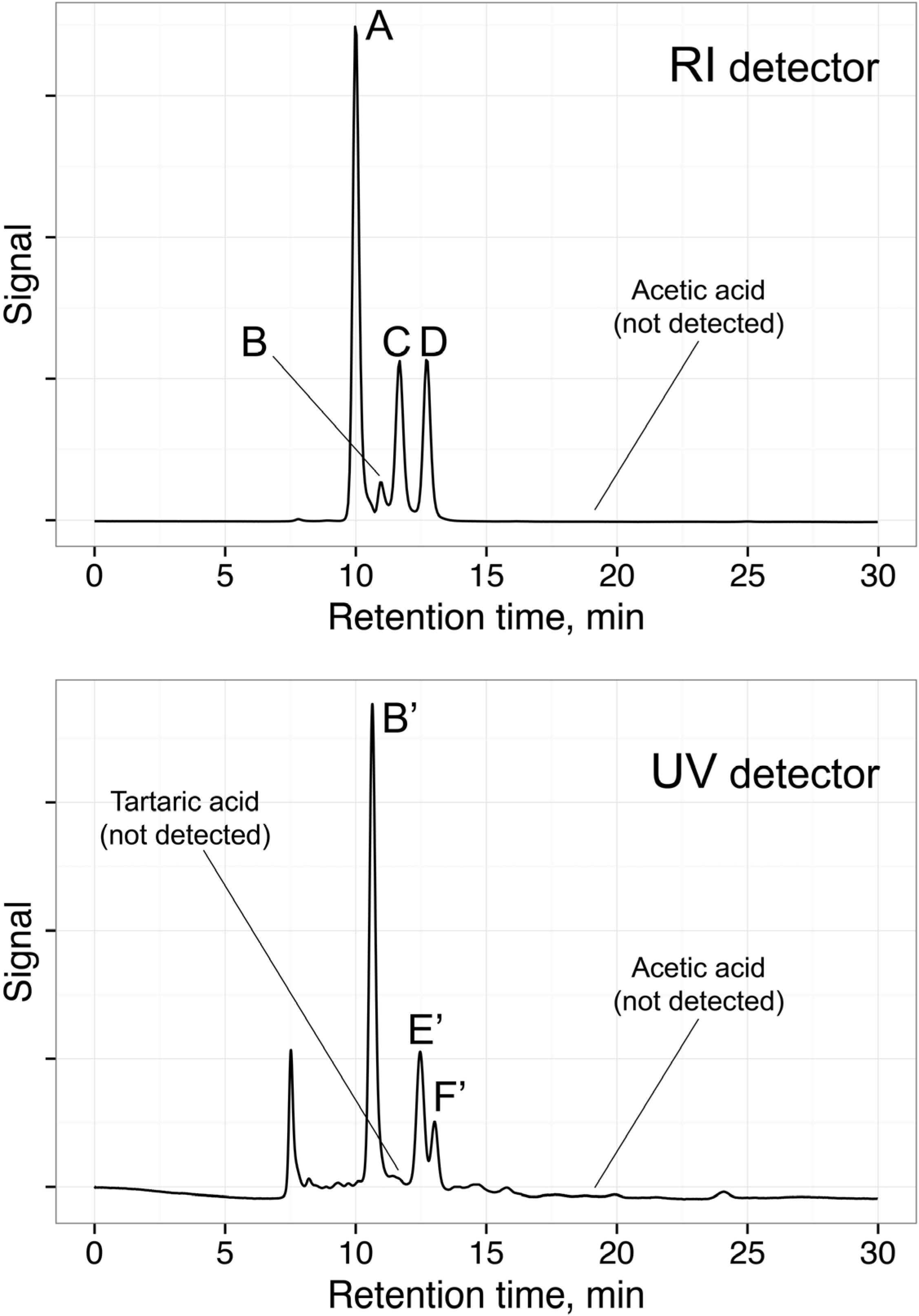
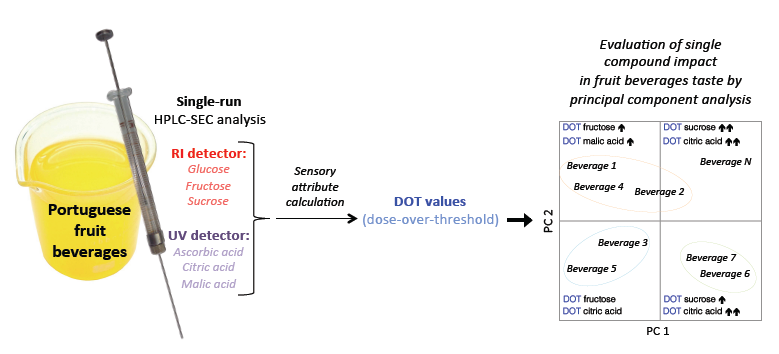
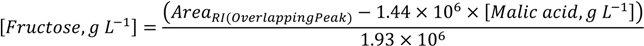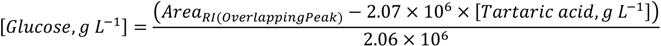3.3. DOT Values: Contribution for Unsupervised Classification of Beverage Samples
DOT values were calculated for each beverage sample according to Keutgen and Pawelzik [
3] and are shown in
Table 6, allowing the evaluation of each compound’s contribution to the beverage global taste perception. Compounds with DOT values greater than one have a significant individual sensory impact. The analysis of the DOT values clearly shows that, with the exception of glucose, all of the other compounds, when present in a specific beverage, have a significant sensory influence (DOT values higher than one). Furthermore, it should be noticed that the use of DOT values enables the verification of the real influence of each compound on the global taste perception of a specific beverage, revealing that a compound present in a low concentration can have a greater impact on the beverage taste than a more concentrated compound, which is the case for organic acids. For example, citric acid is present in the studied fruit beverages in concentrations lower than any of the sugars (2 < (citric acid, g L
−1) < 8; 1.4 < (sugar, g L
−1) < 85), but in most of the samples, citric acid has a greater individual sensory influence towards the global taste perception of the beverage (2.1 < DOT
citric acid < 14.5; 0.2 < DOT
sugar < 9.5).
Table 6.
Dose-over-threshold calculated values for each individual sugar and organic acid present in each beverage.
Table 6.
Dose-over-threshold calculated values for each individual sugar and organic acid present in each beverage.
| Sample Number | DOT Values |
|---|
| Sucrose | Glucose | Fructose | Citric Acid | Malic Acid | Ascorbic Acid |
|---|
| 1 | 1.7 | 0.7 | 1.7 | 9.1 | -- | 1.6 |
| 2 | 1.2 | 0.7 | 2.4 | 4.0 | 4.1 | 1.9 |
| 3 | 1.6 | 0.8 | 1.6 | 9.9 | 2.2 | 1.2 |
| 4 | 0.2 | 0.6 | 2.0 | 5.1 | 4.7 | 1.1 |
| 5 | 1.8 | 0.8 | 2.2 | 6.5 | 3.6 | 1.1 |
| 6 | 9.0 | 1.1 | 1.7 | 2.5 | 4.9 | 1.6 |
| 7 | 1.4 | 2.0 | 6.0 | 3.8 | 0.7 | 1.1 |
| 8 | 7.3 | 1.2 | 2.8 | 4.8 | -- | 2.2 |
| 9 | 1.6 | 1.8 | 7.4 | 2.2 | 9.5 | 1.2 |
| 10 | -- | 0.9 | 1.8 | 5.4 | 2.1 | 1.8 |
| 11 | 4.3 | 1.5 | 3.2 | 14.4 | -- | 3.0 |
| 12 | 1.9 | 1.0 | 1.8 | 3.7 | 2.9 | 1.2 |
| 13 | 5.8 | 0.7 | 2.8 | 2.2 | -- | 1.2 |
| 14 | -- | 0.7 | 1.5 | 6.3 | -- | 1.7 |
| 15 | 6.9 | 1.5 | 3.0 | 5.9 | -- | 1.5 |
| 16 | 8.8 | 1.2 | 2.4 | 5.8 | -- | 1.7 |
| 17 | 5.1 | 2.0 | 4.1 | 6.0 | -- | 1.5 |
| 18 | 4.3 | 1.8 | 3.4 | 4.5 | -- | -- |
| 19 | 5.2 | 1.7 | 3.6 | 6.7 | -- | 1.1 |
| 20 | 8.2 | 1.3 | 2.5 | 4.0 | -- | 1.4 |
| 21 | 8.3 | 1.1 | 2.3 | 6.8 | -- | 1.5 |
| 22 | 4.0 | 2.0 | 2.5 | 3.2 | -- | 1.3 |
| 23 | 4.7 | 2.2 | 3.2 | 2.3 | -- | 1.2 |
| 24 | 3.7 | 2.6 | 3.0 | 3.4 | -- | 1.1 |
| 25 | 3.1 | 0.7 | 1.4 | 4.8 | -- | 1.2 |
| 26 | 1.2 | 3.5 | 4.8 | 3.3 | -- | -- |
| 27 | 2.4 | 1.4 | 2.9 | 6.7 | -- | 1.3 |
| 28 | 3.8 | 3.3 | 9.0 | 6.8 | -- | 1.6 |
| 29 | 9.4 | 1.6 | 3.1 | 13.5 | -- | 1.7 |
| 30 | 0.3 | 0.6 | 1.8 | 3.1 | -- | 1.3 |
| ANOVA a | ade | bc | abd | abe | bcde | bc |
Welch’s ANOVA showed that there were significant differences between the DOT values of the compounds (
p-value lower than 0.001). The multiple comparison test allowed one to reveal which compounds had DOT values with significant differences (
Table 6). The results showed that there were more observed mean differences within compounds’ DOT values than those found in the analytical results.
Furthermore, in this study, the DOT values of the main compounds detected in the beverage samples (glucose, fructose and sucrose; ascorbic, citric and malic acids) were used in an attempt to verify the existence of similarity among different fruit beverages of six Portuguese brands, containing different amounts of added juice from one or more fruits (e.g., apple, banana, grape, kiwi, mango, orange, passion fruit, peach, pear, pineapple and/or strawberry, among other). This purpose was accomplished using PCA, an unsupervised statistical technique, after dividing into two main groups: beverages containing one or two fruits (17 samples, including two fruit juices, eight fruit nectars and seven soft-drinks) and multi-fruits beverages (13 samples, including four fruit nectars and nine soft-drinks), containing a mixture of four to nine fruits.
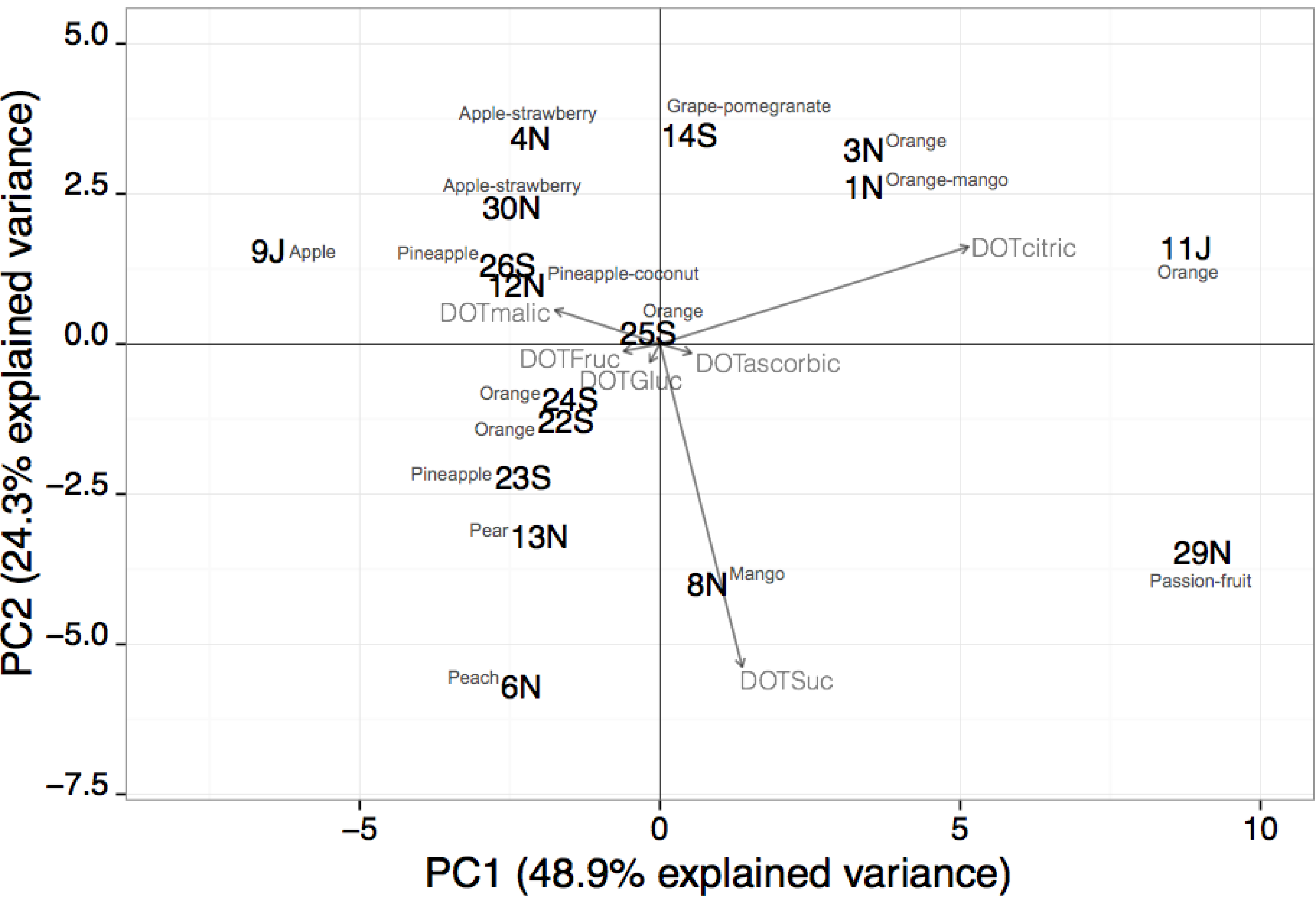
The PCA applied to beverages containing one to two fruits showed that the first four functions explained 98.9% of the total data variance (48.9%, 24.3%, 19.8% and 5.9%, respectively). In
Figure 2, the two-dimensional spatial sample distribution considering the first and second principal component functions is shown.
As can be observed, samples were unsupervised and assembled into four main groups. The first group (located in the first quadrant, the positive regions of PC1 and PC2) includes four samples from the three types of beverages, mainly of orange fruit, for which citric acid has a significant impact on the beverage global taste (DOT values varying from 6.3 to 14.4). On the contrary, sugars have a medium to low contribution to the overall taste perception (DOT values between 0.7 and 4.3). In the second quadrant (negative and positive regions of PC1 and PC2, respectively), the formed group contains five beverage samples of pineapple, apple and/or strawberry, also from the three types of beverages, being characterized by average to high citric and/or malic acids DOT values (from 2.2 to 9.5) and a medium to low contribution of sugars towards taste (DOT values ranging from 0.2 to 4.8), with the exception of Sample 9, for which fructose has a high individual sensory impact (DOT equal to 7.4). In the third quadrant (negative regions of PC1 and PC2), for the five samples (fruit nectars and soft-drinks) of one fruit (peach, pear, pineapple or orange), sucrose has a high individual taste contribution (DOT values ranging from 3.7 to 9.4) and citric acid has a medium sensory impact (DOT values between 2.5 and 3.5). Finally, two samples are in the fourth quadrant (positive and negative regions of PC1 and PC2, respectively) for which, although sucrose has also a strong taste influence (DOT values equal to 7.3 and 9.4), as in the previous quadrant, citric acid has a significant individual sensory impact (DOT values equal to 4.8 and 13.5). Globally, the unsupervised groups that emerged considering the individual sensory contribution of sugars and organic acids may be tentatively related to the beverage’s well-balanced flavor, which results from achieving an equilibrium between its sweetness and acidity: high acidity and low sweetness beverages; medium acidity and low sweetness beverages; low acidity and high sweetness beverages; and high acidity and high sweetness beverages. As can be observed, the naturally formed groups appeared not to be related with beverage types or the kind of fruit present in the beverage.
Figure 2.
Representation of the two first principal component factor scores obtained for beverages containing one to two fruits using the sugars and organic acids DOT values (samples are identified by the number and type of fruit beverage: S, soft-drinks; N, fruit nectars; J, fruit juices).
Figure 2.
Representation of the two first principal component factor scores obtained for beverages containing one to two fruits using the sugars and organic acids DOT values (samples are identified by the number and type of fruit beverage: S, soft-drinks; N, fruit nectars; J, fruit juices).
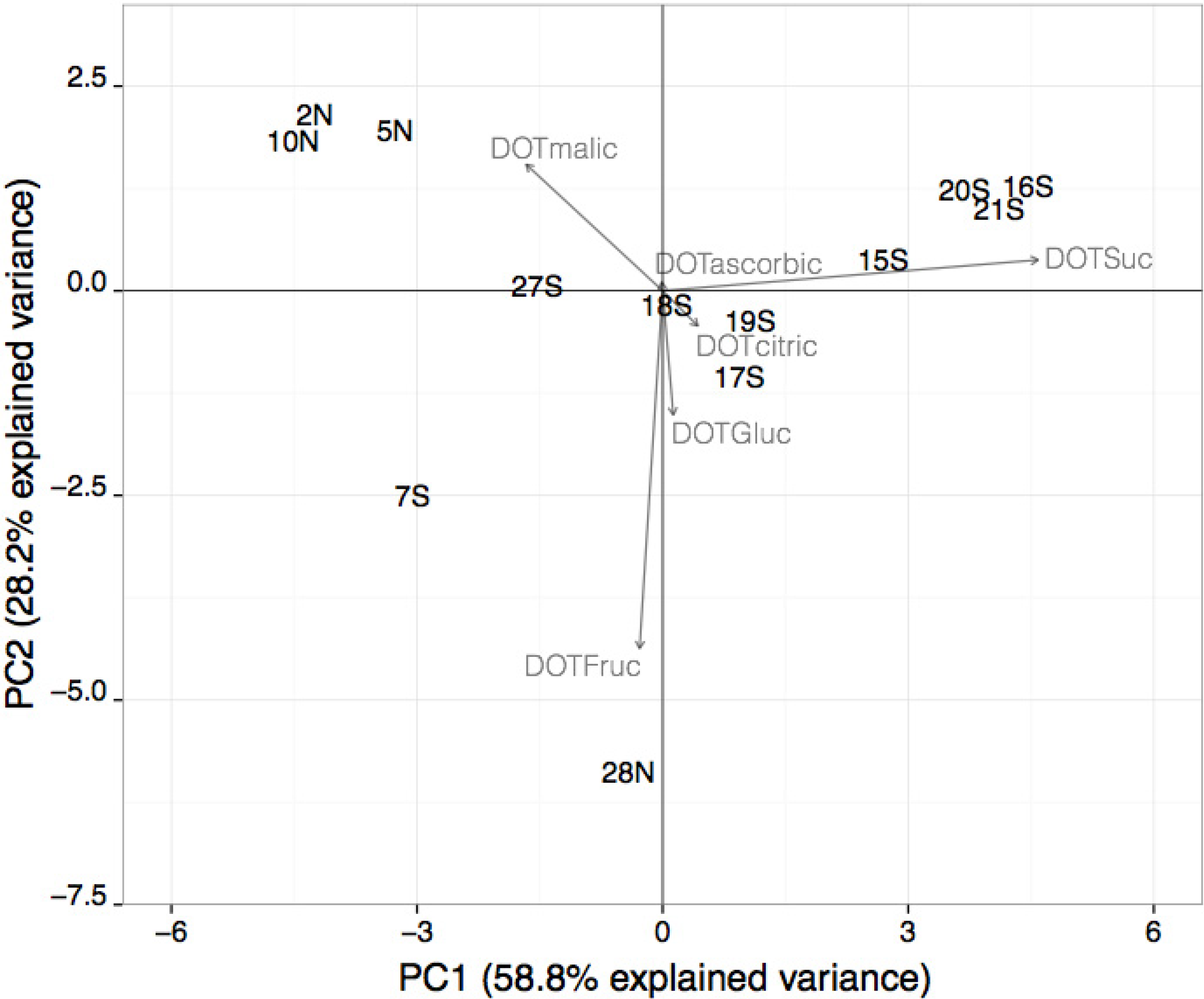
The PCA was also applied to multi-fruit beverages, containing a mixture of four to nine fruits. The scree-plot (data not shown) allowed verifying that only the first three functions should be selected, which explained 94.3% of the total data variance (58.8%, 28.2% and 7.3%, respectively). In
Figure 3, the two-dimensional spatial distribution of the multi-fruits beverage samples, considering the first and second principal component functions, is shown. As can be seen, the 13 samples are distributed in the four quadrants considering the two first principal components functions, mainly due to the individual sensory impact of sucrose, fructose and/or malic acid. The first principal component function split samples (located in the positive or negative region), taking into account sucrose individual sensory contribution to the beverage global taste perception (DOT values ranging from 4.3 to 8.8 and from 0 to 3.8, respectively). The second function separates samples according to the influence of fructose towards the beverage’s overall sensory perception, its impact being low or high for samples located in the positive or negative regions, respectively. The significance of the individual sensory effect of malic acid allowed for refining the samples spatial distribution into each one of the four quadrants. Samples located in the first and fourth quadrants do not contain malic acid, and those located in the second and third quadrants have low to medium malic acid contents, corresponding to low to medium DOT values or low values, respectively. According to the previous discussion, it can be noticed that, in general, beverages located in the first, third and fourth quadrants present a well-balanced taste, due to the similar significant sensory contribution of sugars and organic acids, whereas beverages located in the second quadrant may possess a greater acidity.
Figure 3.
Representation of the two first principal component factor scores obtained for the multi-fruit beverages containing four to nine fruits using the sugars and organic acids DOT values (samples are identified by the number and type of fruit beverage: S, soft-drinks; N, fruit nectars; J, fruit juices).
Figure 3.
Representation of the two first principal component factor scores obtained for the multi-fruit beverages containing four to nine fruits using the sugars and organic acids DOT values (samples are identified by the number and type of fruit beverage: S, soft-drinks; N, fruit nectars; J, fruit juices).