Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthetic Design of OSM
2.3. Instrumental Analysis
3. Results and Discussion
3.1. Building Design of OSM Architectures
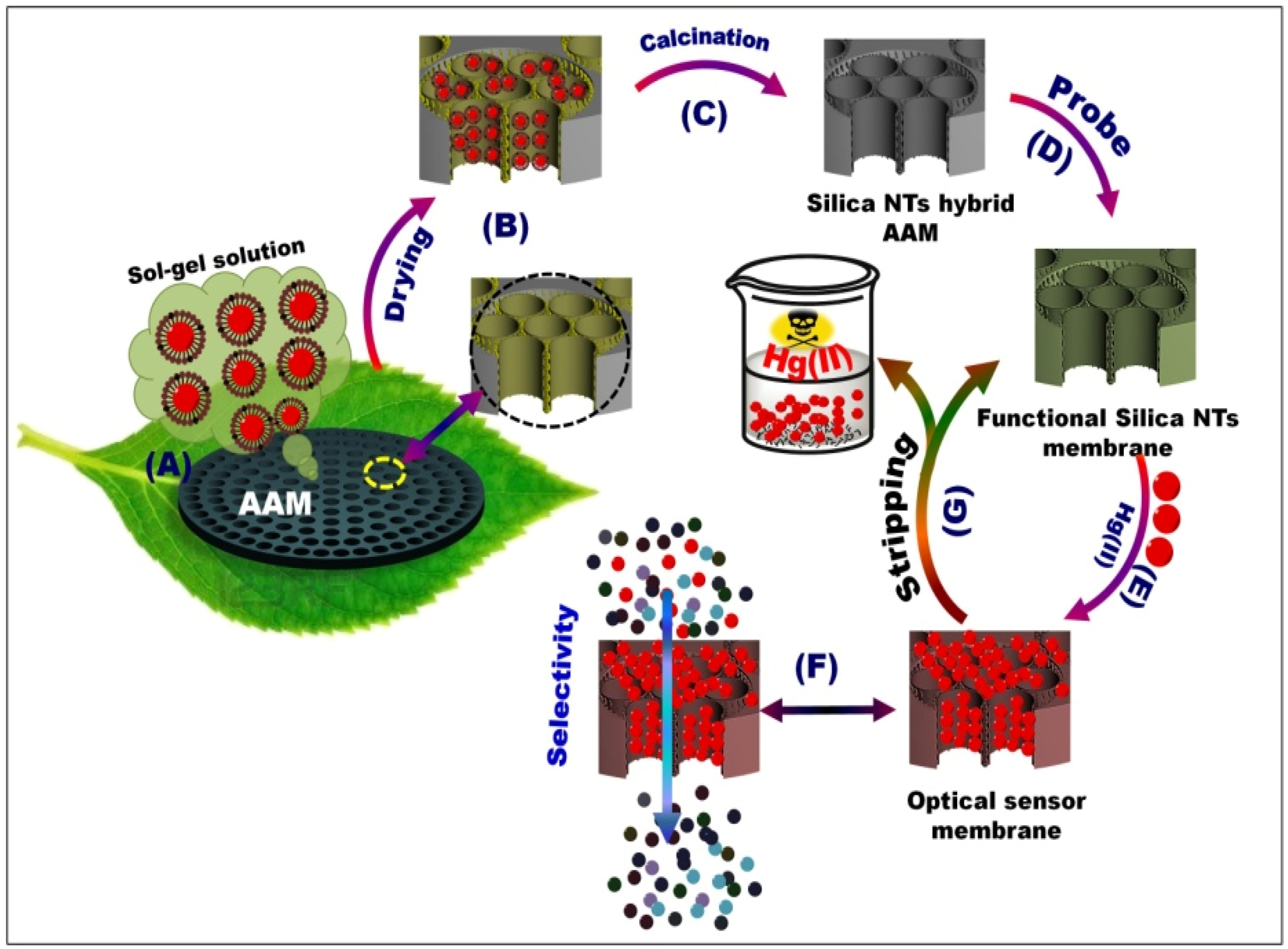
3.2. Characterization of the Fabricated OSM Architectures
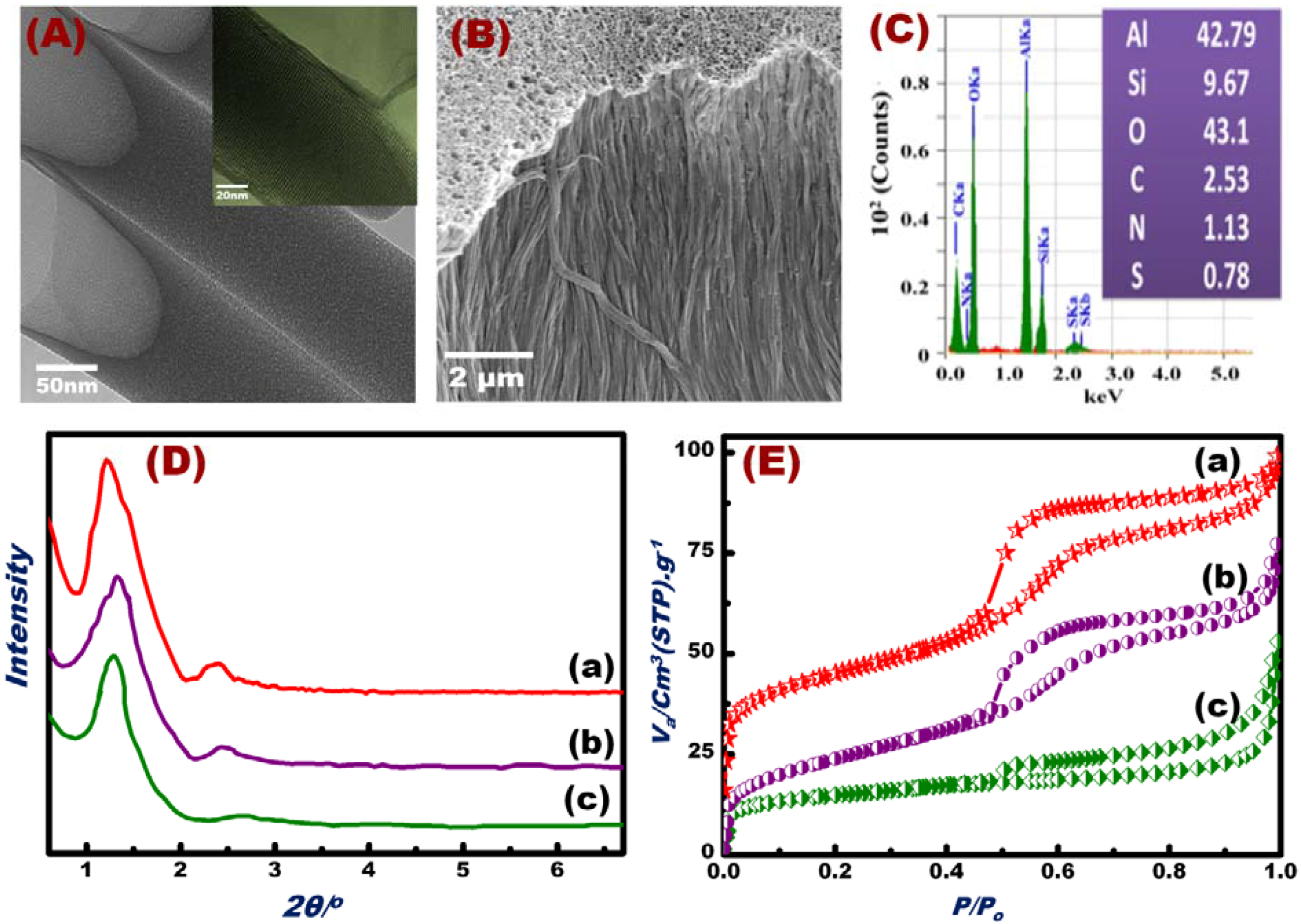
| Material | S(BET) m2/g | Vp cm3/g | D/nm |
|---|---|---|---|
| Silica NTs | 130 | 0.66 | 6.1 |
| Silica NT-Captor | 70 | 0.60 | 5.7 |
| Silica NT-Captor/Hg | 40 | 0.40 | 5.2 |
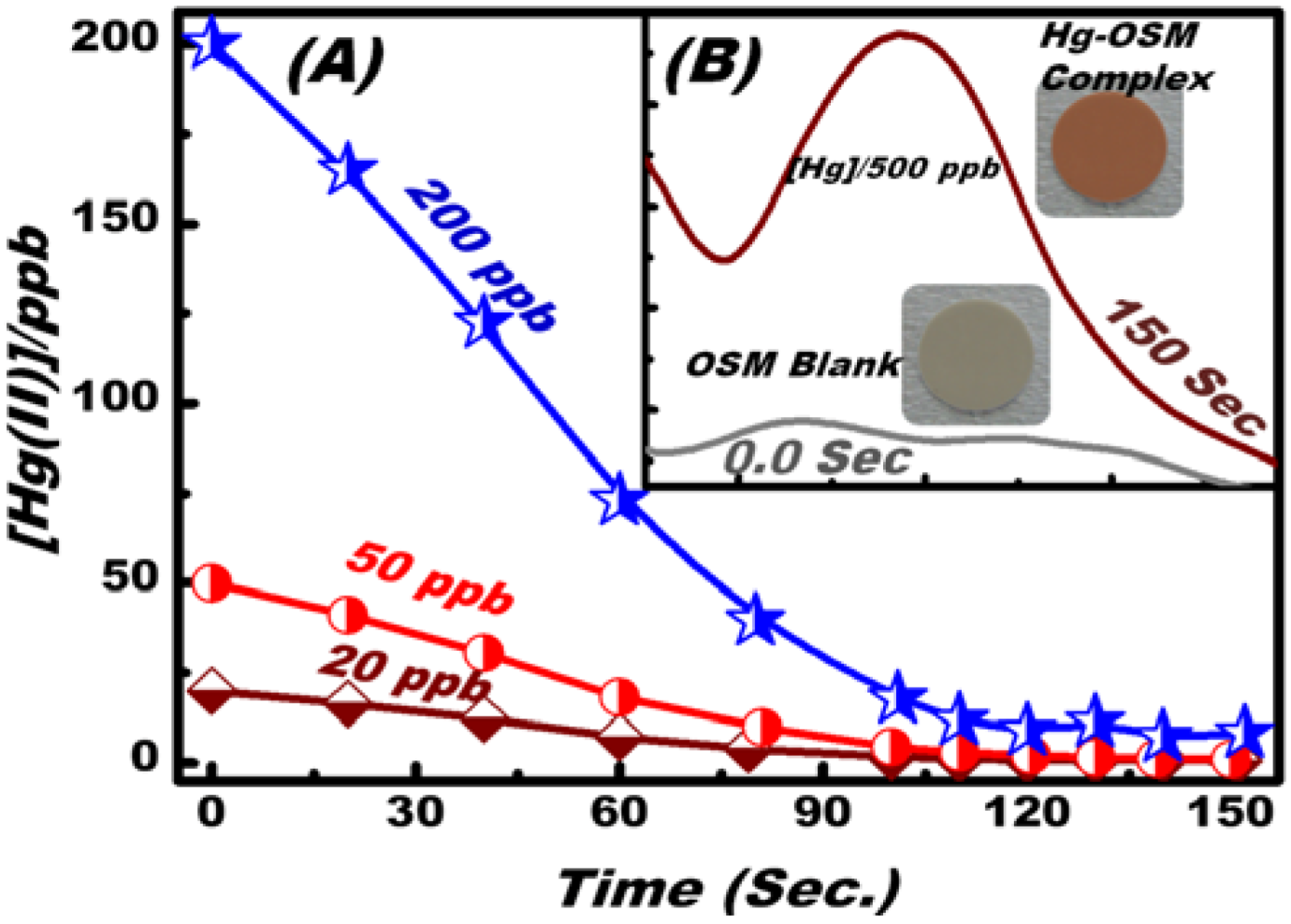
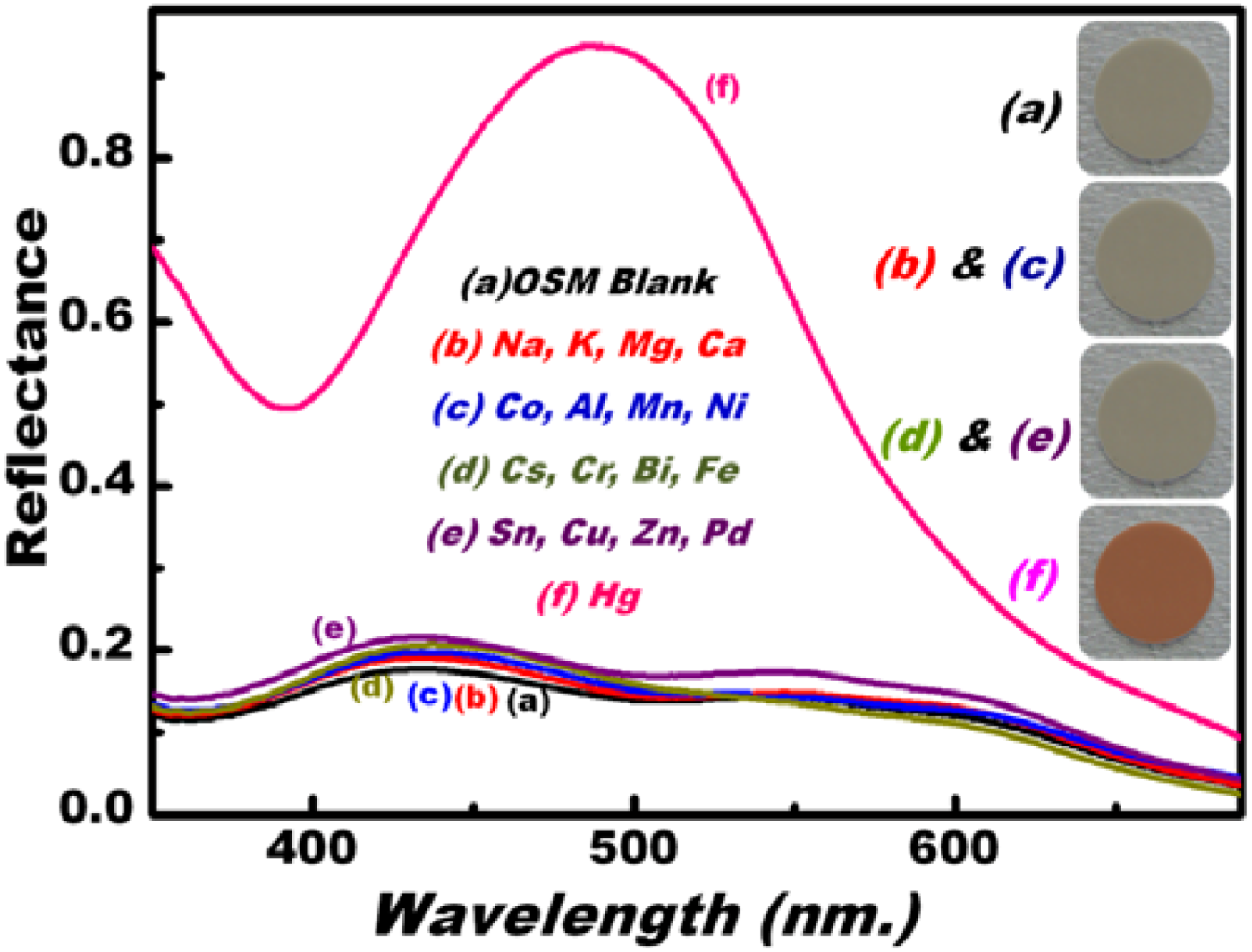
3.3. Optical Screening/Sensing of Hg(II) Ions Using OSM
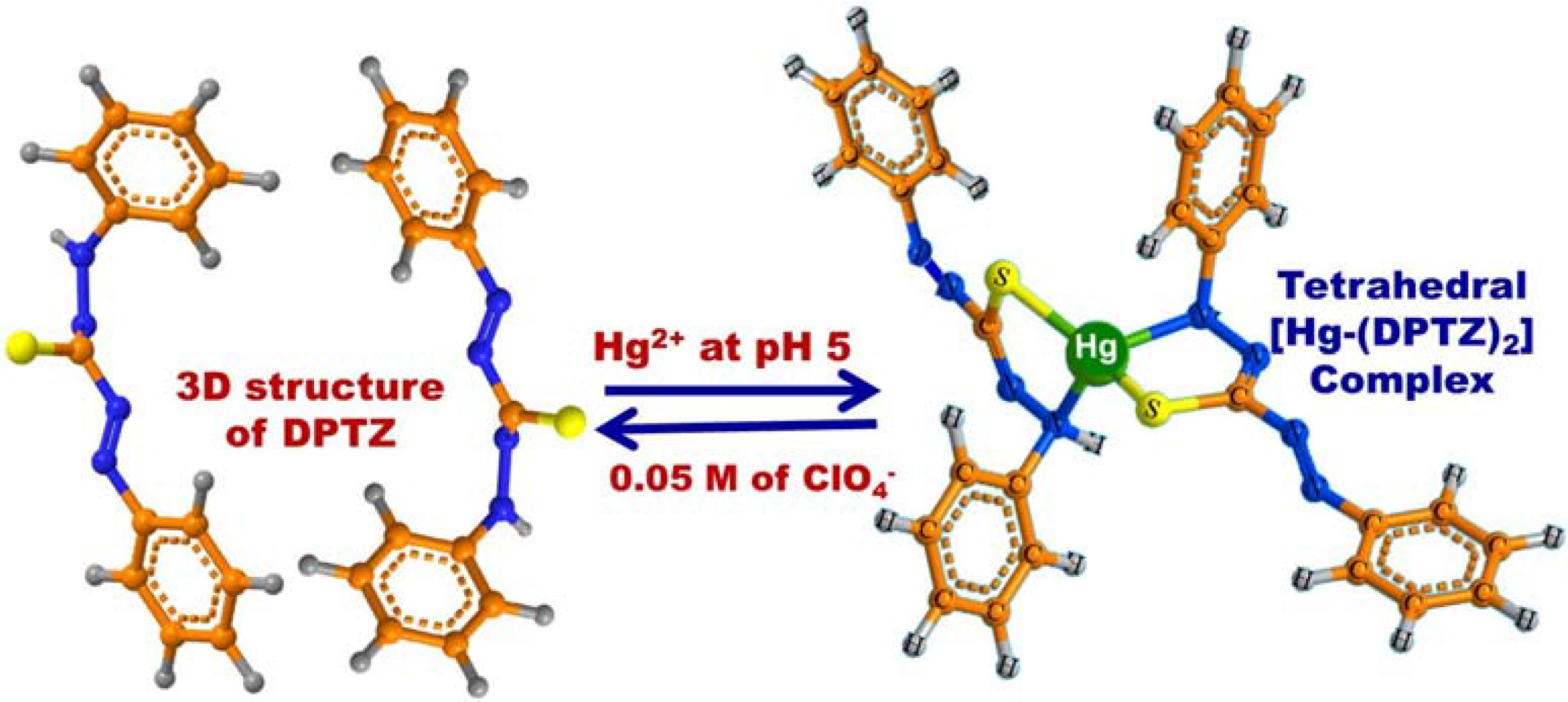
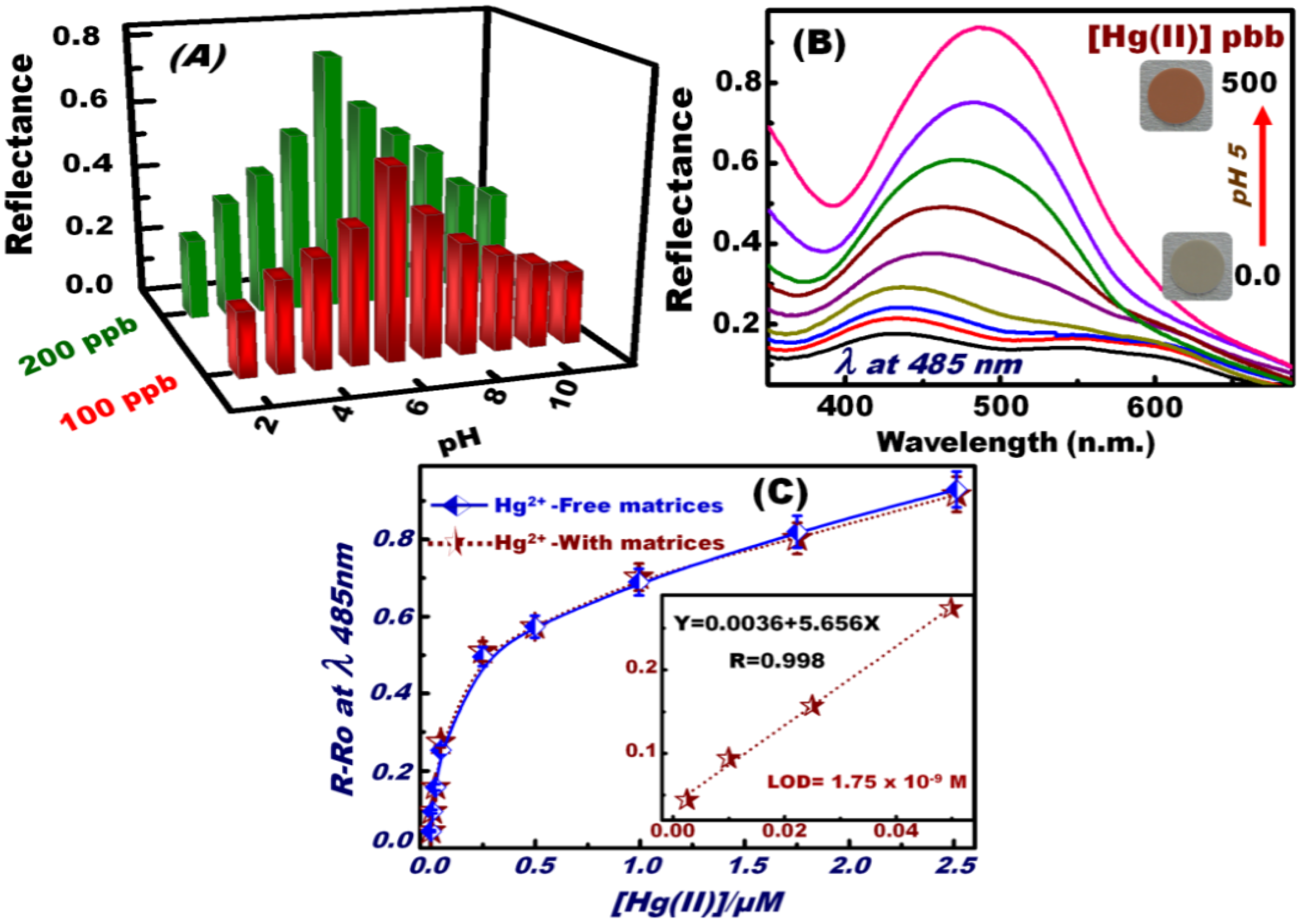
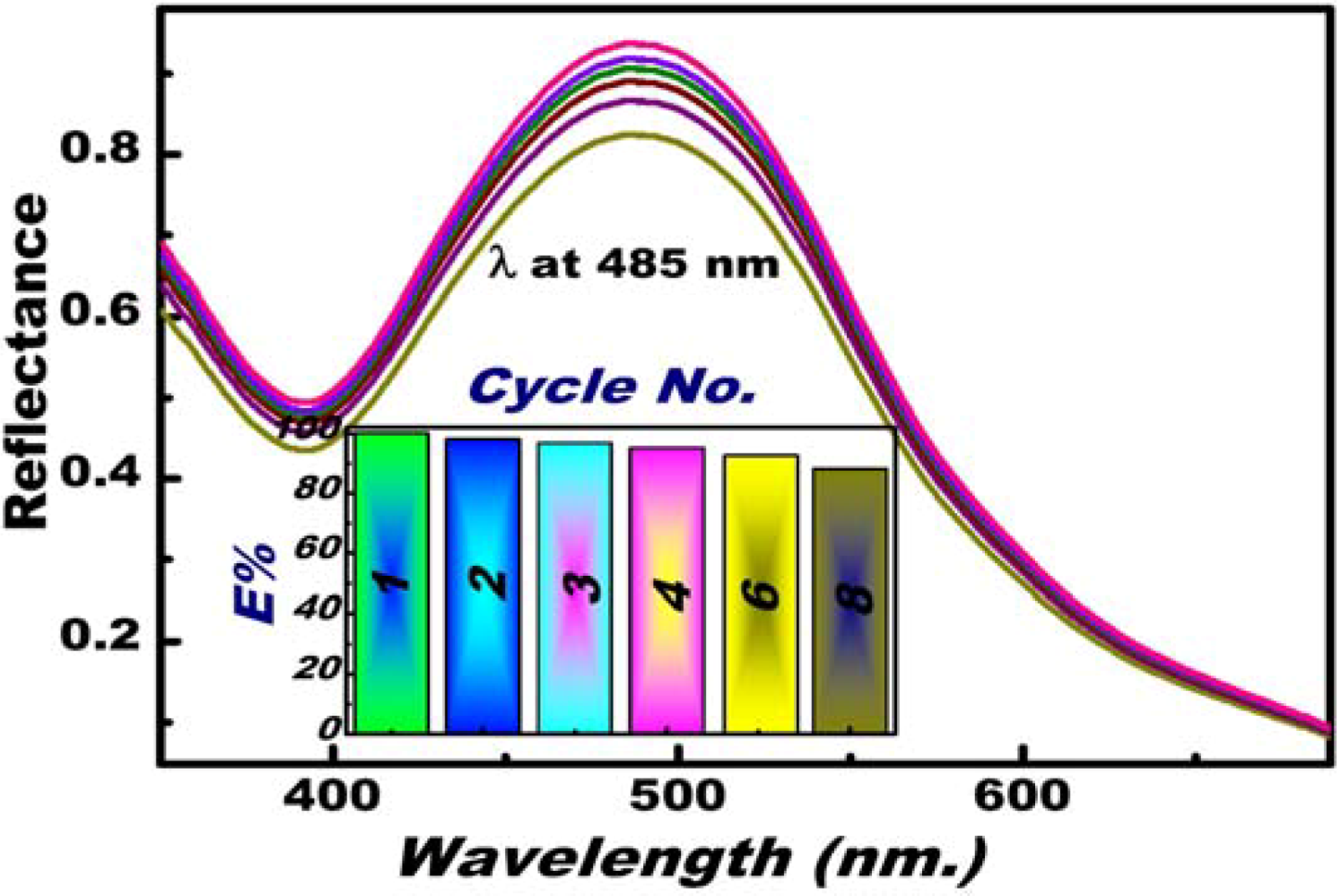
3.4. Feature and Functionality of the Hg(II) Ion–OSM Sensing System
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bessbousse, H.; Rhlalou, T.; Verchere, J.-F.; Lebrun, L. Sorption and filtration of Hg (II) ions from aqueous solutions with a membrane containing poly (ethyleneimine) as a complexing polymer. J. Membr. Sci. 2008, 325, 997–1006. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Prabhakaran, D.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Three-dimensional wormhole and ordered mesostructures and their applicability as optically ion-sensitive probe templates. Chem. Mater. 2008, 20, 2644–2654. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Ismael, M.; Khairy, M.; Awual, M.R. Optical mesosensors for monitoring and removal of ultra-trace concentration of Zn(II) and Cu(II) ions from water. Analyst 2012, 137, 5208–5214. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A. Envirnmental remediation and monitoring of cadmium. TrAC Trends Anal. Chem. 2014, 62, 56–68. [Google Scholar]
- El-Safty, S.A.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Optical nanosensor design with uniform pore geometry and large particle morphology. Chem. Eur. J. 2007, 13, 9245–9255. [Google Scholar]
- Elshehy, E.A.; El-Safty, S.A.; Shenashen, M.A.; Khairy, M. Design and evaluation of optical mesocaptor for the detection/recovery of Au(III) from an urban mine. Sensor Actuat. B-chem. 2014, 203, 363–374. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Mercury-ion optical sensors. TrAC Trend Anal. Chem. 2012, 38, 98–115. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Organic-Inorganic Mesoporous Monolithic Scaffolds and Their Functionality in Ion-Sensitive Removal of Mercury Ions. In Mercury; Source, Application and Health Impacts; NOVA Science Publishers: New York, USA, 2013; pp. 25–65. [Google Scholar]
- Basu, N.; Kwan, M.; Chan, H.M. Mercury but not organochlorines inhibits muscarinic cholinergic receptor binding in the cerebrum of ringed seals (Phoca hispida). J. Toxicol. Environ. Health Part A 2006, 69, 1133–1143. [Google Scholar] [CrossRef]
- Ros-Lis, J.V.; Marcos, M.D.; Mártinez-Mánez, R.; Rurack, K.; Soto, J. A regenerative chemodosimeter based on metal induced dye formation for the highly selective and sensitive optical determination of Hg2+ ions. Angew. Chem. Int. Ed. 2005, 44, 4405–4407. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- World Health Organization. Mercury in Drinking-Water: 2005. WHO/SDE/WSH/05.08/10.
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters-A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef]
- Cerutti, S.; Silva, M.F.; Gásquez, J.A.; Olsina, R.A.; Martinez, L.D. On-line preconcentration/determination of cadmium in drinking water on activated carbon using 8-hydroxyquinoline in a flow injection system coupled to an inductively coupled plasma optical emission spectrometer. Spectrochim. Acta Part B: At. Spectrosc. 2003, 58, 43–50. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, I.; Lobana, T.S. A mercury (II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta 2003, 59, 101–105. [Google Scholar] [CrossRef]
- Puk, R.; Weber, J.H. Determination of mercury (II), monomethylmercury cation, dimethylmercury and diethylmercury by hydride generation, cryogenic trapping and atomic absorption spectrometric detection. Anal. Chim. Acta 1994, 292, 175–183. [Google Scholar] [CrossRef]
- Bloxham, M.J.; Hill, S.J.; Worsfold, P.J. Atomic spectrometry update advances in atomic absorption and fluorescence spectrometry and related techniques. J. Anal. At. Spectrom. 1996, 11, 511–514. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Abdelllatef, A.; Ismeal, M.; Shahat, A. Optical Nanosphere Sensor Design based Shell-By-Shell Fabrication for Removal of Toxic Metals from Human Blood. Adv. Healthcare Mater. 2013, 2, 854–862. [Google Scholar] [CrossRef]
- El-Safty, S.A. Organic–inorganic hybrid mesoporous monoliths for selective discrimination and sensitive removal of toxic mercury ions. J. Mater. Sci. 2009, 44, 6764–6774. [Google Scholar] [CrossRef]
- Balaji, T.; El-Safty, S.A.; Matsunaga, H.; Hanaoka, T.; Muzukami, F. Optical sensors based on nanostructured cage materials for the detection of toxic metal ions. Angew. Chem. Int. Ed. 2006, 45, 7260–7266. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Khairy, M. Optical detection/collection of toxic Cd(II) ions using cubic Ia3d aluminosilica mesocage sensors. Talanta 2012, 98, 69–78. [Google Scholar] [CrossRef]
- Henke, K.R.; Robertson, D.; Krepps, M.K.; Atwood, D.A. Chemistry and stability of precipitates from aqueous solutions of 2,4,6-trimercaptotriazine, trisodium salt, nonahydrate (TMT-55) and mercury (II) chloride. Water Res. 2000, 34, 3005–3013. [Google Scholar] [CrossRef]
- Chiarle, S.; Ratto, M.; Rovatti, M. Mercury removal from water by ion exchange resins adsorption. Warer Res. 2000, 34, 2971–2978. [Google Scholar] [CrossRef]
- Chen, K.H.; Lu, G.H.; Chang, J.B.; Mao, S.; Yu, K.H.; Cui, S.M.; Chen, J.H. Hg (II) ion detection using thermally reduced graphene oxide decorated with functionalized gold nanoparticles. Anal. Chem. 2012, 84, 4057–4062. [Google Scholar] [CrossRef]
- Evans, O.; McKee, G.D. Determination of mercury (II) and organomercury compounds by reversed-phase liquid chromatography with reductive electrochemical detection. Analyst 1988, 113, 243–246. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, R.S. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membrane Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Q.; Yuan, R.; Zhou, X.; Liu, H.; Shan, H.; Song, Q. A new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta 2007, 71, 68–72. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Ismael, M.; Shahat, A.; Shenashen, M.A. Mesoporous hexagonal and cubic aluminosilica adsorbents for toxic nitroanilines from water. Envir. Sci. Poll. Res. 2013, 20, 3863–3876. [Google Scholar] [CrossRef]
- Campbell, T.; Corker, J.M.; Dent, A.J.; El-Safty, S.A.; Evans, J.; Fiddy, S.G.; Newton, M.A.; Ship, C.P.; Turin, S. Synthesis, Characterization and Chemistry of Transition metals in mesoporous silica. Stud. Surf. Sci. Catal. 2001, 132, 667–672. [Google Scholar]
- El-Safty, S.A.; Hanaoka, T. Synthesis of monolithic nanostructured silicate family materials through the lyotropic liquid crystalline mesophases of non-ionic surfactant. Stud. Surf. Sci. Catal. 2003, 146, 173–176. [Google Scholar]
- El-Safty, S.A.; Mizukami, F.; Hanaoka, T. Monolithic ordered silica with large cage and cylindrical structures, and hydrothermal stable frameworks. Stud. Surf. Sci. Catal. 2005, 158A, 431–438. [Google Scholar]
- Khairy, M.; El-Safty, S.A.; Ismael, M. Mesoporous nanomagnet supercaptors for selective heme-proteins from human cells. Chem. Commun. 2012, 48, 10832–10834. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A. Selective encapsulation of hemoproteins from mammalian cells using mesoporous metal oxide nanoparticles. Coll. Surfaces B: Biointerfaces 2013, 111, 460–468. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Khairy, M. Trapping of biological macromolecules in the three-dimensional mesocage pore cavities of monolith adsorbents. J. Porous Mater. 2013, 20, 679–692. [Google Scholar] [CrossRef]
- Das, S.K.; El-Safty, S.A. Development of Mesoscopically Assembled Sulfated Zirconia Nanoparticles as Promising Heterogeneous and Recyclable Biodiesel Catalysts. ChemCatChem 2013, 5, 3050–3059. [Google Scholar] [CrossRef]
- El-Safty, S.A. Synthesis, Characterization and Catalytic Activity of Highly Ordered Hexagonal and Cubic Composite Monoliths. J. Colloid Interface Sci. 2008, 319, 477–488. [Google Scholar] [CrossRef]
- Hoa, N.D.; El-Safty, S.A. Highly sensitive and selective volatile organic compound gas sensors based on mesoporous nanocomposite monoliths. Anal. Methods 2011, 3, 1948–1956. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A. Hemoproteins–nickel foam hybrids as effective supercapacitors. Chem. Commun. 2014, 50, 1356. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Mizukami, F.; Hanaoka, T. Adsorption of Aniline onto Hexagonal Mesoporous Silicate Monoliths (HOM-2). Int. J. Envir. Poll 2008, 33, 1–4, 97–112. [Google Scholar]
- Yang, Z.L.; Niu, Z.W.; Cao, X.Y.; Yang, Z.Z.; Lu, Y.F.; Hu, Z.B.; Han, C.C. Template synthesis of uniform 1D mesostructured silica materials and their arrays in anodic alumina membranes. Angew. Chem. Int. Ed. 2003, 42, 4201–4203. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Sirkar, K.K. Membrane Handbook; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Martin, C.R. Nanomaterials-a membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Gong, Z.H.; Ji, G.B.; Zheng, M.B.; Chang, X.F.; Dai, W.J.; Pan, L.J.; Shi, Y.; Zheng, Y.D. Structural characterization of mesoporous silica nanofibers synthesized within porous alumina membranes. Nanoscale Res. Lett. 2009, 4, 1257–1262. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shahat, A.; Warkocki, W.; Ohnuma, M. Building-block-based mosaic cage silica nanotubes for molecular transport and separation. Small 2011, 7, 62–65. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Hoa, N.D.; Shenashen, M.A. Topical developments of nanoporous membrane filters for ultrafine noble metal nanoparticles. Eur. J. Inorg. Chem. 2012, 9, 2288–2296. [Google Scholar]
- Anthony, Y.K.; Seth, T.T.; Sergio, M.L. Mesoporous silica composites containing multiple regions with distinct pore size and complex pore organization. J. Am. Chem. Soc. 2005, 127, 6934–6935. [Google Scholar] [CrossRef]
- Keilbach, A.; Moses, J.; Khn, R.; Dblinger, M.; Bein, T. Electrodeposition of copper and silver nanowires in hierarchical mesoporous silica/anodic alumina nanostructures. Chem. Mater. 2010, 22, 5430–5436. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Size-selective separations of biological macromolecules on mesocylinder silica arrays. Anal. Chim. Acta 2011, 694, 151–161. [Google Scholar] [CrossRef]
- Sandell, E.B. Colorimetric Determination of Traces of Metals, 3th ed.; Interscience Publisher: New York, NY, USA, 1959; p. 326. [Google Scholar]
- El-Safty, S.A.; Shenashen, M.A.; Shahat, A. Tailor-made micro-object optical sensor based on mesoporous pellets for visual monitoring and removal of toxic metal ions from aqueous media. Small 2013, 9, 2288–2296. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A. Hierarchical inorganic–organic multi-shell nanospheres for intervention and treatment of lead-contaminated blood. Nanoscale 2013, 5, 7920–7927. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Elshehy, E.A.; El-Safty, S.A.; Khairy, M. Visual monitoring and removal of divalent copper, cadmium, and mercury ions from water by using mesoporous cubic Ia3d aluminosilica sensors. Sep. Purif. Technol. 2013, 116, 73–86. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Architecture of optical sensor for recognition of multiple toxic metal ions from water. J. Hazard. Mater. 2013, 260, 833–843. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Optical mesosensor for capturing of Fe(III) and Hg(II) ions from water and physiological fluids. Sensor Actuat. B-chem. 2013, 183, 58–70. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Shahat, A.; El-Safty, S.A. Ultra-trace recognition and removal of toxic chromium (VI) ions from water using visual mesocaptor. J. Hazard. Mater. 2013, 244–245, 726–735. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A. Simultaneous detection and removal of cadmium ions from different environmental matrices. J. Life Cycle Assessment 2014, 10, 126–141. [Google Scholar]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Ravikovitch, P.I.; de Jong, K.P.; Benjelloun, M.; van Bavel, E.; Janssen, A.H.; Neimark, A.V.; Weckhuysen, B.M.; Vansant, E.F. A New templated ordered structure with combined micro- and mesopores and internal silica nanocapsules. J. Phys. Chem. B 2002, 106, 5873–5877. [Google Scholar] [CrossRef]
- Cruz-Chu, E.R.; Aksimentiev, A.; Schulten, K. Ionic current rectification through silica nanopores. J. Phys. Chem. C 2009, 113, 1850–1862. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Ismael, M.; Khairy, M.; Awual, M.R. Mesoporous aluminosilica sensors for the visual removal and detection of Pd(II) and Cu(II) ions. Micropor. Mesopor. Mater. 2013, 166, 195–205. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshehy, E.A.; EL-Safty, S.A.; Shenashen, M.A. Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions. Chemosensors 2014, 2, 219-234. https://doi.org/10.3390/chemosensors2040219
Elshehy EA, EL-Safty SA, Shenashen MA. Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions. Chemosensors. 2014; 2(4):219-234. https://doi.org/10.3390/chemosensors2040219
Chicago/Turabian StyleElshehy, Emad A., Sherif A. EL-Safty, and Mohamed A. Shenashen. 2014. "Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions" Chemosensors 2, no. 4: 219-234. https://doi.org/10.3390/chemosensors2040219
APA StyleElshehy, E. A., EL-Safty, S. A., & Shenashen, M. A. (2014). Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions. Chemosensors, 2(4), 219-234. https://doi.org/10.3390/chemosensors2040219






