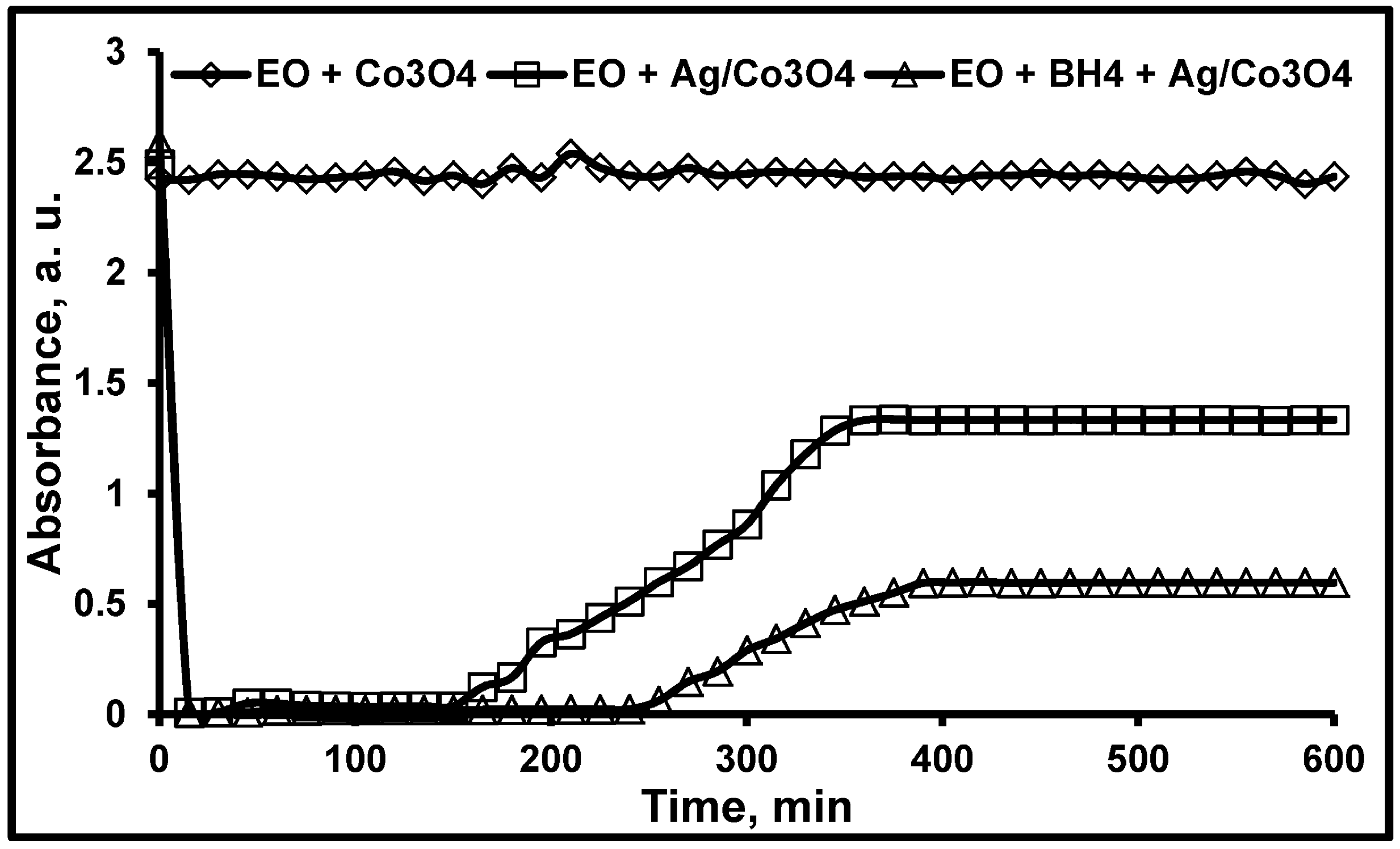

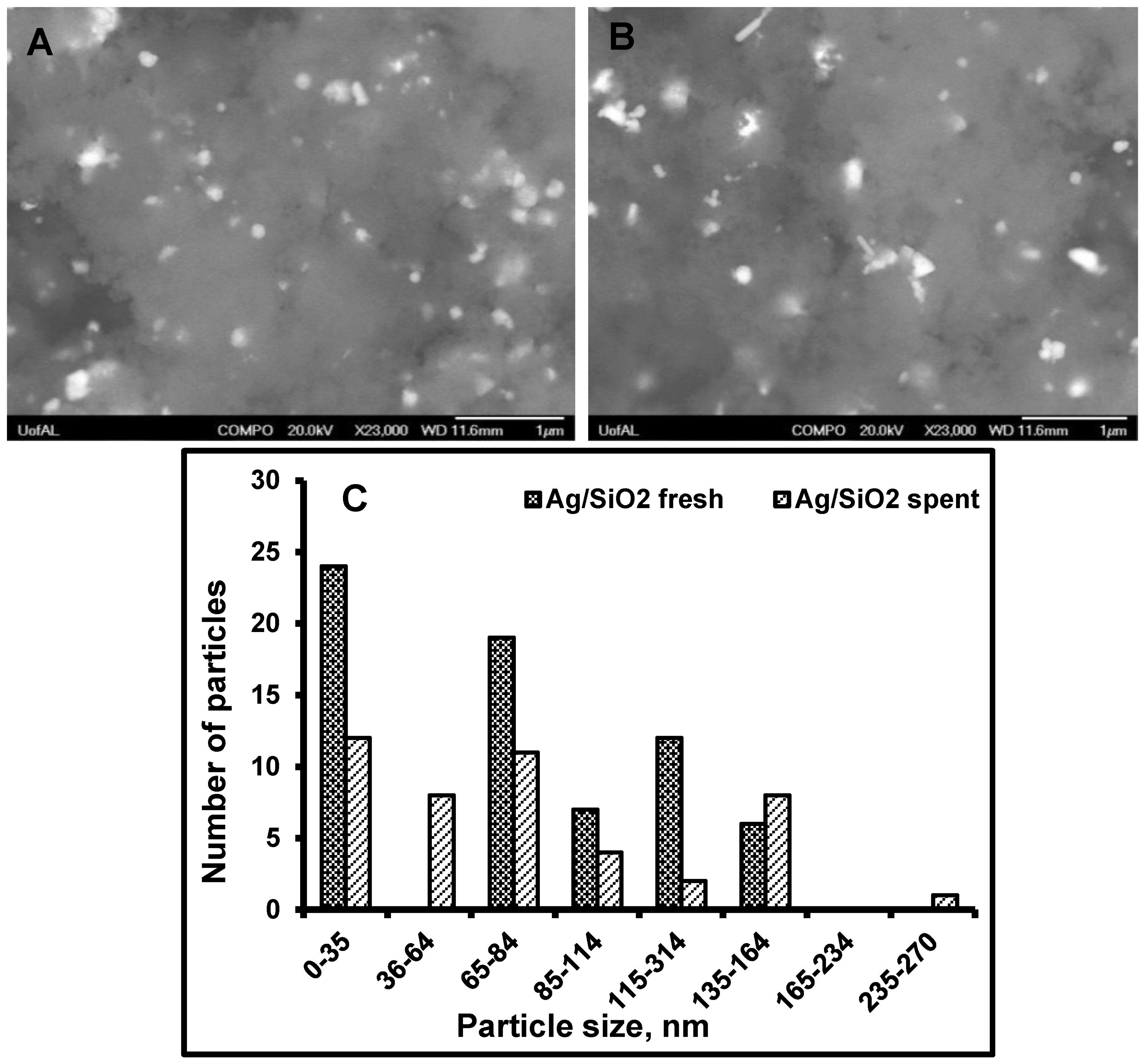
3.3. Particle Size Distribution and Washing/Leaching Studies

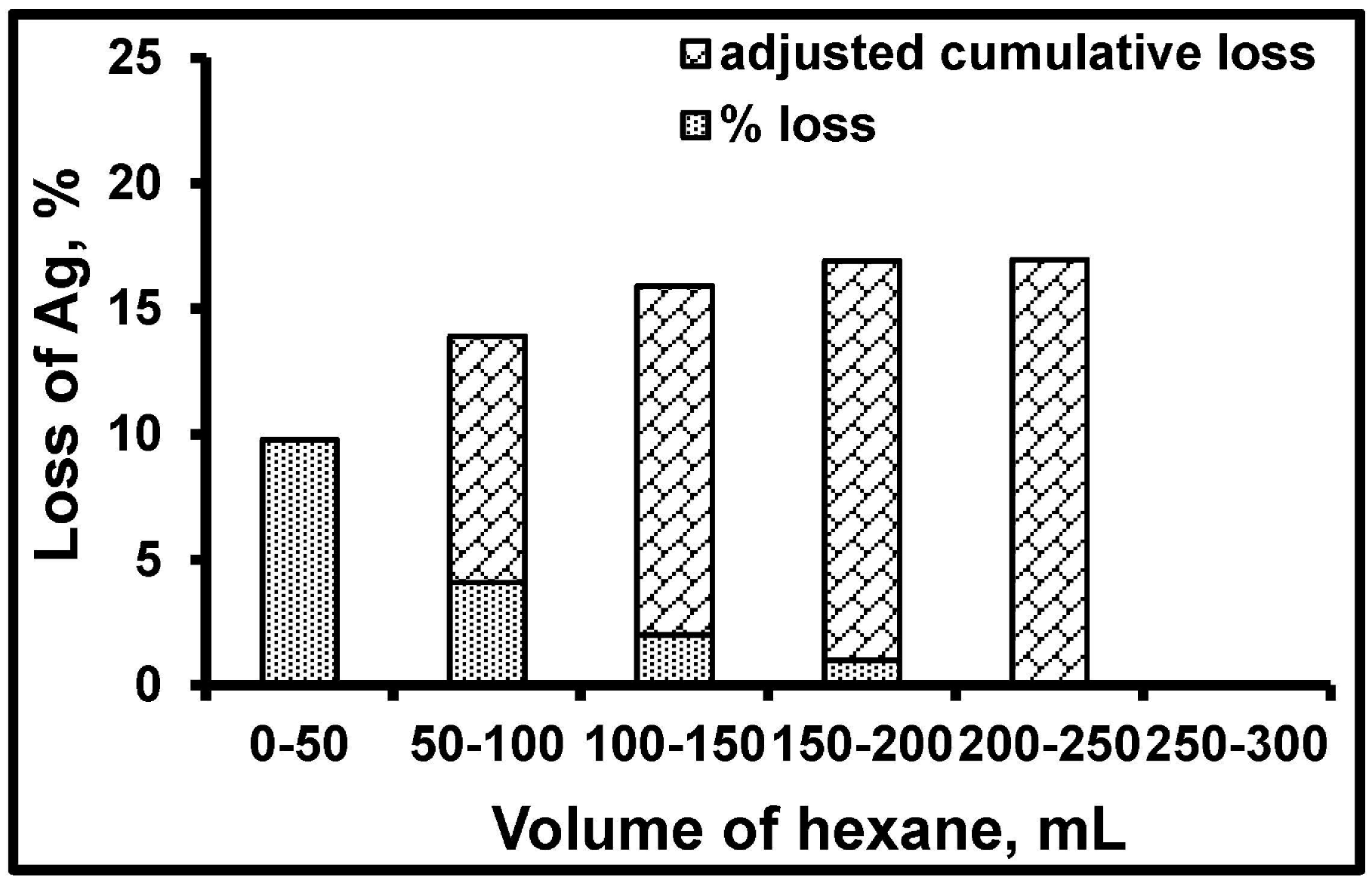
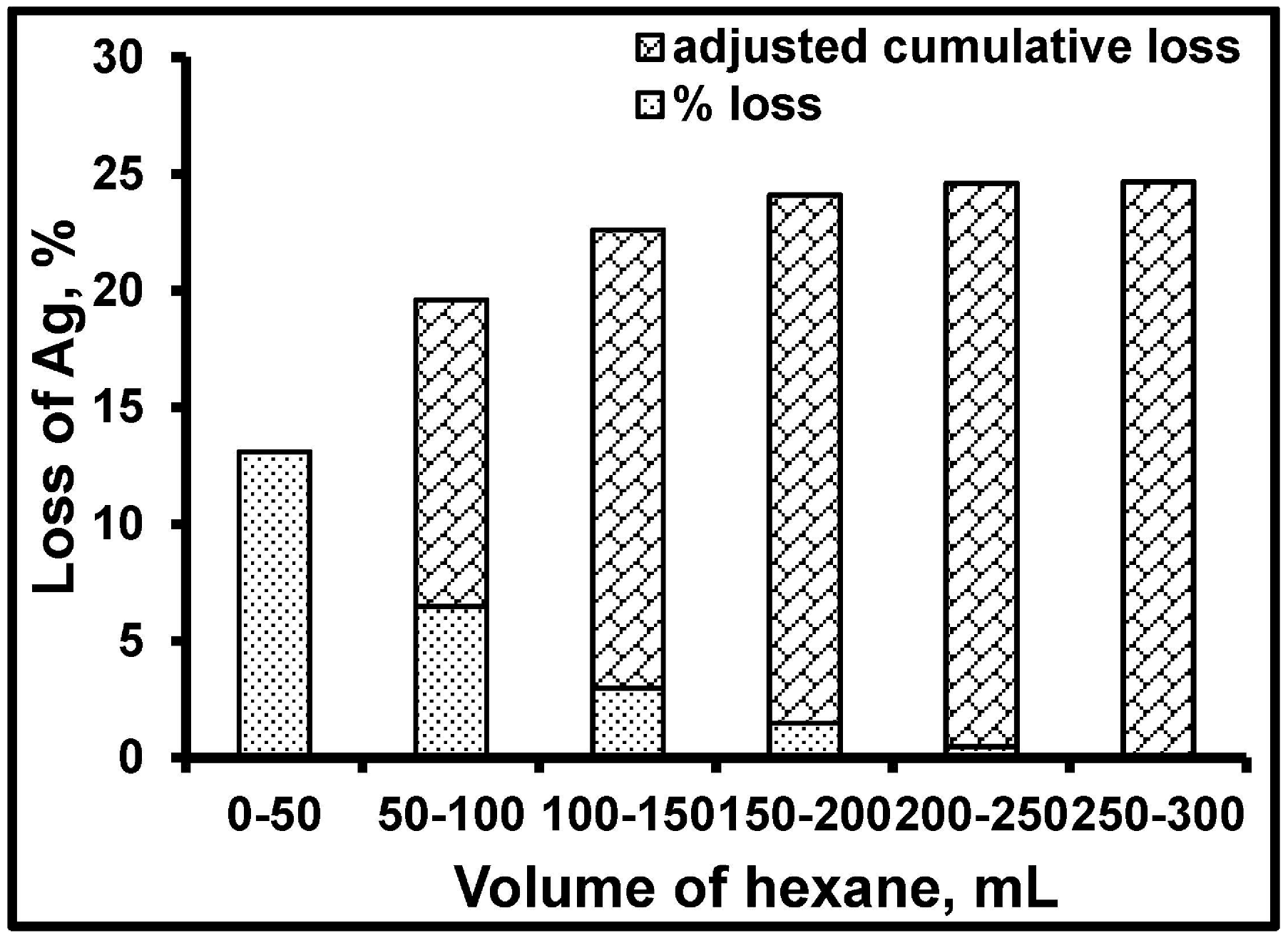
| Monolithic Catalyst | Number of Ag Nanoparticles Lost from Support, % | Ag Leached from Support in Aqueous Reaction Medium, % | |
|---|---|---|---|
| Polar Solvent (Water) | Non-Polar Solvent (Hexane) | ||
| Ag/SiO2 | 27 | 17 | 10 |
| Ag/Co3O4 | 44 | 25 | 19 |
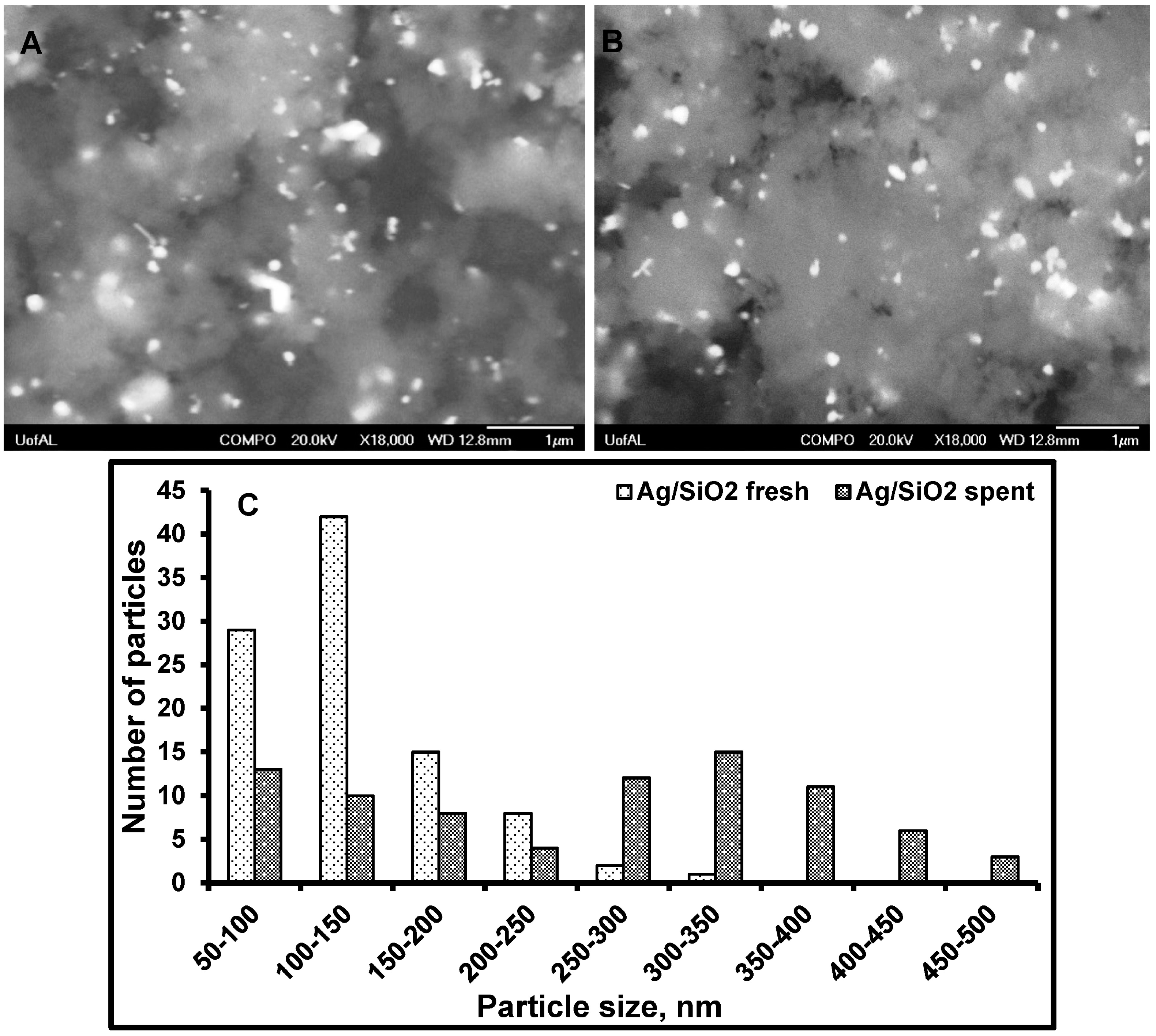
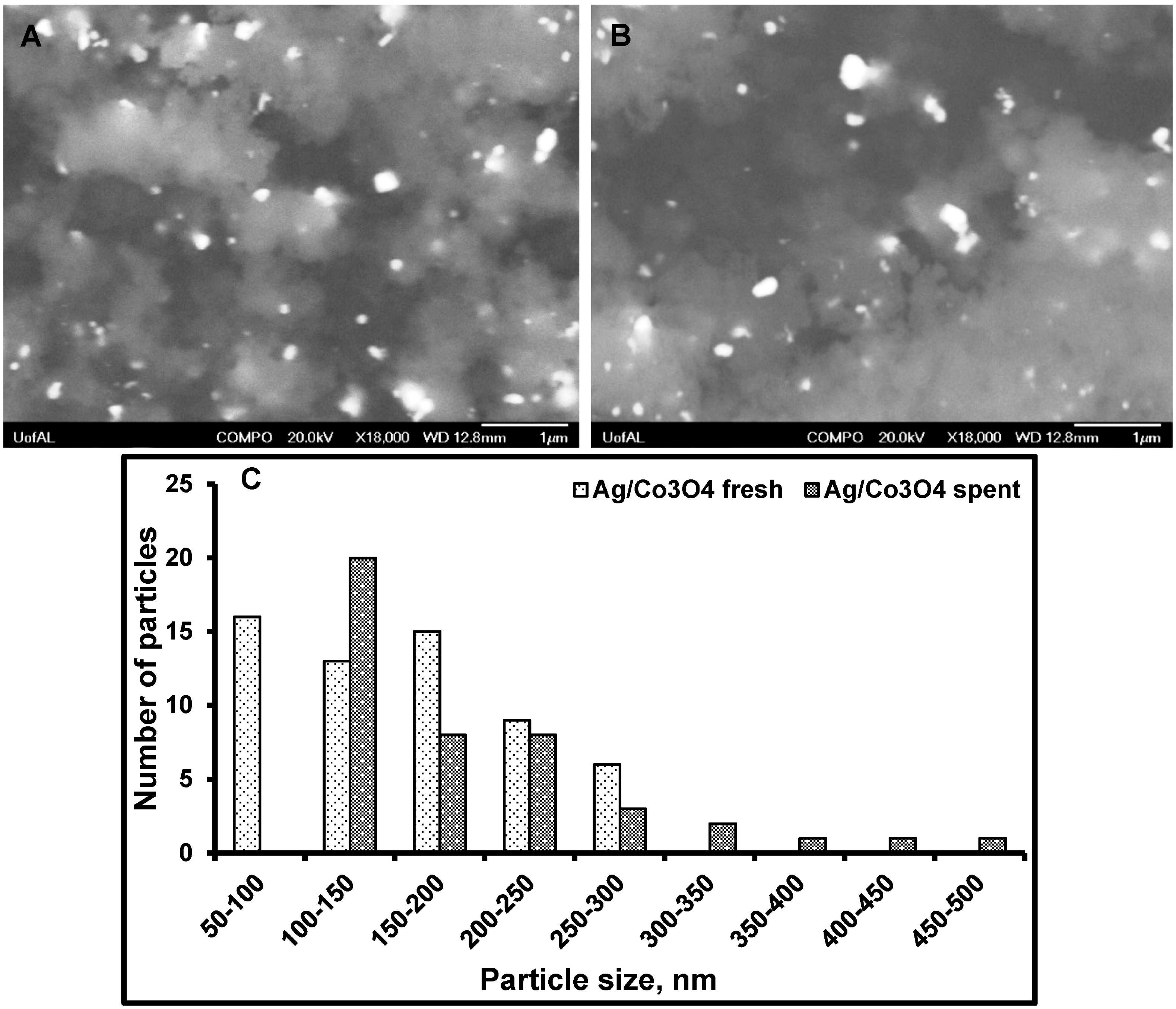
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wirth, T. Microreactors in Organic Chemistry and Catalysis, 2nd ed.; Wiley: New York, NY, USA, 2013; p. 478. [Google Scholar]
- Liu, X.; Unal, B.; Jensen, K.F. Heterogeneous catalysis with continuous flow microreactors. Catal. Sci. Technol. 2012, 2, 2134–2138. [Google Scholar] [CrossRef]
- Escriba, M.; Ibhadon, A.O. Recent developments in catalytic micro process engineering for fine chemicals synthesis. Recent Patents Catal. 2013, 2, 101–115. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical porous materials: Catalytic applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Rooke, J.C.; Chen, L.; Su, B.L. Synthesis and applications of hierarchically porous catalysts. Chin. J. Catal. 2013, 34, 22–47. [Google Scholar] [CrossRef]
- Chen, L.-H.; Li, X.-Y.; Rooke, J.C.; Zhang, Y.-H.; Yang, X.-Y.; Tang, Y.; Xiao, F.-S.; Su, B.-L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Nakanishi, K. Pore structure control of silica gels based on phase separation. J. Porous Mat. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Smått, J.-H.; Sayler, F.M.; Grano, A.; Bakker, M.G. Formation of hierarchically porous metal oxide and metal monoliths by nanocasting into silica monoliths. Adv. Eng. Mat. 2012, 14, 1059–1073. [Google Scholar] [CrossRef]
- Grano, A.J.; Sayler, F.M.; Smått, J.-H.; Bakker, M.G. Hierarchically porous monoliths of carbon and metal oxides with ordered mesopores. J. Porous Mat. 2014, 21, 1113–1122. [Google Scholar] [CrossRef]
- Sayler, F.M.; Grano, A.J.; Smått, J.-H.; Lindén, M.; Bakker, M.G. Nanocasting of hierarchically porous NiO, Co3O4, Ni, Co and Ag monoliths: Impact of processing conditions on fidelity of replication. Micropor. Mesopor. Mater. 2014, 184, 141–150. [Google Scholar] [CrossRef]
- Grano, A.J.; Sayler, F.M.; Smått, J.-H.; Bakker, M.G. Alternative etching methods to expand nanocasting, and use in the synthesis of hierarchically porous nickel oxide, zinc oxide and copper monoliths. J. Mater. Res. 2013, 28, 2483–2489. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic tio2 with controlled multiscale porosity via a template-free sol-gel process accompanied by phase separation. Chem. Mater. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801–10812. [Google Scholar] [CrossRef]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. Hierarchical nanostructured carbons with meso-macroporosity: Design, characterization, and applications. Acc. Chem. Res. 2013, 46, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, M.E.; Niemelä, M.K.; Krause, A.O.I.; Vaara, T.; Vuori, A.I. Rh/C catalysts for methanol hydrocarbonylation i. Catalyst characterisation. Appl. Catal. A 2001, 205, 37–49. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.N.C.B.; Calvete, M.R.J.F.; Pinho e Melo, T.M.V.D.; Pereira, M.M. Immobilized catalysts for hydroformylation reactions: A versatile tool for aldehyde synthesis. Eur. J. Org. Chem. 2012, 2012, 6309–6320. [Google Scholar] [CrossRef]
- Thomas, J.M.; Johnson, B.F.G.; Raja, R.; Sankar, G.; Midgley, P.A. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 2003, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Riente, P.; Yus, M. Nickel nanoparticles in hydrogen transfer reactions. Acc. Chem. Res. 2010, 44, 379–391. [Google Scholar] [CrossRef]
- De Jong, K.P. Synthesis of Solid Catalysts; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Takahashi, R.; Sato, S.; Tomiyama, S.; Ohashi, T.; Nakamura, N. Pore structure control in Ni/SiO2 catalysts with both macropores and mesopores. Micropor. Mesopor. Mater. 2006, 98, 107–114. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Zhou, S.; Zhao, D.; Wu, L. Facile synthesis of hierarchically ordered porous carbon via in situ self-assembly of colloidal polymer and silica spheres and its use as a catalyst support. Chem. Mater. 2010, 22, 3433–3440. [Google Scholar] [CrossRef]
- El Kadib, A.; Chimenton, R.; Sachse, A.; Fajula, F.; Galarneau, A.; Coq, B. Functionalized inorganic monolithic microreactors for high productivity in fine chemicals catalytic synthesis. Angew. Chem. Int. Ed. 2009, 121, 5069–5072. [Google Scholar] [CrossRef]
- Numata, M.; Takahashi, R.; Yamada, I.; Nakanishi, K.; Sato, S. Sol–gel preparation of Ni/TiO2 catalysts with bimodal pore structures. Appl. Catal. A 2010, 383, 66–72. [Google Scholar] [CrossRef]
- Linares, N.; Hartmann, S.; Galarneau, A.; Barbaro, P. Continuous partial hydrogenation reactions by Pd@unconventional bimodal porous titania monolith catalysts. ACS Catal. 2012, 2, 2194–2198. [Google Scholar] [CrossRef]
- Hakat, Y.; Kotbagi, T.V.; Bakker, M.G. Catalytic activity of Ag/SiO2 and Ag/Co3O4 hierarchically porous monoliths for hydrogenation of dyes. Curr. Catal. 2014, 3, 286–295. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.W.; Thiel, P.A. A little chemistry helps the big get bigger. Science 2010, 330, 599–600. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakat, Y.; Kotbagi, T.V.; Bakker, M.G. Ag/SiO2- and Ag/Co3O4-Based Monolithic Flow Microreactors for Hydrogenation of Dyes: Their Activity and Stability. Processes 2015, 3, 98-112. https://doi.org/10.3390/pr3010098
Hakat Y, Kotbagi TV, Bakker MG. Ag/SiO2- and Ag/Co3O4-Based Monolithic Flow Microreactors for Hydrogenation of Dyes: Their Activity and Stability. Processes. 2015; 3(1):98-112. https://doi.org/10.3390/pr3010098
Chicago/Turabian StyleHakat, Yasemin, Trupti V. Kotbagi, and Martin G. Bakker. 2015. "Ag/SiO2- and Ag/Co3O4-Based Monolithic Flow Microreactors for Hydrogenation of Dyes: Their Activity and Stability" Processes 3, no. 1: 98-112. https://doi.org/10.3390/pr3010098