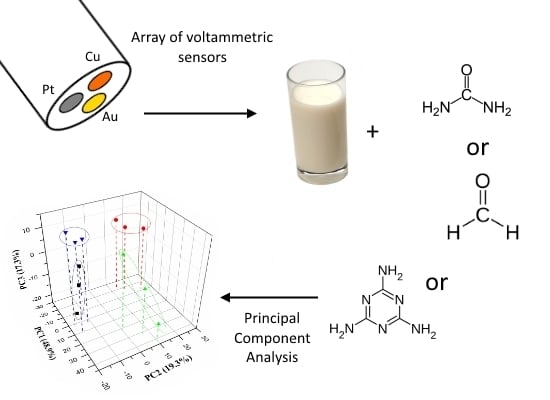
Voltammetric Electronic Tongue for Discrimination of Milk Adulterated with Urea, Formaldehyde and Melamine
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals, Materials, and Samples
2.2. Electrodes and Instrumentation
2.3. Electrochemical Quartz Crystal Microbalance (EQCM) Measurements
2.4. Electrochemical Analysis
2.5. Chemometric Analyses
3. Results and Discussion
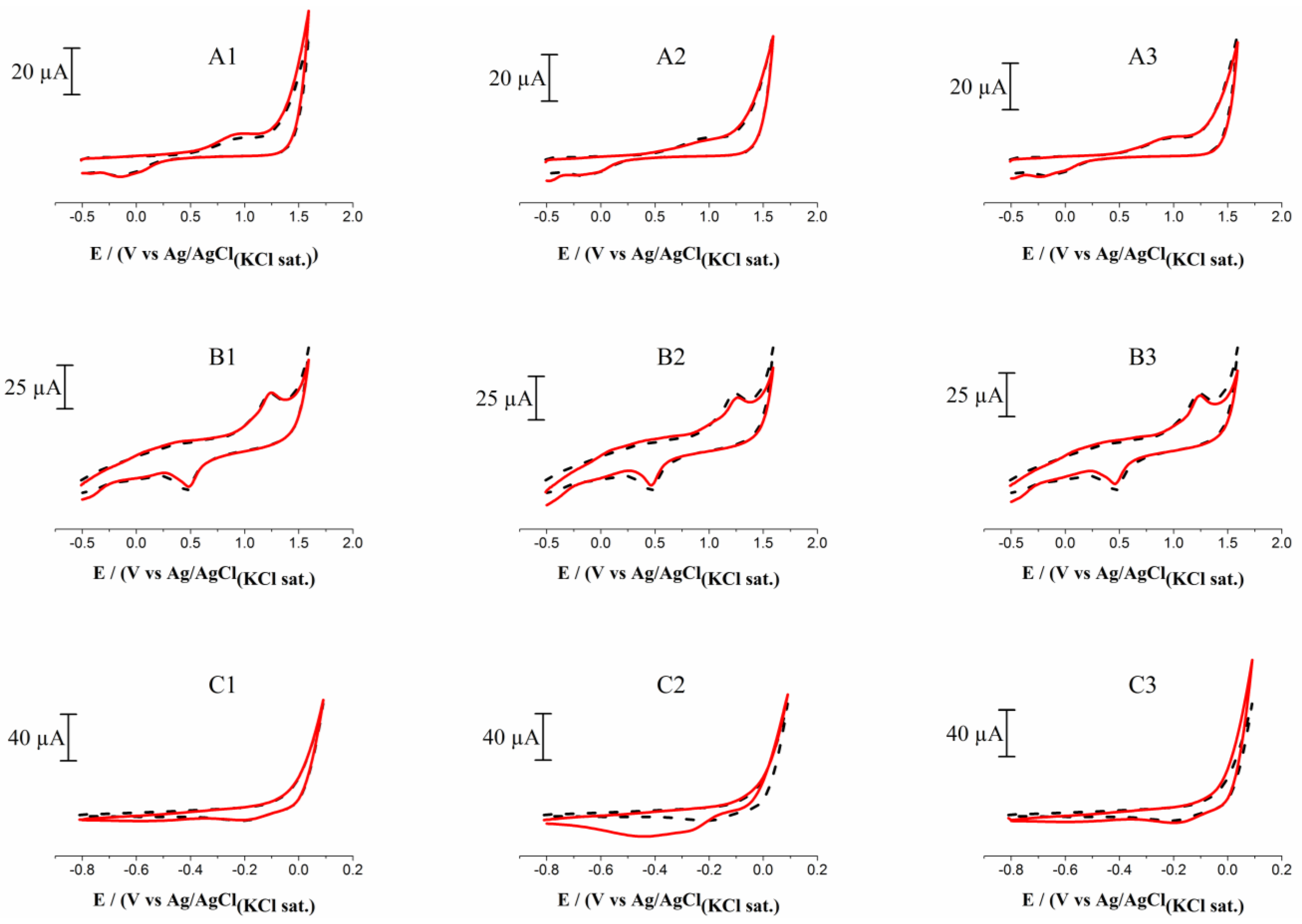
3.1. Electrochemical Studies in a Whole Milk Sample
3.2. EQCM Studies
3.3. Discrimination of Milk Samples
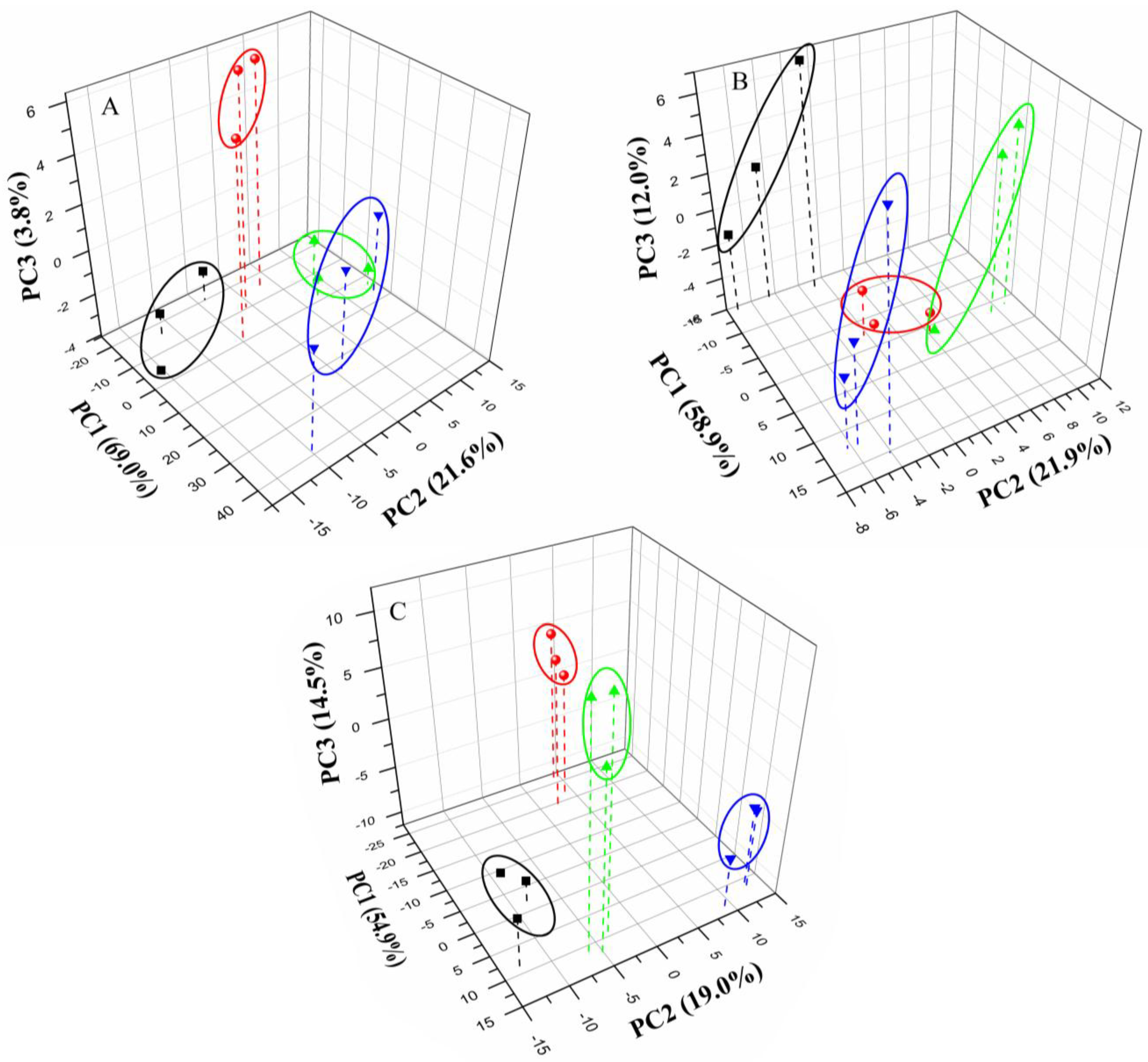

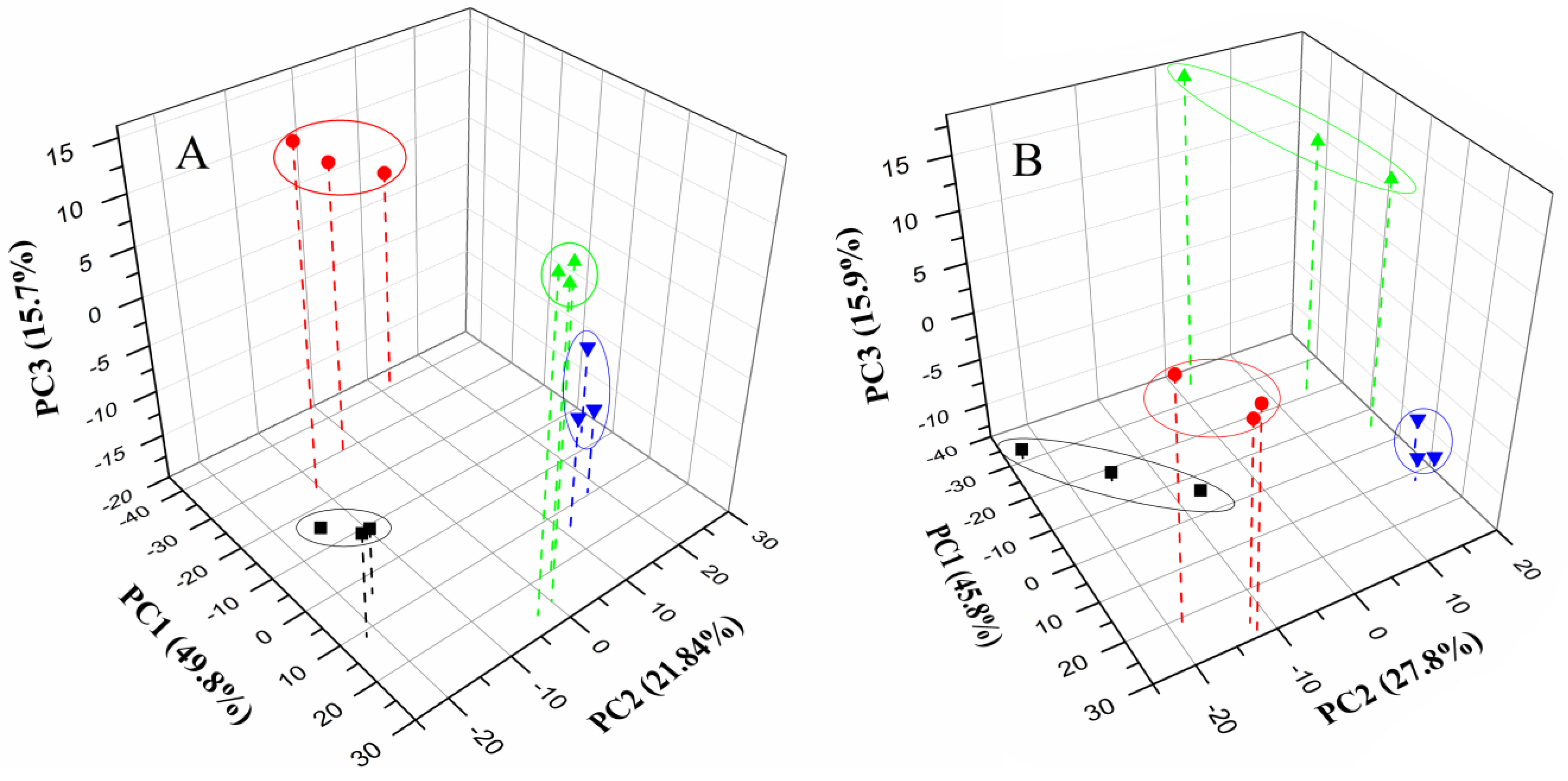
4. Conclusions
Acknowledgments
Author Contributes
Conflicts of Interest
References
- Spink, J.; Moyer, D.C. Defining the public health threat of food fraud. J. Food Sci. 2011, 76, R157–R163. [Google Scholar] [CrossRef] [PubMed]
- Fuca, N.; Pasta, C.; Impoco, G.; Caccamo, M.; Licitra, G. Microstructural properties of milk fat globules. Int. Dairy J. 2013, 31, 44–50. [Google Scholar] [CrossRef]
- Wilbey, R.A. Milk Quality. Int. J. Dairy Technol. 1996, 49, 36–36. [Google Scholar] [CrossRef]
- Abernethy, G.; Higgs, K. Rapid detection of economic adulterants in fresh milk by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1288, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Veloso, A.C.A.; Teixeira, N.; Ferreira, I.M.P.L.V.O.; Ferreira, M.A. Detecção de Adulterações em Produtos Alimentares Contendo Leite e/ou Proteínas Lácteas. Quim. Nova 2002, 25, 609–615. (In Portuguese) [Google Scholar] [CrossRef]
- Hilding-Ohlsson, A.; Fauerbach, J.A.; Sacco, N.J.; Celina Bonetto, M.; Corton, E. Voltamperometric Discrimination of Urea and Melamine Adulterated Skimmed Milk Powder. Sensors 2012, 12, 12220–12234. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Medhe, S.; Raj, K.R.; Srivastava, M.M. High performance thin layer chromatography for routine monitoring of adulterants in milk. Natl. Acad. Sci. Lett. 2012, 35, 309–313. [Google Scholar] [CrossRef]
- Jonker, J.S.; Kohn, R.A.; Erdman, R.A. Using milk urea nitrogen to predict nitrogen excretion and utilization efficiency in lactating dairy cows. J. Dairy Sci. 1998, 81, 2681–2692. [Google Scholar] [PubMed]
- Sharma, R.; Rajput, Y.S.; Kaur, S.; Tomar, S.K. A method for estimation of urea using ammonia electrode and its applicability to milk samples. J. Dairy Res. 2008, 75, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, U.B.; Lakshminarayana, D.; Kothari, I.L.; Patel, N.G.; Kapse, H.N.; Makhija, K.K.; Patel, R.B.; Panchal, C.J. Potentiometric biosensor for urea determination in milk. Sens. Actuators B Chem. 2009, 140, 260–266. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Methenitis, C. Construction of a simple optical sensor based on air stable lipid film with incorporated urease for the rapid detection of urea in milk. Anal. Chim. Acta 2010, 675, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.D.; Devi, R. Cytokinesis blocked micronucleus assay of peripheral lymphocytes revealing the genotoxic effect of formaldehyde exposure. Clin. Anat. 2014, 27, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Cheng, D.; Mao, L. Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem. 2010, 122, 895–900. [Google Scholar] [CrossRef]
- Su, H.; Fan, H.; Ai, S.; Wu, N.; Fan, H.; Bian, P.; Liu, J. Selective determination of melamine in milk samples using 3-mercapto-1-propanesulfonate-modified gold nanoparticles as colorimetric probe. Talanta 2011, 85, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Stone, R. Chinese probe unmasks high-tech adulteration with melamine. Science 2008, 322, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Dorne, J.L.; Doerge, D.R.; Vandenbroeck, M.; Fink-Gremmels, J.; Mennes, W.; Knutsen, H.K.; Vernazza, F.; Castle, L.; Edler, L.; Benford, D. Recent advances in the risk assessment of melamine and cyanuric acid in animal feed. Toxicol. Appl. Pharmacol. 2013, 270, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Thiagarajan, S.; Chen, S.-M. Detection of melamine in milk powder and human urine. J. Agric. Food Chem. 2010, 58, 4537–4544. [Google Scholar] [CrossRef] [PubMed]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, MA, USA, 1990. [Google Scholar]
- Liang, X.; Wei, H.; Cui, Z.; Deng, J.; Zhang, Z.; You, X.; Zhang, X.-E. Colorimetric detection of melamine in complex matrices based on cysteamine-modified gold nanoparticles. Analyst 2011, 136, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Atwal, A.S.; Mahadevan, S. Determination of formaldehyde in fresh and retail milk by liquid column chromatography. J. AOAC Int. 1993, 76, 1010–1013. [Google Scholar] [PubMed]
- Torell, D.T. Adding formalin to milk helps in raising orphan lambs. Calif. Agric. 1972, 26, 8–10. [Google Scholar]
- Cheng, Y.; Qin, J.; Lim, J.; Chan, D.E.; Kim, M.S.; Chao, K. An investigation of FT-Raman spectroscopy for quantification of additives to milk. In Sensing for Agriculture and Food Quality and Safety IV; Kim, M.S., Tu, S.I., Chao, K., Eds.; SPIE: Baltimore, MD, USA, 2012. [Google Scholar] [CrossRef]
- He, B.; Liu, R.; Yang, R.; Xu, K. Adulteration detection in milk using infrared spectroscopy combined with two-dimensional correlation analysis. In Optical Diagnostics and Sensing X: Toward Point-of-Care Diagnostics; Cote, G.L., Ed.; SPIE: San Francisco, CA, USA, 2010. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S. Detecting multiple adulterants in dry milk using Raman chemical imaging. In Sensing for Agriculture and Food Quality and Safety IV; Kim, M.S., Tu, S.I., Chao, K., Eds.; SPIE: Baltimore, MD, USA, 2012. [Google Scholar] [CrossRef]
- Czerwenka, C.; Muller, L.; Lindner, W. Detection of the adulteration of water buffalo milk and mozzarella with cow’s milk by liquid chromatography-mass spectrometry analysis of beta-lactoglobulin variants. Food Chem. 2010, 122, 901–908. [Google Scholar] [CrossRef]
- Krska, R.; Becalski, A.; Braekevelt, E.; Koerner, T.; Cao, X.L.; Dabeka, R.; Godefroy, S.; Lau, B.; Moisey, J.; Rawn, D.F.K.; et al. Challenges and trends in the determination of selected chemical contaminants and allergens in food. Anal. Bioanal. Chem. 2012, 402, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Huo, D.Q.; Jiang, Y.H.; Hou, C.J.; Zhang, S.Y. Monitoring the adulteration of milk with melamine: A visualised sensor array approach. Food Addit. Contam. Part A Chem. 2013, 30, 786–795. [Google Scholar] [CrossRef]
- Xing, H.B.; Zhan, S.S.; Wu, Y.G.; He, L.; Zhou, P. Sensitive colorimetric detection of melamine in milk with an aptamer-modified nanogold probe. RSC Adv. 2013, 3, 17424–17430. [Google Scholar] [CrossRef]
- Bueno, L.; Paixão, T.R.L.C. A single platinum microelectrode for identifying soft drink samples. Int. J. Electrochem. 2012, 2012. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Bertotti, M. Fabrication of disposable voltammetric electronic tongues by using Prussian Blue films electrodeposited onto CD-R gold surfaces and recognition of milk adulteration. Sens. Actuator B Chem. 2009, 137, 266–273. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Vilas-Boas, M.; Rocha, M.A.; Estevinho, L.; Machado, A. An electronic tongue for honey classification. Microchim. Acta 2008, 163, 97–102. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Seleznev, B.; Vlasov, Y.; Velikzhanin, V. Electronic tongue for recognition of flesh food. In Artificial Chemical Sensing: Olfaction and the Electronic Nose; Stetter, J.R., Penrose, W.R., Eds.; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2001; Volume 2001, pp. 76–81. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Corbo, D.; Bertotti, M. Amperometric determination of ethanol in beverages at copper electrodes in alkaline medium. Anal. Chim. Acta 2002, 472, 123–131. [Google Scholar] [CrossRef]
- Pedrotti, J.J.; Angnes, L.; Gutz, I.G.R. Miniaturized reference electrodes with microporous polymer junctions. Electroanalysis 1996, 8, 673–675. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazyte, J.; Juodiene, T.; Lukinskas, A. Determination of Au (III) in the surface layers formed anodically on the gold electrode. J. Electroanal. Chem. 1998, 441, 19–24. [Google Scholar] [CrossRef]
- De Araujo, W.R.; Paixão, T.R.L.C. Use of copper electrode for melamine quantification in milk. Electrochim. Acta 2014, 117, 379–384. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-order chemical sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef] [PubMed]
- Finete, V.D.M.; Gouvea, M.M.; Marques, F.F.D.; Netto, A.D.P. Is it possible to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem. 2013, 141, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S.; Talpur, F.N.; Sherazi, S.T.H.; Nizamani, S.M.; Khaskheli, A.A. Rapid detection of melamine adulteration in dairy milk by SB-ATR-Fourier transform infrared spectroscopy. Food Chem. 2013, 141, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Pereira-Filho, E.R.; Rodriguez-Saona, L.E. Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem. 2013, 138, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhao, H.; Zeng, L.; Wang, J.; Wang, R.; Qiu, X.; He, Y. Electrochemical determination of melamine using oligonucleotides modified gold electrodes. Talanta 2009, 80, 484–488. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, L.; De Araujo, W.R.; Salles, M.O.; Kussuda, M.Y.; Paixão, T.R.L.C. Voltammetric Electronic Tongue for Discrimination of Milk Adulterated with Urea, Formaldehyde and Melamine. Chemosensors 2014, 2, 251-266. https://doi.org/10.3390/chemosensors2040251
Bueno L, De Araujo WR, Salles MO, Kussuda MY, Paixão TRLC. Voltammetric Electronic Tongue for Discrimination of Milk Adulterated with Urea, Formaldehyde and Melamine. Chemosensors. 2014; 2(4):251-266. https://doi.org/10.3390/chemosensors2040251
Chicago/Turabian StyleBueno, Lígia, William R. De Araujo, Maiara O. Salles, Marcos Y. Kussuda, and Thiago R. L. C. Paixão. 2014. "Voltammetric Electronic Tongue for Discrimination of Milk Adulterated with Urea, Formaldehyde and Melamine" Chemosensors 2, no. 4: 251-266. https://doi.org/10.3390/chemosensors2040251