Particle Based Modeling of Electrical Field Flow Fractionation Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods
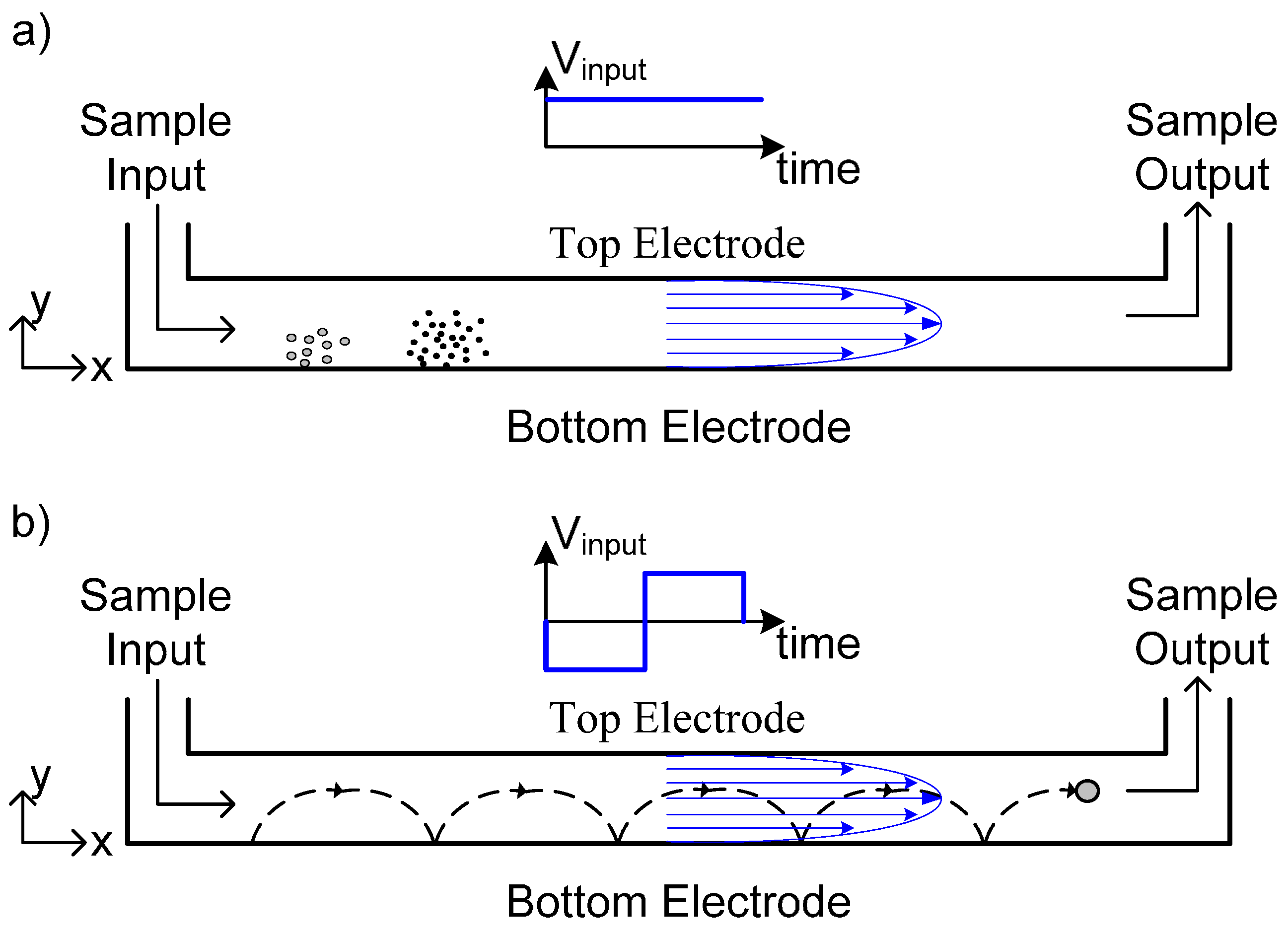

2.1.1. Simulation and Experiment 1—Modeling of Normal ElFFF Operation
2.1.2. Simulation and Experiment 2—Modeling of Cyclical ELFFF Operation
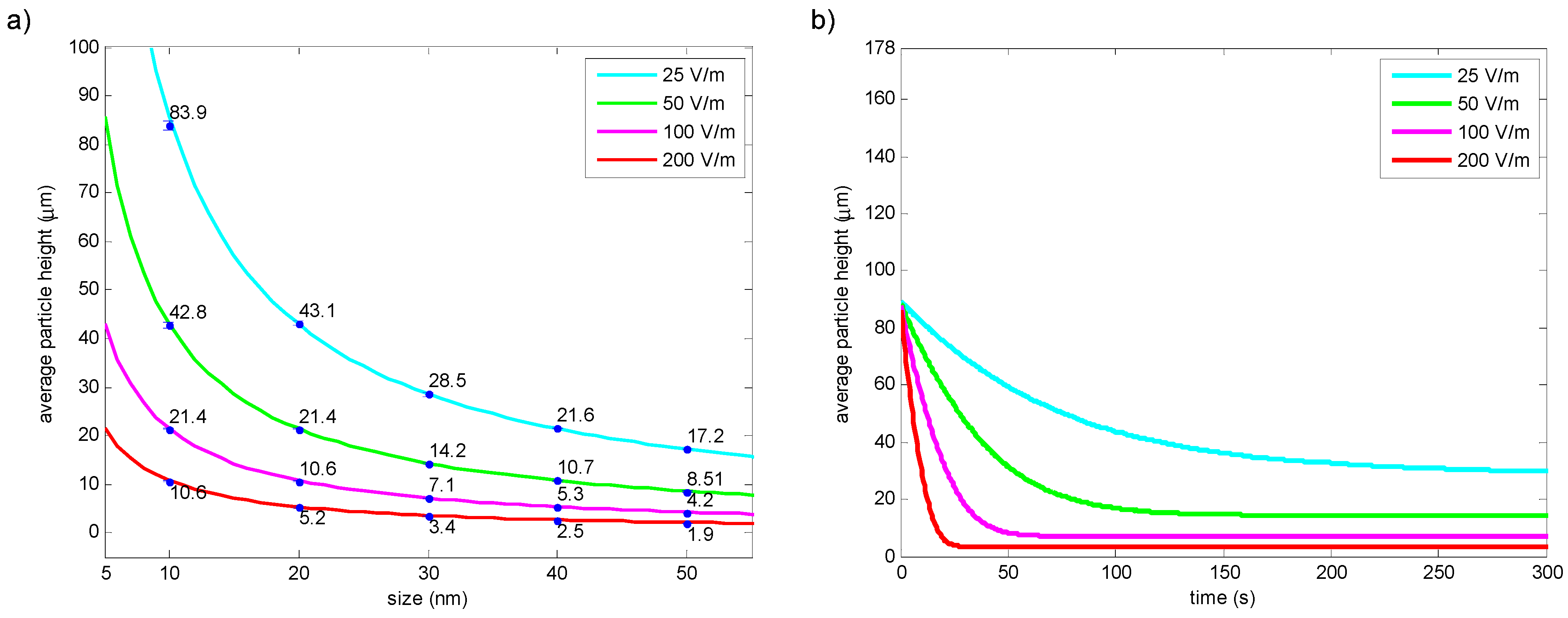
2.1.3. Simulation 3—Investigation of the Particle Retention Time for Equal Duty and High Duty Cycle Input Voltages
2.1.4. Simulation 4—Investigation of the Separation Efficiency for Even Duty and High Duty Cycle Input Voltages
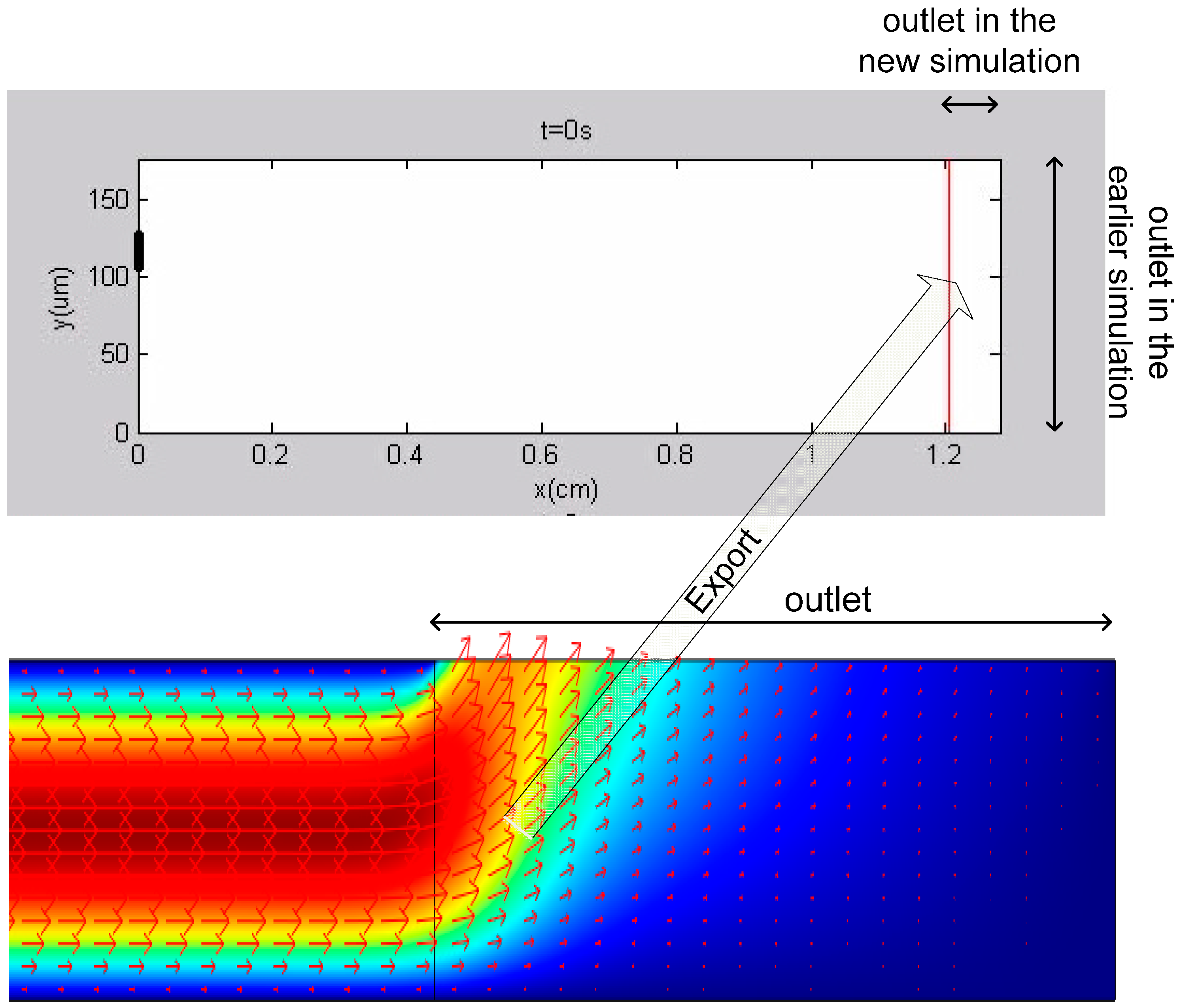
2.1.5. Simulation 5—Detailed Modeling of the Channel Outlet
3. Results and Discussion
3.1. Results of the Simulation and Experiment 1—Modeling of Normal ElFFF Operation

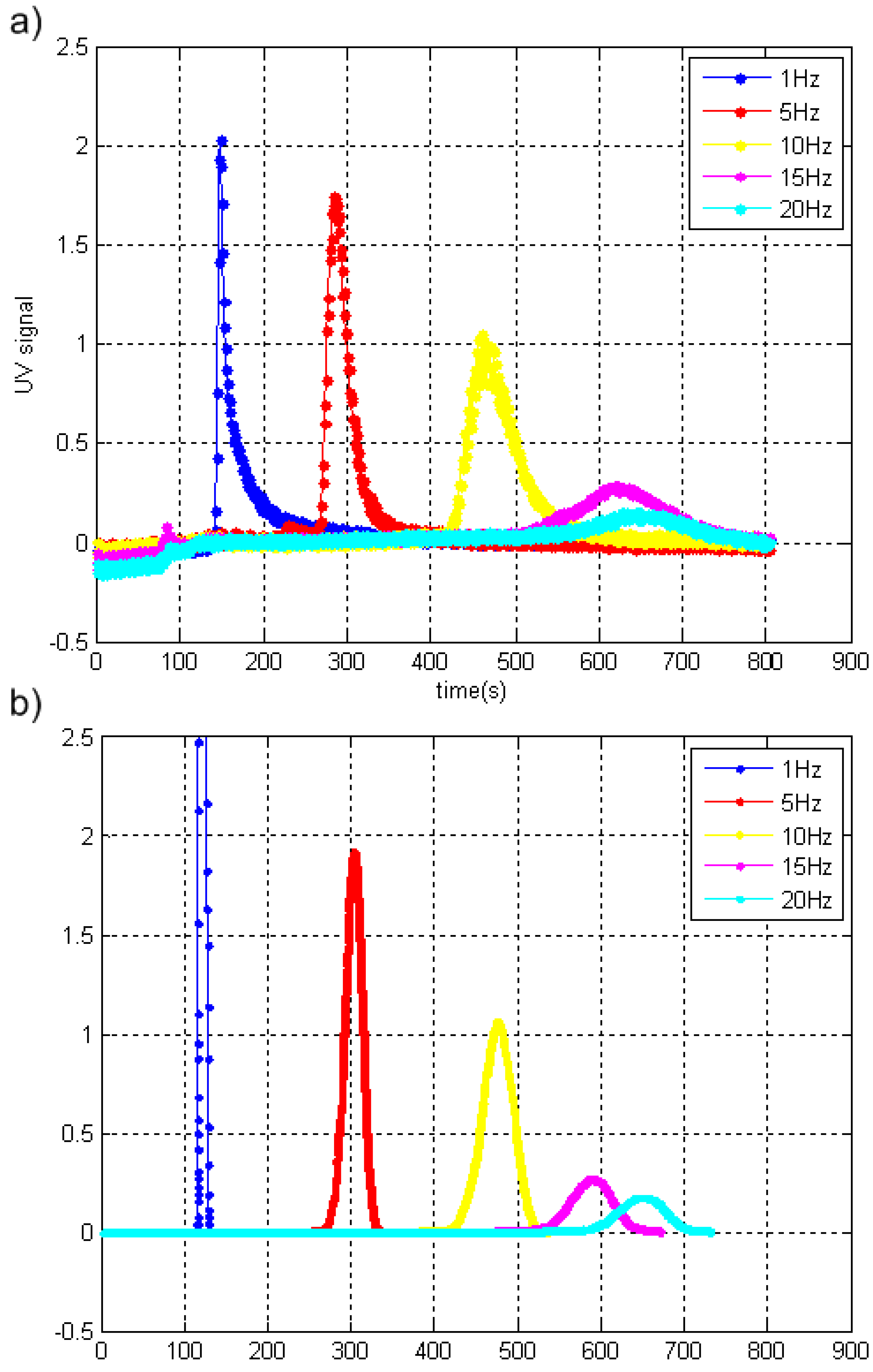
3.2. Results of the Simulation and Experiment 2—Modeling of Cyclical ELFFF Operation
3.3. Results of Simulation 3—Investigation of the Particle Retention Time for Equal Duty and High Duty Cycle Input Voltages
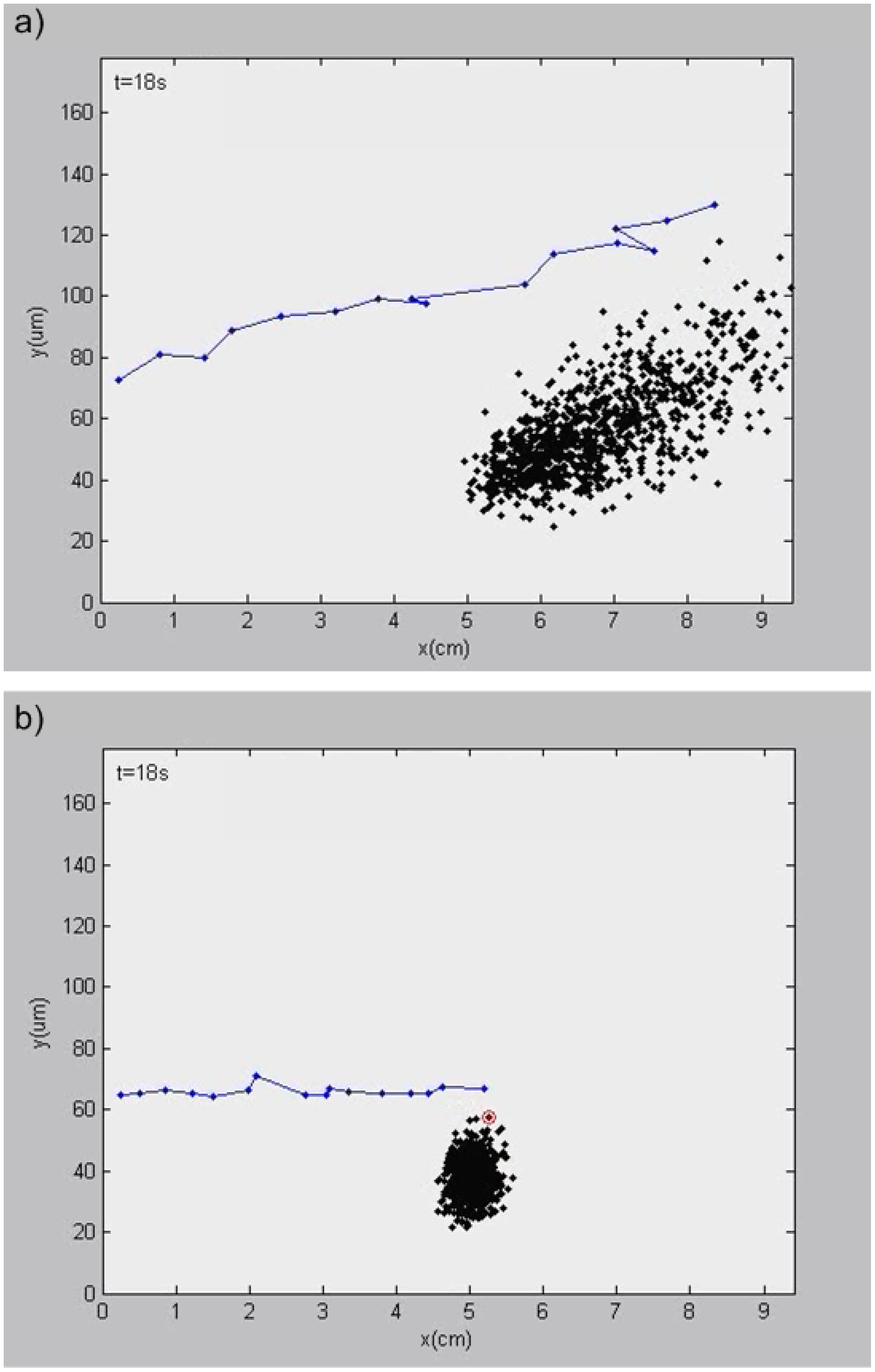
3.4. Results of Simulation 4—Investigation of the Separation Efficiency for Even Duty and High Duty Cycle Input Voltages


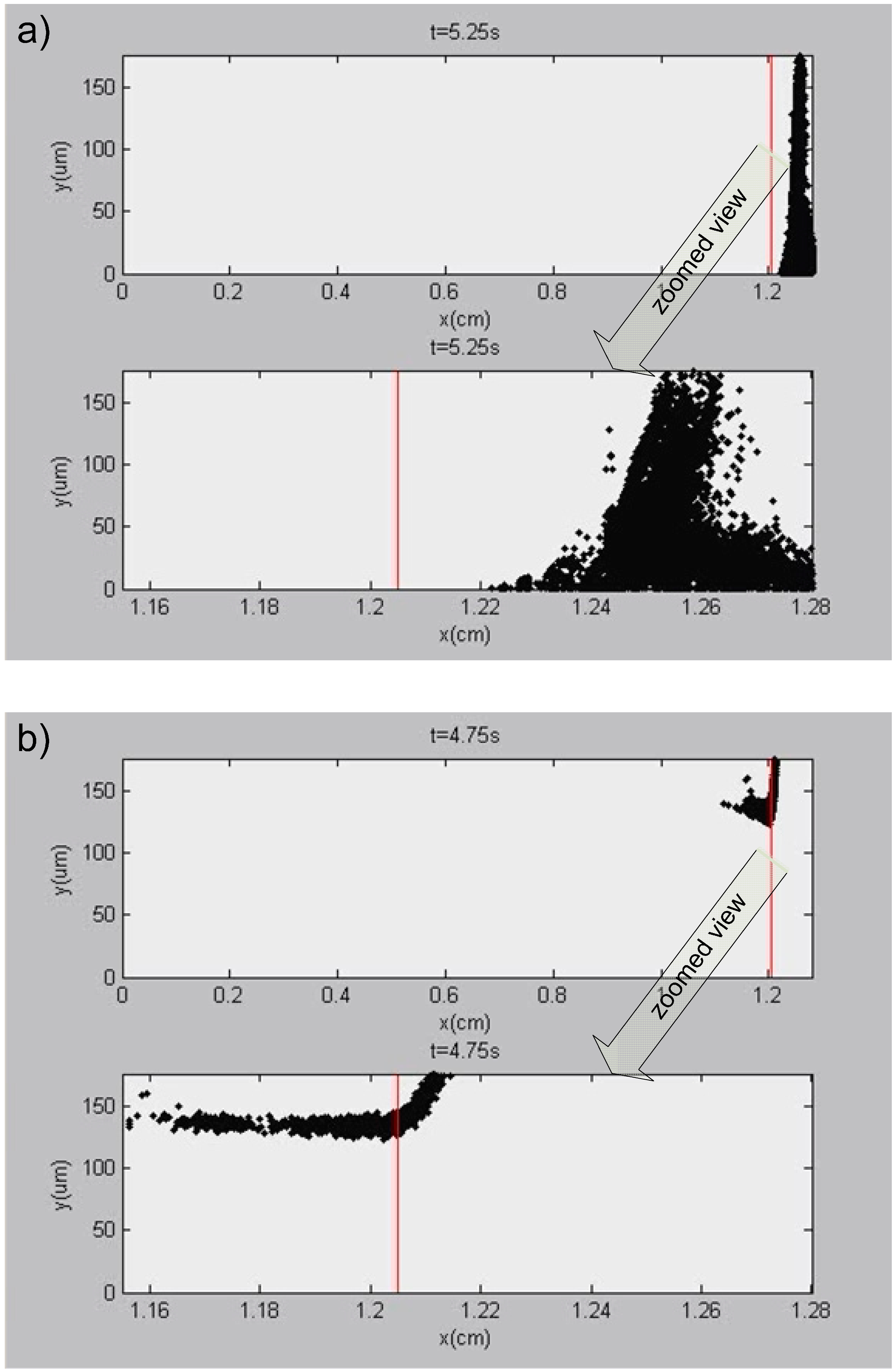
3.5. Results of Simulation 5—Detailed Modeling of the Channel Outlet
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giddings, J.C. A New Separation Concept Based on a Coupling of Concentration and Flow Nonuniformities. Sep. Sci. 1966, 1, 123–125. [Google Scholar] [CrossRef]
- Caldwell, K.D.; Kesner, L.F.; Myers, M.N.; Giddings, J.C. Electrical Field-Flow Fractionation of Proteins. Science 1972, 176, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.D.; Gao, Y.S. Electrical field-flow fractionation in particle separation. 1. Monodisperse standards. Anal. Chem. 1993, 65, 1764–1772. [Google Scholar] [PubMed]
- Gale, B.K.; Srinivas, M. Cyclical electrical field flow fractionation. Electrophoresis 2005, 26, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Tasci, T.O.; Johnson, W.P.; Fernandez, D.P.; Manangon, E.; Gale, B.K. Circuit modification in electrical field flow fractionation systems generating higher resolution separation of nanoparticles. J. Chromatogr. A 2014, 1365, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tri, N.; Caldwell, K.; Beckett, R. Development of electrical field-flow fractionation. Anal. Chem. 2000, 72, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Lao, A.I.K.; Trau, D.; Hsing, I.M. Miniaturized Flow Fractionation Device Assisted by a Pulsed Electric Field for Nanoparticle Separation. Anal. Chem. 2002, 74, 5364–5369. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Sant, H.J.; Gale, B.K. Optimization of cyclical electrical field flow fractionation. Electrophoresis 2010, 31, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Palkar, S.A.; Schure, M.R. Mechanistic Study of Electrical Field Flow Fractionation. 1. Nature of the Internal Field. Anal. Chem. 1997, 69, 3223–3229. [Google Scholar]
- Biernacki, J.J.; Mellacheruvu, P.M.; Mahajan, S.M. Electric Circuit Model for Electrical Field Flow Fractionation. Anal. Chem. 2006, 78, 4998–5005. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chauhan, A. Electrochemical response and separation in cyclic electric field-flow fractionation. Electrophoresis 2007, 28, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Caldwell, K.D.; Frazier, A.B. Geometric Scaling Effects in Electrical Field Flow Fractionation. 1. Theoretical Analysis. Anal. Chem. 2001, 73, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, J.J.; Vyas, N. A one-dimensional transient model of electrical field flow fractionation. Electrophoresis 2005, 26, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chauhan, A. Separation of charged colloids by a combination of pulsating lateral electric fields and poiseuille flow in a 2D channel. J. Colloid Interf. Sci. 2005, 282, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kantak, A.; Merugu, S.; Gale, B.K. Improved theory of cyclical electrical field flow fractionation. Electrophoresis 2006, 27, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Gale, B.K.; Le Hecho, I.; Lespes, G. Nanoparticle characterization by cyclical electrical field-flow fractionation. Anal. Chem. 2011, 83, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Kantak, A.; Srinivas, M.; Gale, B. Characterization of a microscale cyclical electrical field flow fractionation system. Lab Chip 2006, 6, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Kantak, A.S.; Srinivas, M.; Gale, B.K. Effect of Carrier Ionic Strength in Microscale Cyclical Electrical Field-Flow Fractionation. Anal. Chem. 2006, 78, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Sant, H.J.; Gale, B.K. Microscale field-flow fractionation: Theory and practice. In Microfluidic Technologies for Miniaturized Analysis Systems; Springer: New York, NY, USA, 2007; pp. 471–521. [Google Scholar]
- Somchue, W.; Siripinyanond, A.; Gale, B.K. Electrical Field-Flow Fractionation for Metal Nanoparticle Characterization. Anal. Chem. 2012, 84, 4993–4998. [Google Scholar] [CrossRef] [PubMed]
- Tasci, T.O.; Manangon, E.; Fernandez, D.P.; Johnson, W.P.; Gale, B.K. Separation of magnetic nanoparticles by cyclical electrical field flow fractionation. IEEE Transac. Magnet. 2013, 49, 331–335. [Google Scholar] [CrossRef]
- Sutherland, W. LXXV. A dynamical theory of diffusion for non-electrolytes and the molecular mass of albumin. Philos. Mag. Series 6 1905, 9, 781–785. [Google Scholar] [CrossRef]
- O'neill, M.E. A sphere in contact with a plane wall in a slow linear shear flow. Chem. Eng. Sci. 1968, 23, 1293–1298. [Google Scholar] [CrossRef]
- Goldman, A.J.; Cox, R.G.; Brenner, H. Slow viscous motion of a sphere parallel to a plane wall—II Couette flow. Chem. Eng. Sci. 1967, 22, 653–660. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Pashley, R.M. Measurement of the hydrophobic interaction between two hydrophobic surfaces in aqueous electrolyte solutions. J. Colloid Interf. Sci. 1984, 98, 500–514. [Google Scholar] [CrossRef]
- Merugu, S.; Sant, H.J.; Gale, B.K. A novel method for effective field measurements in electrical field-flow fractionation. Electrophoresis 2012, 33, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sant, H.J.; Chakravarty, S.; Merugu, S.; Ferguson, C.G.; Gale, B.K. Characterization of Polymerized Liposomes Using a Combination of dc and Cyclical Electrical Field-Flow Fractionation. Anal. Chem. 2012, 84, 8323–8329. [Google Scholar] [CrossRef] [PubMed]
- Tasci, T.O.; Johnson, W.P.; Fernandez, D.P.; Manangon, E.; Gale, B.K. Biased Cyclical Electrical Field Flow Fractionation for Separation of Sub 50 nm Particles. Anal. Chem. 2013, 85, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasci, T.O.; Johnson, W.P.; Fernandez, D.P.; Manangon, E.; Gale, B.K. Particle Based Modeling of Electrical Field Flow Fractionation Systems. Chromatography 2015, 2, 594-610. https://doi.org/10.3390/chromatography2040594
Tasci TO, Johnson WP, Fernandez DP, Manangon E, Gale BK. Particle Based Modeling of Electrical Field Flow Fractionation Systems. Chromatography. 2015; 2(4):594-610. https://doi.org/10.3390/chromatography2040594
Chicago/Turabian StyleTasci, Tonguc O., William P. Johnson, Diego P. Fernandez, Eliana Manangon, and Bruce K. Gale. 2015. "Particle Based Modeling of Electrical Field Flow Fractionation Systems" Chromatography 2, no. 4: 594-610. https://doi.org/10.3390/chromatography2040594
APA StyleTasci, T. O., Johnson, W. P., Fernandez, D. P., Manangon, E., & Gale, B. K. (2015). Particle Based Modeling of Electrical Field Flow Fractionation Systems. Chromatography, 2(4), 594-610. https://doi.org/10.3390/chromatography2040594




