Photonic Crystal-Based Sensing and Imaging of Potassium Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Particles
2.2.2. Determination of Particle Size and Zeta Potentials
2.2.3. Preparation of Sensor Films
2.2.4. Assembly of the Sensor Films
2.2.5. Measurements of Reflected Light of the Ion-Selective Photonic Crystal
2.2.6. Microscope Imaging Technique for Sensor Films
3. Results and Discussion
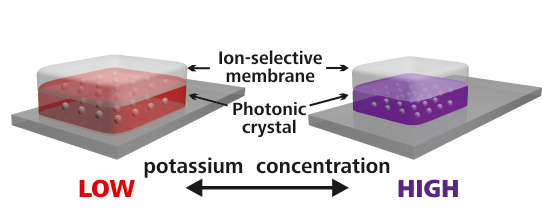
3.1. Choice of Materials and Sensor Layout
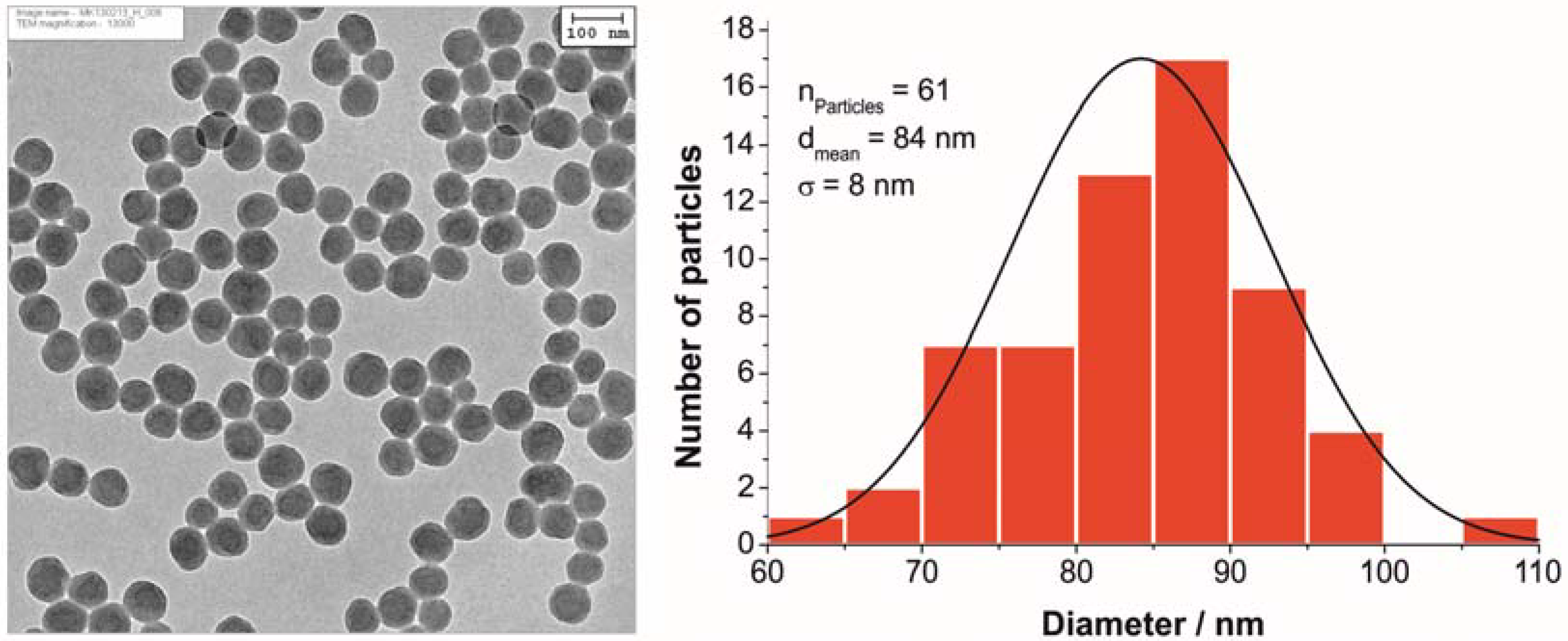
3.2. Characterization of Polystyrene Nanoparticles
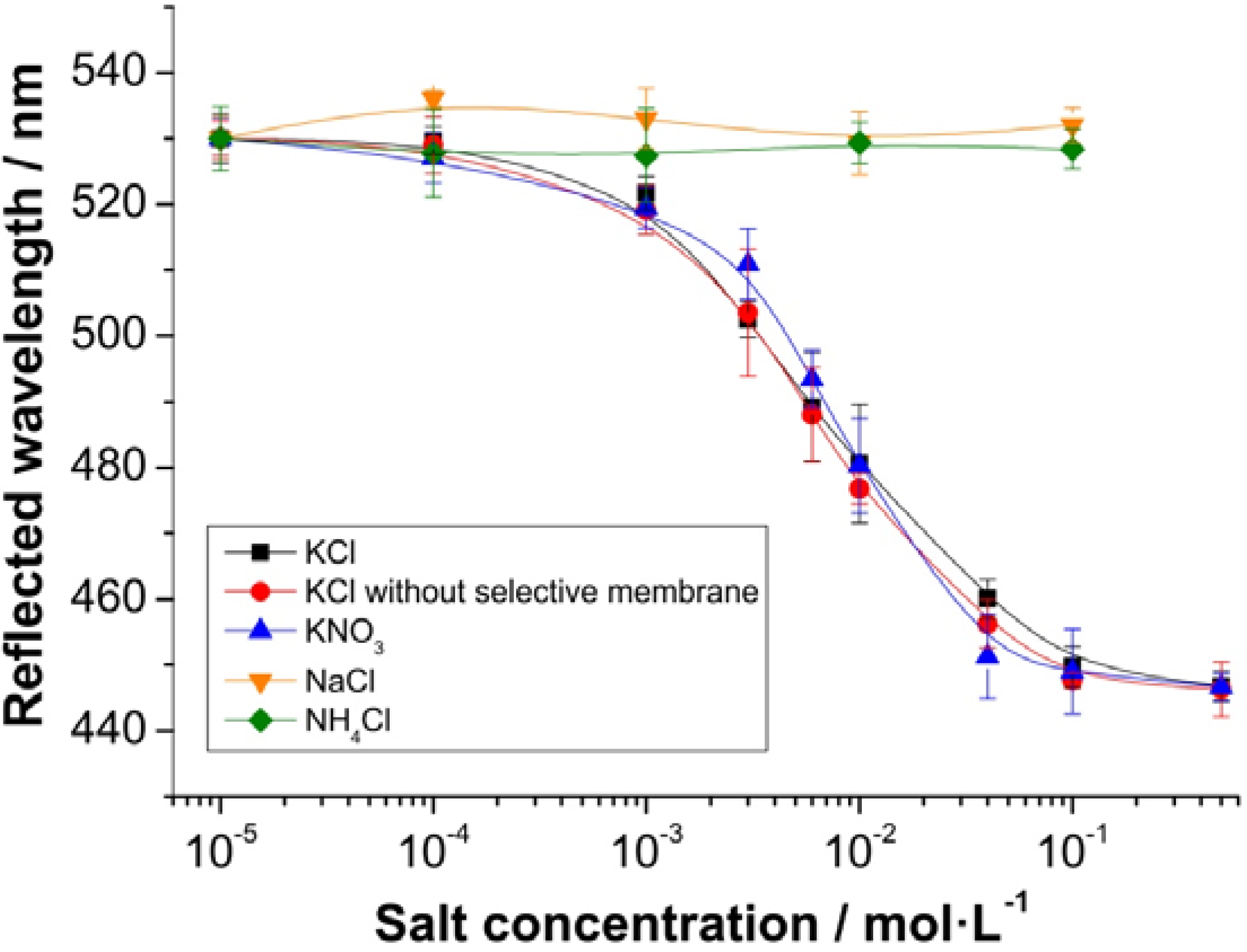
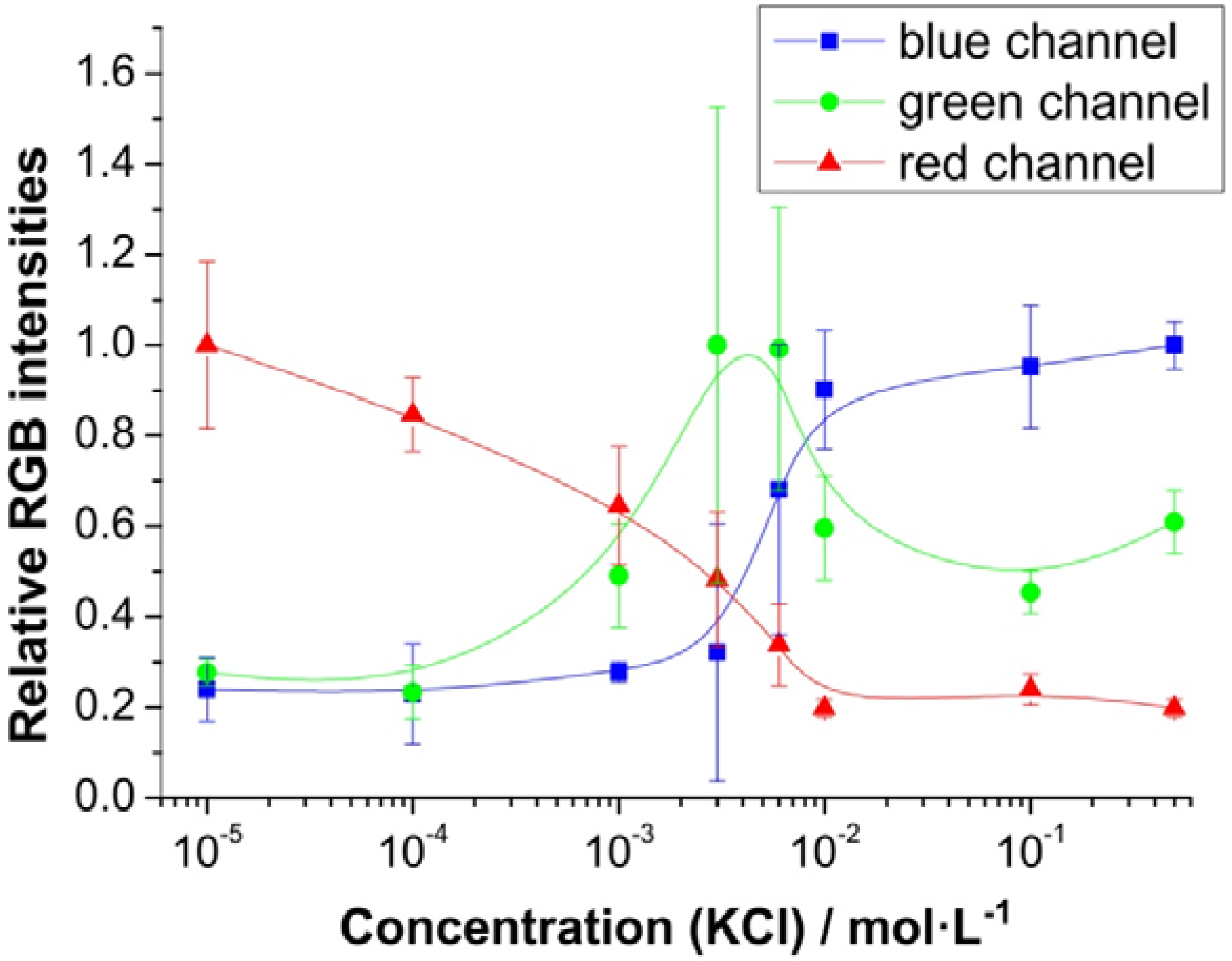
3.3. Sensing Performance
3.4. Imaging Potassium Concentrations
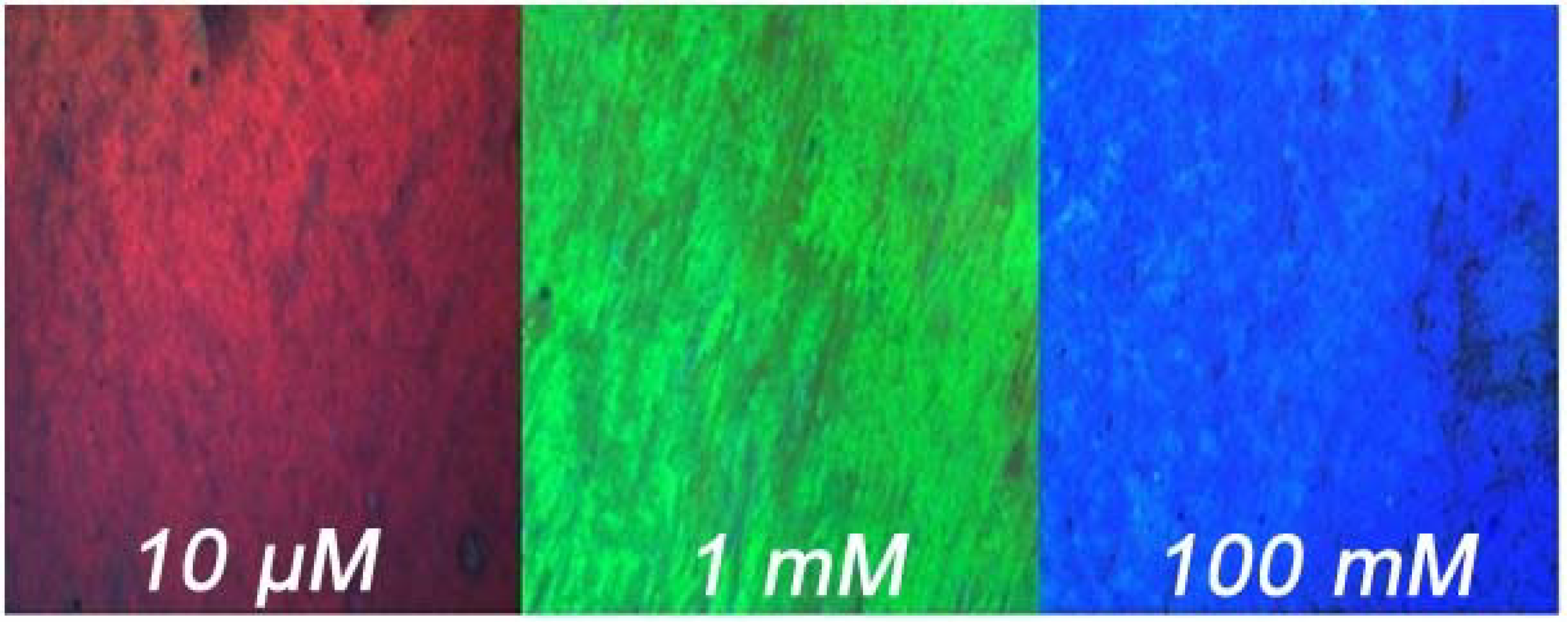
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, H.; Li, X.; Li, Y.; Fan, L.; Kraatz, H.-B. A novel colorimetric potassium sensor based on the substitution of lead from G-quadruplex. Analyst 2013, 138, 856–862. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Mortellaro, M.A.; Leiner, M.J.P.; Fraatz, R.J.; Tusa, J.K. A Fluorescent Sensor with High Selectivity and Sensitivity for Potassium in Water. J. Am. Chem. Soc. 2003, 125, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Teresa, M.; Gomes, S.R.; Tavares, K.S.; Oliveira, J.A.B.P. The quantification of potassium using a quartz crystal microbalance. Analyst 2000, 125, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, M.I.; Cid, L.P.; Barros, L.F.; Sepúlveda, F.V. Modulation of the Two-Pore Domain Acid-sensitive K+ Channel TASK-2 (KCNK5) by Changes in Cell Volume. J. Biol. Chem. 2001, 276, 43166–43174. [Google Scholar] [CrossRef] [PubMed]
- Young, D.B.; Ma, G. Vascular protective effects of potassium. Semin. Nephrol. 1999, 19, 477–486. [Google Scholar] [PubMed]
- Shieh, C.C.; Coghlan, M.; Sullivan, J.P.; Gopalakrishnan, M. Potassium Channels: Molecular Defects, Diseases, and Therapeutic Opportunities. Pharmacol. Rev. 2000, 52, 557–594. [Google Scholar] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, F.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A New Highly Selective Fluorescent K+ Sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef] [PubMed]
- Soldin, S.J.; Brugnara, C.; Wong, E.C. Pediatric Reference Ranges; American Association for Clinical Chemistry: Washington, DC, USA, 2003. [Google Scholar]
- Qin, W.; Zwickl, T.; Pretsch, E. Improved Detection Limits and Unbiased Selectivity Coefficients Obtained by Using Ion-Exchange Resins in the Inner Reference Solution of Ion-Selective Polymeric Membrane Electrodes. Anal. Chem. 2000, 72, 3236–3240. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Werner, T.; Huber, C.; Wolfbeis, O.S.; Leiner, M.J.P. pH-Insensitive Ion Selective Optode: A Coextraction-Based Sensor for Potassium Ions. Anal. Chem. 1999, 71, 1544–1548. [Google Scholar] [CrossRef]
- Crespo, G.A.; Bakker, E. Ionophore-based ion optodes without a reference ion: Electrogenerated chemiluminescence for potentiometric sensors. Analyst 2012, 137, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, H.; Mohr, G.; Kovacs, B.; Werner, T.; Wolfbeis, O.S. Novel type of ion-selective fluorosensor based on the inner filter effect: An optrode for potassium. Anal. Chem. 1993, 65, 123–127. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar]
- Krause, C.; Werner, T.; Huber, C.; Wolfbeis, O.S. Emulsion-Based Fluorosensors for Potassium Featuring Improved Stability and Signal Change. Anal. Chem. 1999, 71, 5304–5308. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar]
- Pal, S.; Fauchet, P.M.; Miller, B.L. 1-D and 2-D Photonic Crystals as Optical Methods for Amplifying Biomolecular Recognition. Anal. Chem. 2012, 84, 8900–8908. [Google Scholar] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O. Photonic Crystal Based Sensor for Organic Solvents and for Solvent-Water Mixtures. Sensors 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals: Molding the Flow of Light (Second Edition); Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Lee, K.; Asher, S.A. Photonic Crystal Chemical Sensors: pH and Ionic Strength. J. Am. Chem. Soc. 2000, 122, 9534–9537. [Google Scholar] [CrossRef]
- Zhang, J.T.; Smith, N.; Asher, S.A. Two-Dimensional Photonic Crystal Surfactant Detection. Anal. Chem. 2012, 84, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kim, H.J.; Sudheendra, L.; Gee, S.J.; Hammock, B.D.; Kennedy, I.M. Photonic Crystal Lab-On-a-Chip for Detecting Staphylococcal Enterotoxin B at Low Attomolar Concentration. Anal. Chem. 2013, 85, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Xu, K.; Sun, Y.; Chen, Y.; Zheng, T.; Li, J. High Sensitive Immunoassay for Multiplex Mycotoxin Detection with Photonic Crystal Microsphere Suspension Array. Anal. Chem. 2013, 85, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Chaudhery, V.; Pokhriyal, A.; George, S.; Polans, J.; Lu, M.; Tan, R.; Zangar, R.C.; Cunningham, B.T. Multiplexed Cancer Biomarker Detection Using Quartz-Based Photonic Crystal Surfaces. Anal. Chem. 2012, 84, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Wilhelm, S.; Hirsch, T.; Wolfbeis, O.S. Optical Sensing of the Ionic Strength Using Photonic Crystals in a Hydrogel Matrix. ACS Appl. Mater. Interfaces 2013, 5, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.A.; Sharma, A.C.; Goponenko, A.V.; Ward, M.M. Photonic Crystal Aqueous Metal Cation Sensing Materials. Anal. Chem. 2003, 75, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Reese, C.E.; Asher, S.A. Photonic Crystal Optrode Sensor for Detection of Pb2+ in High Ionic Strength Environments. Anal. Chem. 2003, 75, 3915–3918. [Google Scholar] [CrossRef] [PubMed]
- Arunbabu, D.; Sannigrahi, A.; Jana, T. Photonic crystal hydrogel material for the sensing of toxic mercury ions (Hg2+) in water. Soft Matter 2011, 7, 2592–2599. [Google Scholar] [CrossRef]
- Saito, H.; Takeoka, Y.; Watanabe, M. Simple and precision design of porous gel as a visible indicator for ionic species and concentration. Chem. Commun. 2003, 2126–2127. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Modern Potentiometry. Angew. Chem. Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.; Landthaler, M.; Babilas, P.; Wolfbeis, O.S. Simultaneous Photographing of Oxygen and pH In-Vivo Using Sensor Films. Angew. Chem. Int. Ed. 2011, 50, 10893–10896. [Google Scholar] [CrossRef]
- Fischer, L.H.; Karakus, C.; Meier, R.J.; Risch, N.; Wolfbeis, O.S.; Holder, E.; Schäferling, M. Referenced Dual Pressure- and Temperature-Sensitive Paint for Digital Color Camera Read Out. Chem. Eur. J. 2012, 18, 15706–15713. [Google Scholar] [PubMed]
- Rajendran, V.K.; Bakthavathsalam, P.; Ali, B.M.J. Smartphone based bacterial detection using biofunctionalized fluorescent nanoparticles. Microchim. Acta 2014, 1–7. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fenzl, C.; Kirchinger, M.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystal-Based Sensing and Imaging of Potassium Ions. Chemosensors 2014, 2, 207-218. https://doi.org/10.3390/chemosensors2030207
Fenzl C, Kirchinger M, Hirsch T, Wolfbeis OS. Photonic Crystal-Based Sensing and Imaging of Potassium Ions. Chemosensors. 2014; 2(3):207-218. https://doi.org/10.3390/chemosensors2030207
Chicago/Turabian StyleFenzl, Christoph, Michael Kirchinger, Thomas Hirsch, and Otto S. Wolfbeis. 2014. "Photonic Crystal-Based Sensing and Imaging of Potassium Ions" Chemosensors 2, no. 3: 207-218. https://doi.org/10.3390/chemosensors2030207