Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion
Abstract
:1. Introduction
1.1. Contemporaneous Monitoring of Inter-Vertebral Passive and Motor Control Systems
1.2. Variable Selection
1.3. Enhanced Functional Assessment
1.4. Repeatability
1.5. Aim of the Study
1.6. Specific Objectives
- To determine whether ratios of inter-level lumbar paraspinal sEMG amplitudes are related to the IV-RoMmax at lumbar inter-vertebral levels.
- To determine whether changes in sEMG amplitudes during different phases of the forward bending cycle are related to IV-RoMmax at lumbar inter-vertebral levels.
2. Experimental Section
2.1. Participants
| Inclusion | Exclusion |
|---|---|
| Males aged 20–40 years | Poor understanding of English |
| Able to understand written information | Having treatment for osteoporosis |
| Willing to participate and able to give informed consent | Recent abdominal or pelvic surgery |
| Consent to GP being informed | Previous lumbar spine surgery |
| BMI < 30 | BMI > 30 |
| No history of low back pain that prevented normal activity for at least one day in the previous year | Any medical radiation exposure in the past year or exposure in the past two years with a dose greater than 8mSv |
| Current involvement in any other research study |
2.2. Kinematic Data Collection and Processing (Quantitative Fluoroscopy)
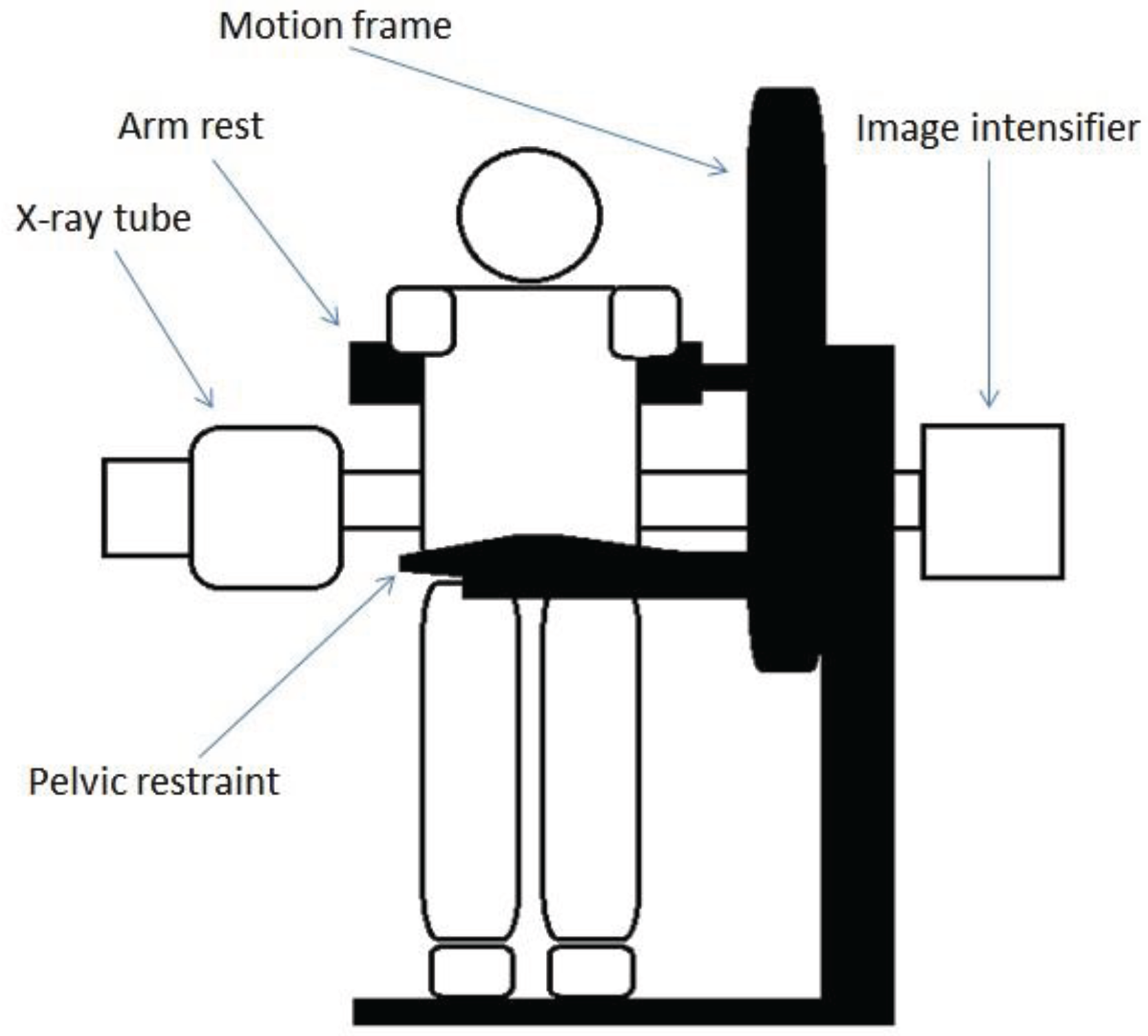
2.3. Electromyography

2.4. Electrode Positioning Accuracy

2.5. The sEMG Equipment
2.6. Reference Contraction
2.7. Synchronisation
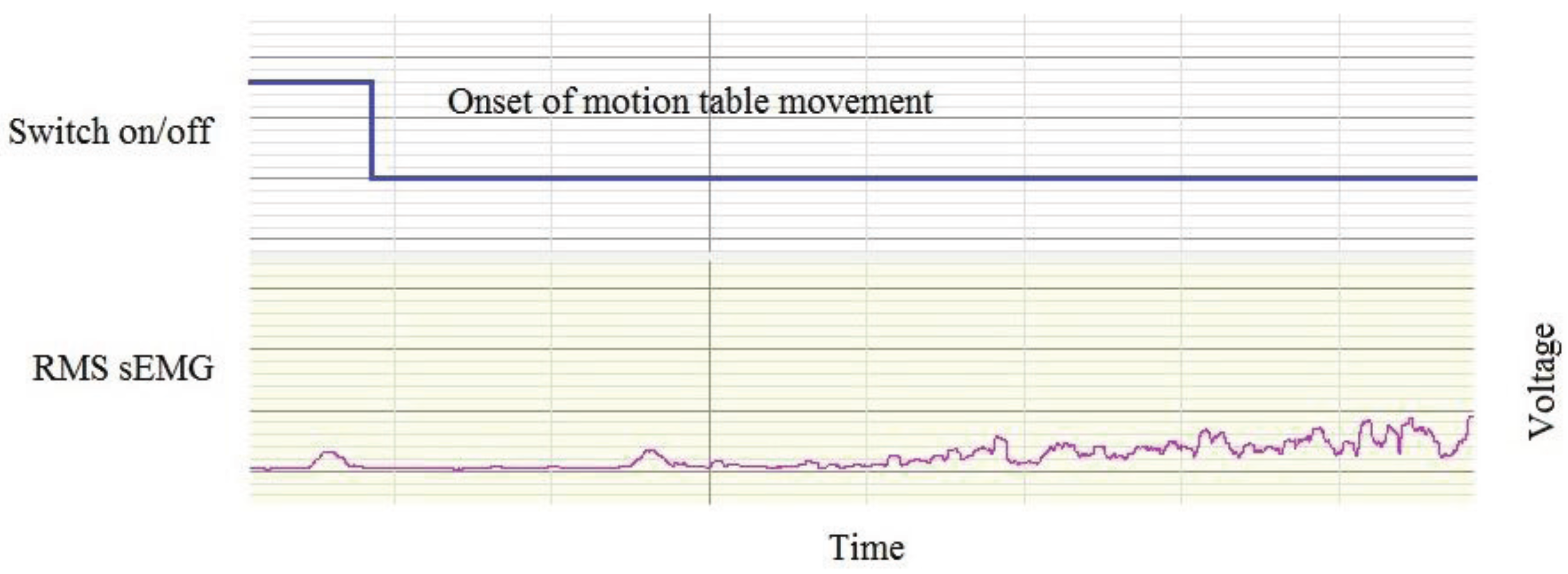
2.8. The sEMG Amplitude Repeatability Study
2.9. Data Analysis
3. Results and Discussion
3.1. Results
3.1.1. Reliability and Agreement
| Intra-Session ICC (3, 1) (95% CI) | Inter-Session ICC (3, 1) (95% CI) | Intra-Session SEM (%) | Inter-Session SEM (%) | |
|---|---|---|---|---|
| TES | 0.996 (0.986–0.999) | 0.895 (0.606–0.974) | 0.5 | 2.7 |
| LES | 0.984 (0.939–0.996) | 0.872 (0.508–0.968) | 1.2 | 3.9 |
| LMU | 0.990 (0.961–0.998) | 0.974 (0.902–0.993) | 1.4 | 2.8 |
3.1.2. Correlations between Muscle Activity Changes and IV-RoMmax
| Inter-Vertebral level | |||||
|---|---|---|---|---|---|
| Muscle activity change | L2-L3 | L3-L4 | L4-L5 | L5-S1 | |
| TES epoch 1-2 | r | 0.404 | 0.316 | −0.164 | 0.224 |
| p | 0.097 | 0.201 | 0.516 | 0.371 | |
| TES epoch 2-3 | r | 0.083 | −0.02 | 0.036 | −0.477 |
| p | 0.743 | 0.938 | 0.888 | 0.045 | |
| TES epoch 3-4* | r | −0.059 | −0.077 | −0.171 | −0.434 |
| p | 0.817 | 0.760 | 0.496 | 0.072 | |
| TES epoch 4-5 | r | −0.124 | −0.194 | −0.134 | −0.103 |
| p | 0.625 | 0.441 | 0.596 | 0.683 | |
| LES epoch 1-2* | r | −0.203 | 0.070 | 0.595 | 0.391 |
| p | 0.418 | 0.782 | 0.009 | 0.108 | |
| LES epoch 2-3 | r | −0.045 | 0.257 | 0.295 | 0.497 |
| p | 0.86 | 0.303 | 0.234 | 0.036 | |
| LES epoch 3-4 | r | −0.117 | −0.118 | 0.211 | 0.266 |
| p | 0.645 | 0.642 | 0.4 | 0.286 | |
| LES epoch 4-5* | r | 0.228 | 0.215 | −0.088 | −0.055 |
| p | 0.362 | 0.392 | 0.729 | 0.829 | |
| LMU epoch 1-2 | r | 0.14 | 0.334 | 0.314 | −0.144 |
| p | 0.58 | 0.176 | 0.204 | 0.567 | |
| LMU epoch 2-3* | r | 0.021 | 0.062 | 0.317 | 0.139 |
| p | 0.935 | 0.807 | 0.200 | 0.581 | |
| LMU epoch 3-4 | r | −0.039 | 0.164 | 0.455 | 0.273 |
| p | 0.877 | 0.517 | 0.058 | 0.272 | |
| LMU epoch 4-5 | r | −0.159 | 0.067 | 0.429 | 0.461 |
| p | 0.53 | 0.793 | 0.076 | 0.027 | |
| Variable | Inter-Vertebral Level | r | p | r² |
|---|---|---|---|---|
| LMU Epoch 4-5 | L5-S1 | 0.461 | 0.027 | 0.212 |
| LES Epoch 2-3 | L5-S1 | 0.497 | 0.036 | 0.247 |
| TES Epoch 2-3 | L5-S1 | −0.477 | 0.045 | 0.227 |
| LES Epoch 1-2* | L4-5 | 0.595 | 0.009 | 0.177 |
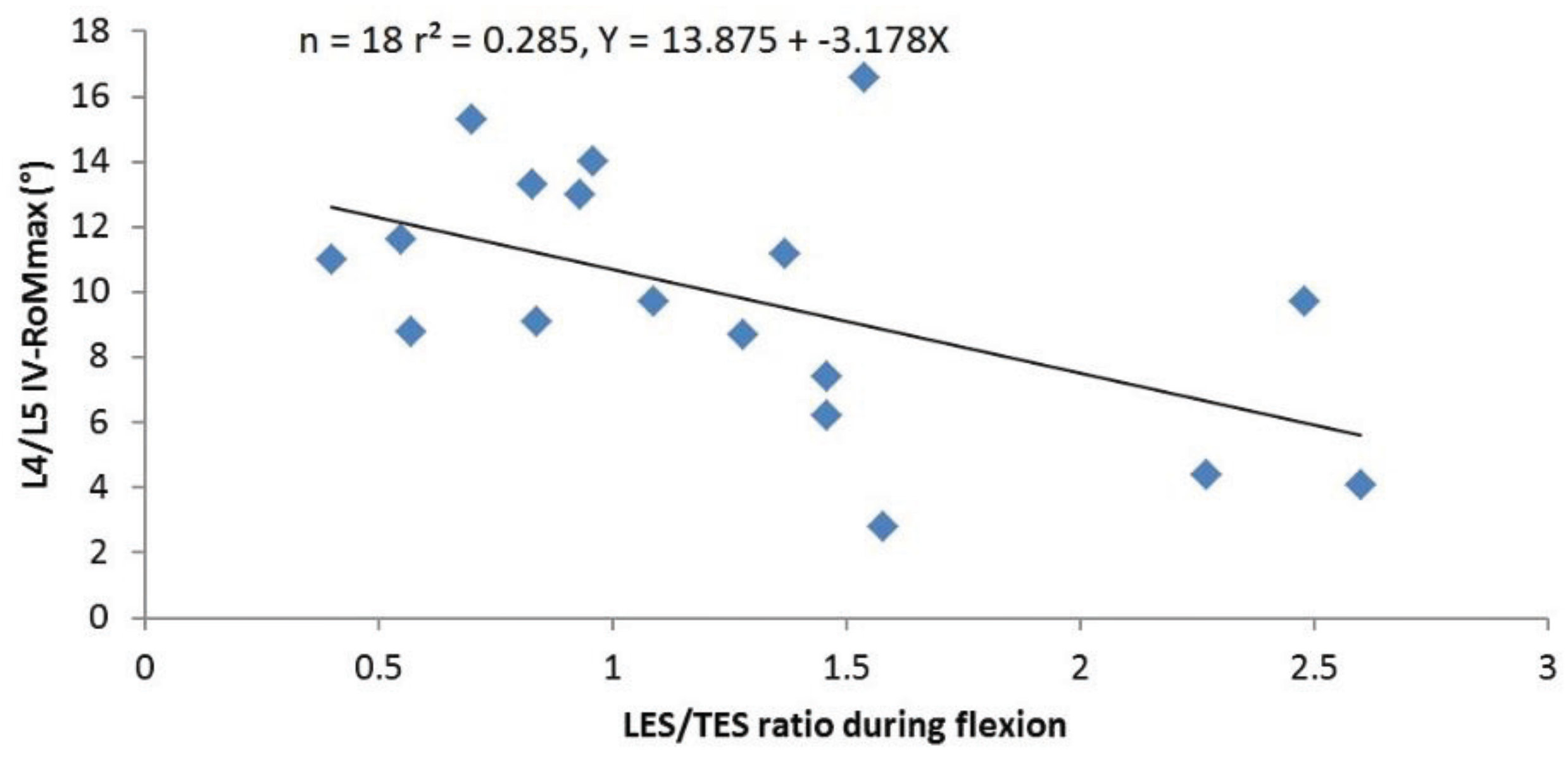
3.1.3. Correlations between sEMG Ratios and IV-RoMmax
| Inter-Vertebral Level | |||||
|---|---|---|---|---|---|
| Ratio | L2-L3 | L3-L4 | L4-L5 | L5-S1 | |
| LMU/TES | r | 0.046 | −0.013 | −0.236 | 0.152 |
| p | 0.856 | 0.958 | 0.345 | 0.548 | |
| LMU/LES | r | −0.209 | 0.04 | 0.263 | 0.37 |
| p | 0.405 | 0.875 | 0.292 | 0.13 | |
| LES/TES | r | 0.095 | −0.217 | −0.533 | −0.242 |
| p | 0.708 | 0.387 | 0.023 | 0.333 | |
3.2. Discussion
3.2.1. Reliability and Agreement
3.2.2. Changes in sEMG Amplitudes at Different Stages of the Flexion Cycle
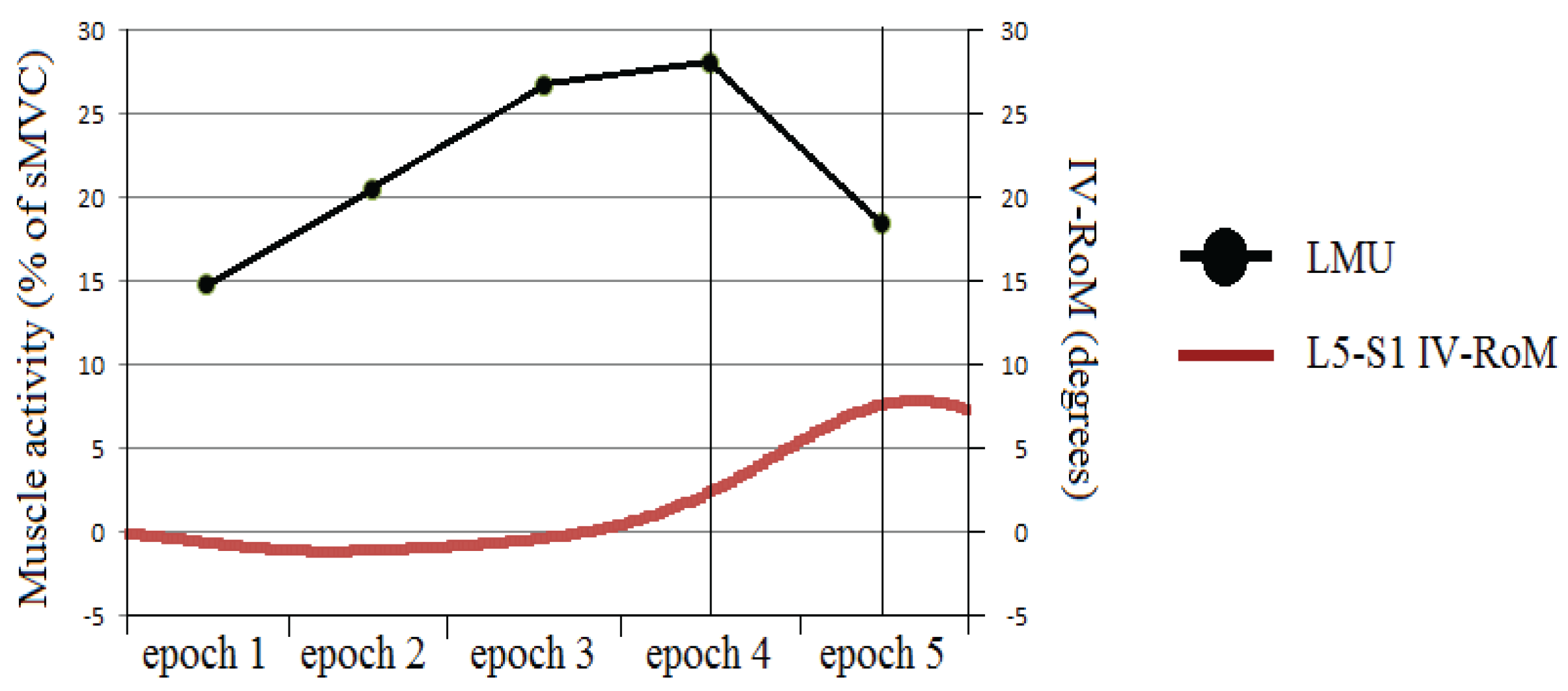
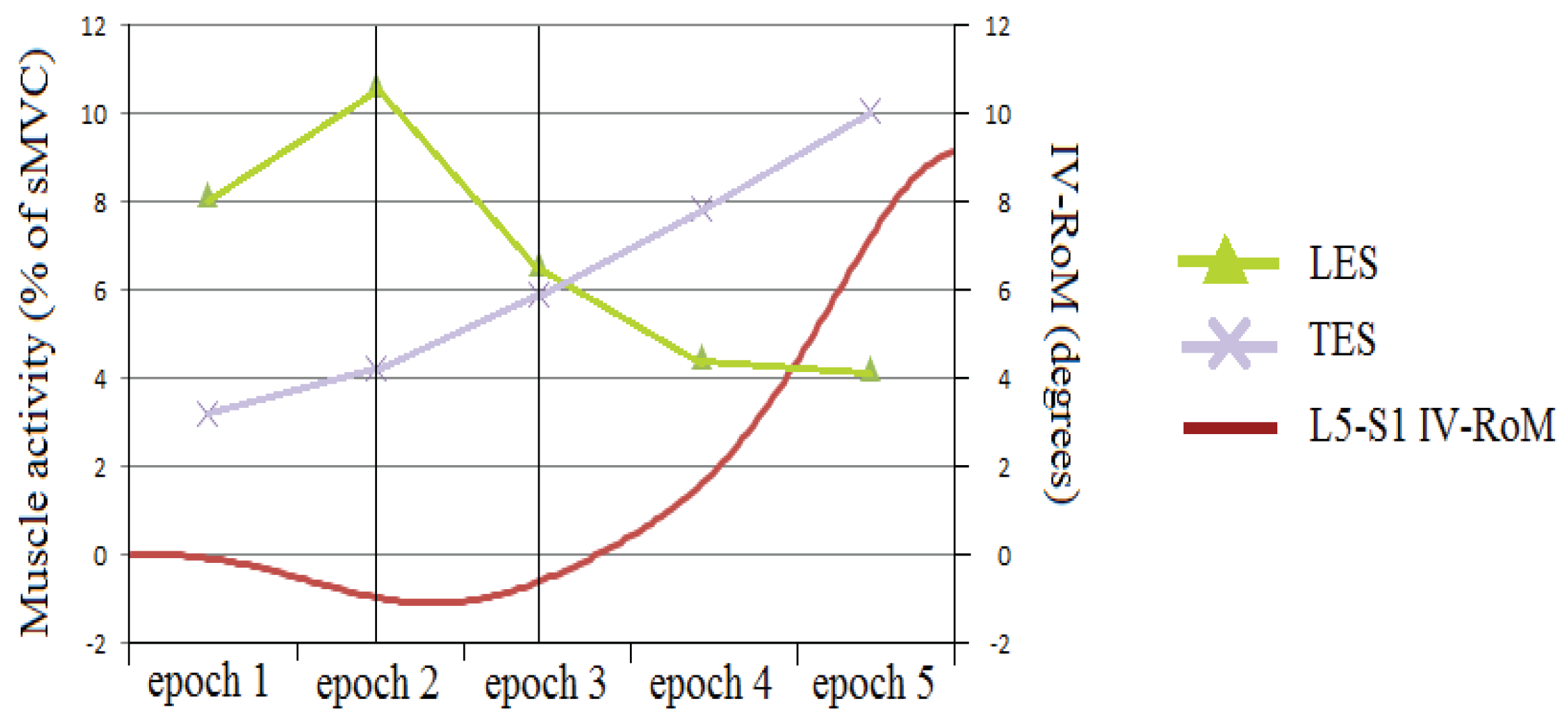

3.2.3. The sEMG Ratios
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AB | Alan Breen |
| ADR | Alister du Rose |
| AECC | Anglo-European College of Chiropractic |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CMRR | Common Mode Rejection Ratio |
| CNS | Central Nervous System |
| EAC | European Academy of Chiropractic |
| EMG | Electromyography |
| FRP | Flexion relaxation phenomenon |
| GP | General Practitioner |
| IAR | Instant axis of rotation |
| ICC | Intraclass Correlation Coefficient |
| IV-RoM | Inter-vertebral Range of Motion |
| IV-RoMmax | Maximum Inter-vertebral Range of Motion |
| LBP | Low Back Pain |
| LES | Lumbar erector spinae |
| LMU | Lumbar multifidus |
| NRES | National Research Ethics Service |
| QF | Quantitative Fluoroscopy |
| RMS | Root Mean Square |
| RoM | Range of Motion |
| SD | Standard Deviation |
| SEM | Standard error of measurement |
| TES | Thoracic erector spinae |
References
- Reeves, N.P.; Narendra, K.S.; Cholewicki, J. Spine stability: The six blind men and the elephant. Clin. Biomech. 2007, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilising system of the spine—Part 1: Function, dysfunction, adaptation and enhancement. J. Spinal Disord. 1992, 5, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilising system of the spine—Part 2: Neutral zone and instability hypothesis. J. Spinal Disord. 1992, 5, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Zuriaga, D.; Lopez-Pascual, J.; Garrido-Jaen, D.; Garcia-Mas, M.A. A comparison of lumbopelvic motion patterns and erector spinae behavior between asymtomatic subjects and patients with recurrent low back pain during pain-free periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yoo, W.G.; Choi, B.R. Differences between two subgroups of low back pain patients in lumbopelvic rotation and symmetry in the erector spinae and hamstring muscles during trunk flexion when standing. J. Electromyogr. Kinesiol. 2013, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Claus, A.P.; Hides, J.A.; Moseley, G.L.; Hodges, P.W. Different ways to balance the spine. Spine 2009, 34, E208–E214. [Google Scholar] [CrossRef] [PubMed]
- Hashemirad, F.; Talebian, S.; Hatef, B.; Kahlaee, A.H. The relationship between flexibility and EMG activity pattern of the erector spinae muscles during trunk flexion-extension. J. Electromyogr. Kinesiol. 2009, 19, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.F.; Cornelius, M.W.; Dankaerts, W.; O’Sullivan, P.B. Spinal kinematics and trunk muscle activity in cyclists: A comparison between healthy controls and non-specific chronic low back pain subjects—A pilot investigation. Man. Ther. 2004, 9, 211–219. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Cholewicki, J.; Peach, J.P. Methodological considerations for using inductive sensors (3SPACE ISOTRAK) to monitor 3-D orthopaedic joint motion. Clin. Biomech. 1997, 12, 190–194. [Google Scholar] [CrossRef]
- Kaigle, A.M.; Wesberg, P.; Hansson, T.H. Muscular and kinematic behavior of the lumbar spine during flexion-extension. J. Spinal Disord. 1998, 11, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.P.; Gunning, J.L.; McGill, S.M. The relationship between lumbar spine load and muscle actitivity during extensor exercises. Phys. Ther. 1998, 78, 8–18. [Google Scholar] [PubMed]
- Peach, J.P.; Sutarno, C.G.; McGill, S.M. Three-dimensional kinematics and trunk muscle myoelectric activity in the young lumbar spine: A database. Arch. Phys. Med. Rehabil. 1998, 79, 663–669. [Google Scholar] [CrossRef]
- Dankaerts, W.; O’Sullivan, P.B.; Burnett, A.F.; Straker, L.M.; Davey, P.; Gupta, R. Discriminating healthy controls and two clinical subgroups of nonspecific chronic low back pain patients using trunk muscle activation and lumbosacral kinematics of postures and movements. Spine 2009, 34, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.C.; Teyhan, D.S.; Mellor, F.E.; Breen, A.C.; Wong, K.W.N.; Deitz, A. Measurement of intervertebral motion using quantitative fluoroscopy: Report of an international forum and proposal for use in the assessment of degenerative disc disease in the lumbar spine. Adv. Orthop. 2012. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, R.; Hodges, P.; Tsao, H.; Hall, L.; MacDonald, D.; Danneels, D. Altered trunk muscle coordination during rapid trunk flexion in people in remission of recurrent low back pain. J. Electromyogr. Kinesiol. 2013, 23, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; van den Hoorn, W.; Dawson, A.; Cholewicki, J. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J. Biomech. 2009, 42, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Luhring, S.; Schinkel-Ivy, A.; Drake, J.D.M. Evaluation of the lumbar kinematic measures that most consistently characterize lumbar muscle activation patterns during trunk flexion: A cross-sectional study. J. Manip. Physiol. Ther. 2015, 38, 44–50. [Google Scholar] [CrossRef] [PubMed]
- McGorry, R.W.; Lin, J.H. Flexion relaxation and its relation to pain and function over the duration of a back pain episode. PLoS ONE 2012, 7, e39207. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Clinical and Radiological Anatomy of the Lumbar Spine, 5th ed.; Churchill Livingstone: London, UK, 2012. [Google Scholar]
- Okawa, A.; Shiomiya, K.; Komori, H.; Muneta, T.; Arai, Y.; Nakai, O. Dynamic motion study of the whole lumbar spine by videofluoroscopy. Spine 1998, 23, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.S.; Flynn, T.W.; Childs, J.D.; Abraham, L.D. Arthrokinematics in a subgroup of patients likely to benefit from a lumbar stabilization exercise program. Phys. Ther. 2007, 87, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Mellor, F.E.; Thomas, P.; Thompson, P.; Breen, A.C. Proportional lumbar spine inter-vertebral motion patterns: A comparison of patients with chronic non-specific low back pain and healthy controls. Eur. Spine J. 2014, 23, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M.; Spratt, K.F.; Genuario, J.; McGough, W.; Kosman, K.; Lurie, J.; Sengupta, D.K. Precision of lumbar intervertebral measurements. Spine 2011, 36, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Teyhen, D.S.; Flynn, T.W.; Bovik, A.C.; Abraham, L.D. A new technique for digital fluoroscopic video assessment of sagittal plane lumbar spine motion. Spine 2005, 30, E406–E413. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.S.; Cook, D.J.; Cheng, B.C. Reliability of computer-assisted lumbar intervertebral measurement using a novel vertebral motion analysis system. Spine J. 2014, 14, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Knutson, L.M.; Soderberg, G.L.; Ballantyne, B.T.; Clarke, W.R. A study of various normalization procedures for within day electromyographic data. J. Electromyogr. Kinesiol. 1994, 4, 47–59. [Google Scholar] [CrossRef]
- Lehman, G.J.; McGill, S.M. The importance of normalization in the interpretation of surface electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Dvorak, J.; Panjabi, M.M.; Chang, D.G.; Theiler, R.; Grob, D. Functional radiographic diagnosis of the lumbar spine. Flexion-extension and lateral bending. Spine 1991, 16, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Wong, E.; Callaghan, J.P. Is muscle co-activation a predisposing factor for low back pain development during standing? A multifactorial approach for early identification of at-risk individuals. J. Electromyogr. Kinesiol. 2010, 20, 256–263. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Roy, J.F.; Descarreaux, M. Changes in flexion-relaxation phenomenon and lumbo-pelvic kinematics following lumbar disc replacement surgery. J. Neuroeng. Rehabil. 2013. [Google Scholar] [CrossRef] [PubMed]
- Anatomy.TV. Available online: https://anatomy.tv/new_home.aspx?startapp=&startres=&startstudyguide=&S=&ReturnUrl=&lpuserid=& (accessed on 2 December 2015).
- Kim, H.W.; Ko, Y.J.; Rhee, W.I.; Lee, J.S.; Lim, J.E.; Lee, S.J.; Im, S.; Lee, J.I. Interexaminer reliability and accuracy of posterior superior iliac spine and iliac crest palpation for spinal level estimations. J. Manip. Physiol. Therap. 2007, 30, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.R.; Kuntz, A.F.; Bohlman, H.H.; Emery, S.E. Changes in the iliac crest-lumbar relationship from standing to prone. Spine J. 2006, 6, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Billis, E.V.; Foster, N.E.; Wright, C.C. Reproducibility and repeatability: Errors of three groups of physiotherapists in locating spinal levels by palpation. Man. Ther. 2003, 8, 223–232. [Google Scholar] [CrossRef]
- Chakraverty, R.; Pynsent, P.; Isaacs, K. Which spinal levels are identified by palpation of the iliac crests and the posterior superior iliac spines? J. Anat. 2007, 210, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Merz, O.; Wolf, U.; Robert, M.; Gesing, V.; Rominger, M. Validity of palpation techniques for the identification of the spinous process L5. Man. Ther. 2013, 18, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, C.; Vanderthommen, M.; Duysens, C.; Crielaard, J.M. Spinal muscle evaluation using the Sorensen test: A critical appraisal of the literature. Joint Bone Spine 2006, 73, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Van Dieen, J.H.; Cholewicki, J.; Radebold, A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine 2003, 28, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.P.; Cholewicki, J.; Silfies, S.P. Muscle activation imbalance and low-back injury in varsity athletes. J. Electromyogr. Kinesiol. 2006, 16, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E. Measurement reliability and agreement in psychiatry. Stat. Methods Med. Res. 1998, 7, 301–317. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Mellor, F.E. An Evaluation of Passive Recumbent Quantitative Fluoroscopy to Measure Mid-Lumbar Intervertebral Motion in Patients with Chronic Non-Specific Low Back Pain and Healthy Volunteers. Ph.D. Thesis, Bournemouth University, Bournemouth, UK, 2014. [Google Scholar]
- Soderberg, G.L.; Knutson, L.M. A guide for use and interpretation of kinesiologic electromyographic data. Phys. Ther. 2000, 80, 485–498. [Google Scholar] [PubMed]
- Geisser, M.E.; Ranavaya, M.; Haig, A.J.; Roth, R.S.; Zucker, R.; Ambroz, C.; Caruso, M. A meta-analytic review of surface electromyography among persons with low back pain and normal, healthy controls. J. Pain 2005, 6, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Van Dieen, J.H.; Selen, L.P.J.; Cholewicki, J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J. Electromyogr. Kinesiol. 2003, 13, 333–351. [Google Scholar] [CrossRef]
- Lariviere, C.; Arsenault, A.B. On the use of EMG-ratios to assess the coordination of back muscles. Clin. Biomech. 2008, 23, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Krag, M.H.; Dimnet, J.C.; Walter, S.D.; Brand, R.A. Thoracic spine centres of rotation in the sagittal plane. J. Orthop. Res. 1984, 1, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kaigle, A.M.; Holm, S.H.; Hansson, T.H. Experimental instability in the lumbar spine. Spine 1995, 20, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Abumi, K.; Kaneda, K.; Tadano, S.; Ukai, T. Phase lag of the intersegmental motion in flexion-extension of the lumbar and lumbosacral spine: An in vivo study. Spine 1996, 21, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, A. Stability of the lumbar spine: A study in mechanical engineering. Acta Orthop. Scand. 1989, 60, 1–54. [Google Scholar] [CrossRef]
- Crisco, J.J.; Panjabi, M.M. The intersegmental and multisegmental muscles of the lumbar spine: A biomechanical model comparing lateral stabilizing potential. Spine 1991, 16, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Cholewicki, J.; van Vliet, J.J., IV. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin. Biomech. 2002, 17, 99–105. [Google Scholar] [CrossRef]
- Pavlova, A.V.; Cooper, K.; Meakin, J.R.; Barr, R.; Aspden, R. Internal lumbar spine motion during lifting. In Proceedings of the 21st Congress of the European Society of Biomechanics, Prague, Czech Republic, 5–8 July 2015.
- Cholewicki, J.; McGill, S.M. Mechanical stability of the in vivo lumbar spine: Implications for injury and chronic low back pain. Clin. Biomech. 1996, 11, 1–15. [Google Scholar] [CrossRef]
- Van den Hoorn, W.; Bruijn, S.M.; Meijer, O.G.; Hodges, P.W.; van Dieen, J.H. Mechanical coupling between transverse plane pelvis and thorax rotations during gait is higher in people with low back pain. J. Biomech. 2012, 45, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Willigenburg, N.W.; Kingma, I.; van Dieen, J.H. Center of pressure trajectories, trunk kinematics and trunk muscle activation during unstable sitting in low back pain patients. Gait Posture 2013, 38, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.V.; Meakin, J.R.; Cooper, K.; Barr, R.J.; Aspden, R.M. The lumbar spine has an intrinsic shape specific to each individual that remains a characteristic throughout flexion and extension. Eur. Spine J. 2014, 23, S26–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, S.M.; Grenier, S.; Kavcic, N.; Cholewicki, J. Coordination of muscle activity to assure stability of the lumbar spine. J. Electromyogr. Kinesiol. 2003, 13, 353–359. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du Rose, A.; Breen, A. Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion. Healthcare 2016, 4, 4. https://doi.org/10.3390/healthcare4010004
Du Rose A, Breen A. Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion. Healthcare. 2016; 4(1):4. https://doi.org/10.3390/healthcare4010004
Chicago/Turabian StyleDu Rose, Alister, and Alan Breen. 2016. "Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion" Healthcare 4, no. 1: 4. https://doi.org/10.3390/healthcare4010004





