Variations in the Life Cycle of Anemone patens L. (Ranunculaceae) in Wild Populations of Canada
Abstract
:1. Introduction
2. Results
2.1. Life Stages
2.2. Growth Patterns
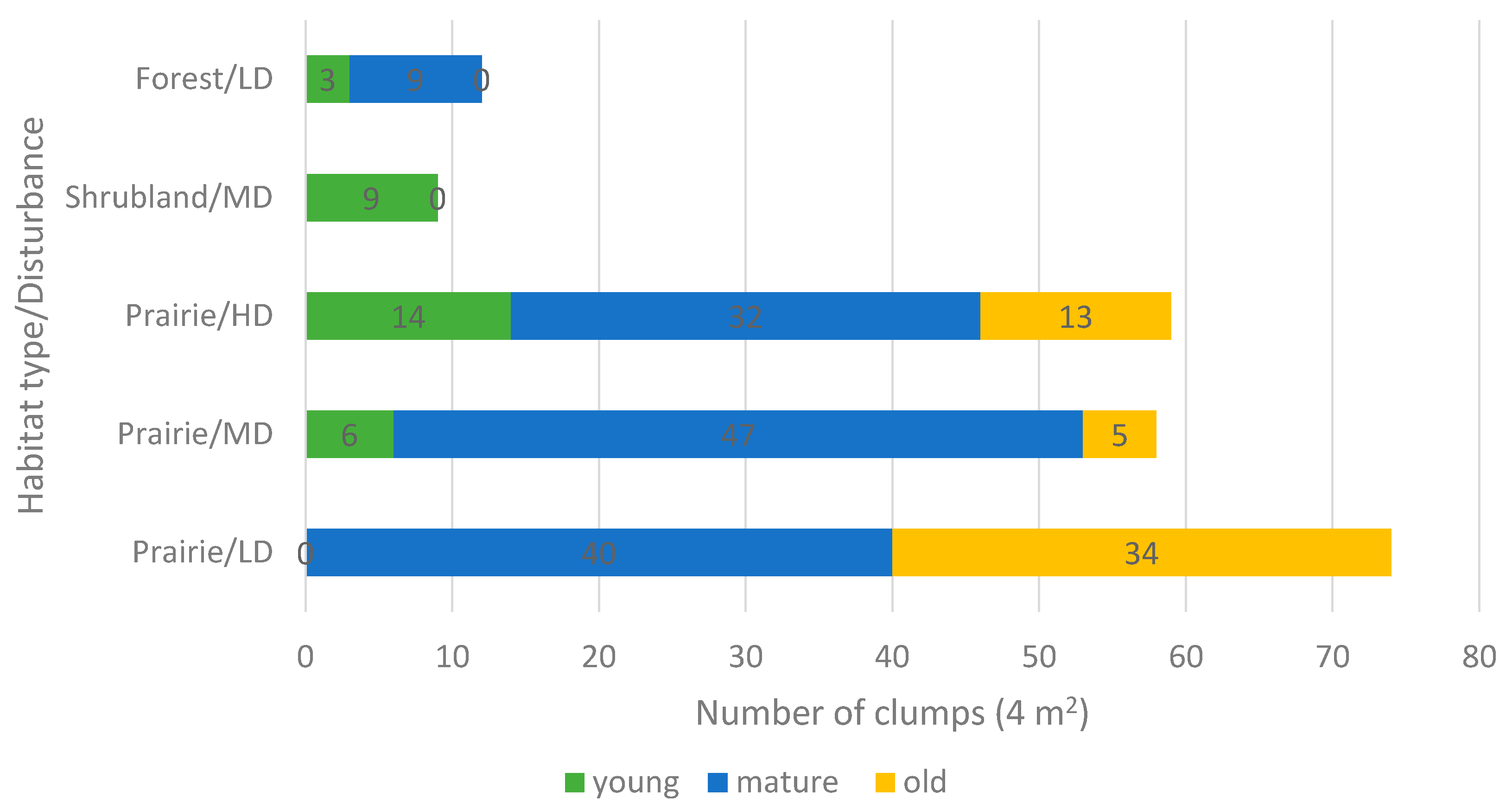
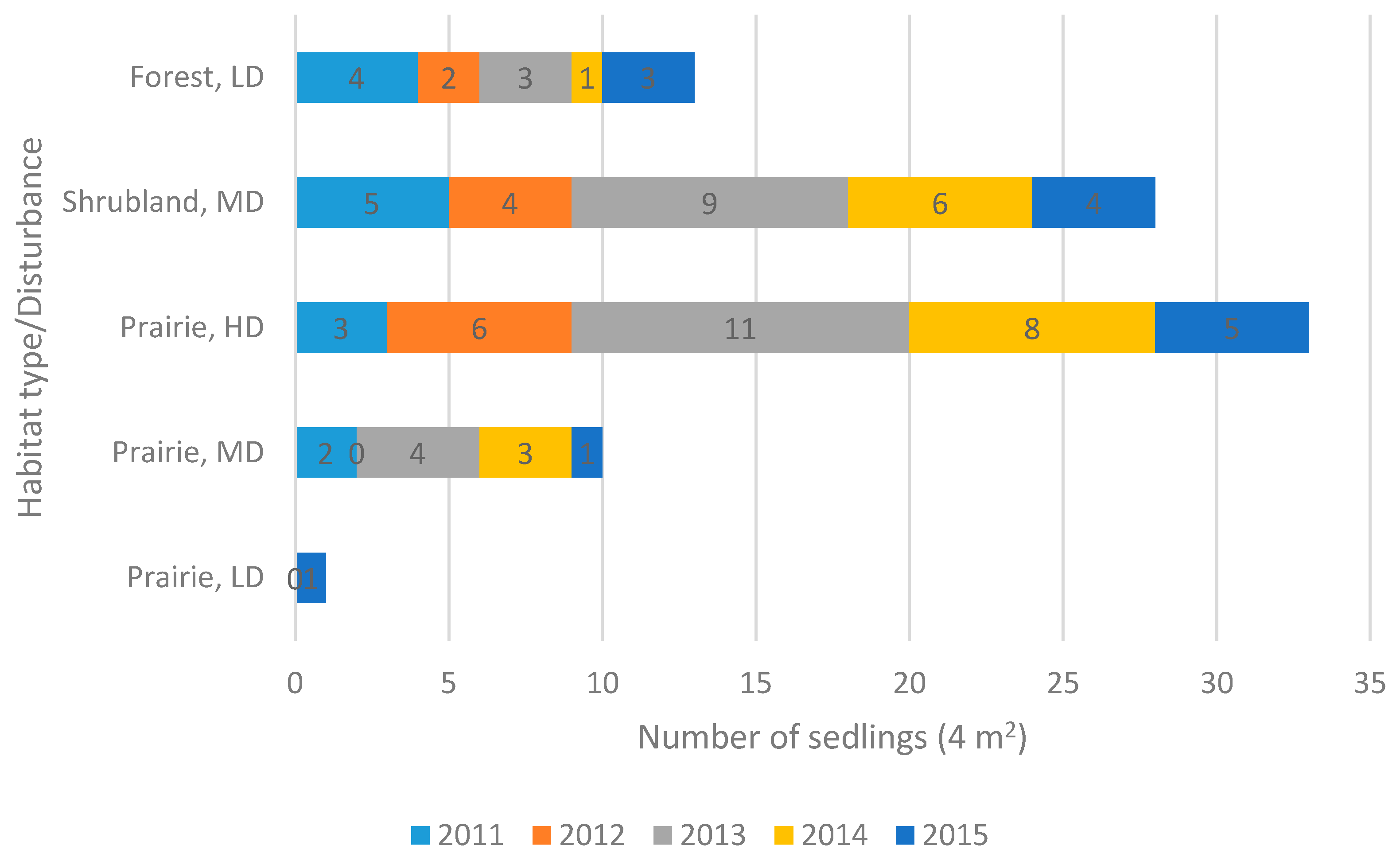
2.3. Reproduction
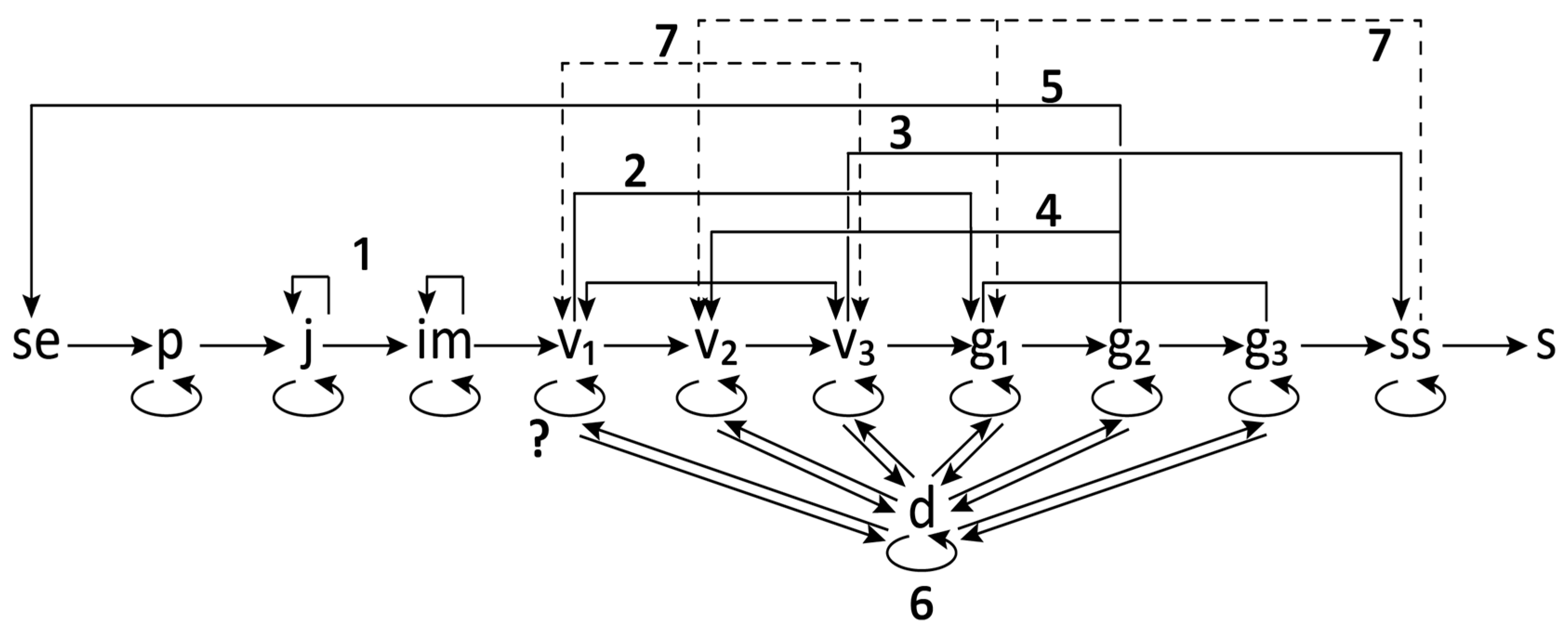
2.4. Ontogeny
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Data Collection
4.3. Data Analysis
Acknowledgments
Conflicts of Interest
References
- Rabotnov, T.A. On coenopopulations of perennial herbaceous plants in natural coenoses. Vegetatio 1969, 19, 87–95. [Google Scholar] [CrossRef]
- Rabotnov, T.A. Dynamics of plant coenotic populations. In The population Structure of Vegetation; White, J., Ed.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1985; Volume 3, pp. 121–142. [Google Scholar]
- Uranov, A.A. Age spectrum of the phytocoenopopulation as a function of time and energetic wave processes. Nauchnye Dokl. Vyss. Shkoly Biol. Nauki 1975, 2, 7–34. (In Russian) [Google Scholar]
- Gatsuk, L.E.; Smirnova, O.V.; Vorontzova, L.I.; Zaugolnova, L.B.; Zhukova, L.A. Age states of plants of various growth forms: A review. J. Ecol. 1980, 68, 675–696. [Google Scholar] [CrossRef]
- Kawano, S. Life history characteristics of temperate woodland plants in Japan. In The Population Structure of Vegetation; White, J., Ed.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1985; Volume 3, pp. 516–549. [Google Scholar]
- Kricsfalusy, V.V. Population studies of Narcissus angustifolius Curt. in the Carpathians. Features of the large life cycle. Nauchnye Dokl. Vyss. Shkoly Biol. Nauki 1987, 3, 89–96. (In Russian) [Google Scholar]
- Kricsfalusy, V.V.; Komendar, V.I. Biology and Ecology of Rare Species: A Case Study of Ephemeroid Plants in the Carpathians; Svit Press at Lvov University: Lvov, Ukraine, 1990. (In Russian) [Google Scholar]
- Oostermeijer, J.G.B.; van’t Veer, R.; den Nijs, J.C.M. Population structure of the rare, long-lived perennial Gentiana pneumonanthe in relation to vegetation and management in The Netherlands. J. Appl. Ecol. 1994, 31, 428–438. [Google Scholar] [CrossRef]
- Hegland, S.J.; van Leeuwen, M.; Oostermeijer, J.G.B. Population structure of Salvia pratensis in relation to vegetation and management of Dutch dry floodplain grasslands. J. Appl. Ecol. 2001, 38, 1277–1289. [Google Scholar] [CrossRef]
- Gregg, K.B.; Kery, M. Comparison of size vs. life-state classification in demographic models for the terrestrial orhchid Cleistes bifaria. Biol. Conserv. 2006, 129, 50–58. [Google Scholar] [CrossRef]
- Deering, R.H.; Vankat, J.L. Forest colonization and developmental growth of the invasive shrub Lonicera maackii. Am. Midl. Nat. 1999, 141, 43–50. [Google Scholar] [CrossRef]
- Paynter, Q.; Downey, P.; Sheppard, A. Age structure and growth of the woody legume weed Cytisus scoparius in native and exotic habitats: Implications for control. J. Appl. Ecol. 2003, 40, 470–480. [Google Scholar] [CrossRef]
- Bühler, C.; Schmid, B. The influence of management regime and altitude on the population structure of Succisa pratensis: Implications for vegetation monitoring. J. Appl. Ecol. 2001, 38, 689–698. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Buss, L.W.; Cook, R.L. (Eds.) Population Biology and Evolution of Clonal Organisms; Yale University Press: New Haven, YA, USA, 1985.
- Fischer, M.; Kleunen, M.V. On the evolution of clonal plant life histories. Ecology and evolutionary biology of clonal plants. Evol. Ecol. 2001, 15, 565–582. [Google Scholar] [CrossRef]
- Klimešová, J.; Pyšek, P. Current topics in clonal plants research: Editorial. Preslia 2011, 83, 275–279. [Google Scholar]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer; Koeltz Scientific Books: Königstein, Germany, 1986; Volume 1. [Google Scholar]
- Gauthier, D.; Wiken, E.B. Monitoring the conservation of grassland habitats, Prairie ecozone, Canada. Environ. Monit. Assess. 2003, 88, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Small, E.; Catling, P.M.; Brookes, B. A Biodiversity Treasure. Official Plant Emblems of Canada; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2012. [Google Scholar]
- Kricsfalusy, V.; Ponomarenko, Y. Notes on taxonomy, biology and ecology of the prairie crocus (Anemone patens L.) in Saskatchewan. Blue Jay 2013, 71, 135–148. [Google Scholar]
- NatureServe. NatureServe Explorer: An Online Encyclopedia of Life. Version 7.0. 2012. Available online: http://www.natureserve.org/explorer (accessed on 23 May 2014).
- Bilz, M.; Kell, S.P.; Maxte, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Lienert, J. Habitat fragmentation effects on fitness of plant populations—A review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Gaston, K.J.; Fuller, R.A. Commonness, population depletion and conservation biology. Trends Ecol. Evol. 2007, 23, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.S.; Shih, R.D.; Balick, M.J. Handbook of Poisonous and Injurious Plants; New York Botanical Garden Press: Bronx, NY, USA, 2007. [Google Scholar]
- Wood, M. The Book of Herbal Wisdom: Using Plants as Medicine; North Atlantic Books: Berkeley, CA, USA, 1997. [Google Scholar]
- Keane, K.; Howarth, D.; Courtney, P. The Standing People: Field Guide of Medicinal Plants for the Prairie Provinces; Root Woman & Dave: Saskatoon, SK, Canada, 2009. [Google Scholar]
- Ye, W.-C.; Ou, B.-X.; Ji, N.-N.; Zhao, S.-X.; Ye, T.; McKervey, M.V.; Stevenson, P. Patensisn, a saponin from Pulsatilla patens var. multifida. Phytochemistry 1995, 4, 937–939. [Google Scholar]
- Royal Canadian Mint. 99999 Gold Coin—Prairie Crocus. 2010. Available online: http://www.mint.ca/store/coin/99999-gold-coin-prairie-crocus-2010-prod820002#.Vms8mUorJmM (accessed on 23 May 2014).
- Bock, J.H.; Peterson, S.J. Reproductive biology of Pulsatilla patens (Ranunculaceae). Am. Midl. Nat. 1975, 94, 476–478. [Google Scholar] [CrossRef]
- Ordway, E. The phenology and population biology of Anemone patens (Ranunculaceae) in Western Minnesota. In Proceedings of the 9th North American Prairie Conference: The Prairie—Past, Present, and Future; Clambey, G.K., Pemble, R.H., Eds.; North Dakota State University Press: Fargo, ND, USA, 1986; pp. 31–34. [Google Scholar]
- Roy, B.A. A plant pathogen influences pollinator behavior and may influence reproduction of non-hosts. Ecology 1996, 77, 2445–2457. [Google Scholar] [CrossRef]
- Beaubien, E.; Hall-Beyer, M. Plant phenology in western Canada: Trends and links to the view from space. Environ. Monit. Assess. 2003, 88, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Beaubien, E.; Hamann, A. Spring flowering response to climate change between 1936 and 2006 in Alberta, Canada. Bioscience 2011, 61, 514–524. [Google Scholar] [CrossRef]
- Zimmerman, J.H. Propagation of spring prairie plants. In Proceedings of the 2nd Midwest Prairie Conference, Parkside, WI, USA, 18–20 September 1972; Zimmerman, J.H., Ed.; University of Wisconsin: Madison, WI, USA, 1972; pp. 153–161. [Google Scholar]
- Greene, H.C.; Curtis, J.T. Germination studies on Wisconsin prairie plants. Am. Midl. Nat. 1950, 43, 186–194. [Google Scholar] [CrossRef]
- Sorensen, J.T.; Holden, D.J. Germination of native prairie forb seeds. J. Range Manag. 1974, 27, 123–126. [Google Scholar] [CrossRef]
- Wildeman, A.G.; Steeves, T.A. The morphology and growth cycle of Anemone patens. Can. J. Bot. 1982, 60, 1126–1137. [Google Scholar] [CrossRef]
- Henderson, R.A. Effects of spring fire timing on pasque-flower (Anemone patens) flower-bud survival. In Proceedings of the 12th North American Prairie Conference: Recapturing a Vanishing Heritage; Smith, D.D., Jacobs, C.A., Eds.; University of Northern Iowa Press: Cedar Falls, IA, USA, 1992; pp. 117–120. [Google Scholar]
- Gross, D.; Romo, J. Temporal changes in species composition in Fescue Prairie: Relationships with burning history, time of burning, and environmental conditions. Plant Ecol. 2010, 208, 137–153. [Google Scholar] [CrossRef]
- Williams, J.L.; Crone, E.E. The impact of invasive grasses on the population growth of Anemone patens, a long-lived native forb. Ecology 2006, 87, 3200–3208. [Google Scholar] [CrossRef]
- Esparrago, J.; Kricsfalusy, V. Traditional grassland management and surrounding land use drive the abundance of a prairie plant species in an urban landscape. Landsc. Urban Plan. 2015, 142, 1–6. [Google Scholar] [CrossRef]
- Kricsfalusy, V.; Li, M.; Gupta, C. Using multiple data sources on species distribution for biodiversity assessment: The prairie crocus (Anemone patens) as a case study. Blue Jay 2015, 73, 102–122. [Google Scholar]
- Borisova, I.V. Pulsatilla patens s.l. In Steppe Vegetation of the Northern Kazakhstan; Lavrenko, E.M., Ed.; Academy of Sciences of the USSR Press: Moscow, Russia, 1961; pp. 84–87. (In Russian) [Google Scholar]
- Uotila, P. Ecology and area of Pulsatilla patens (L.) Mill. in Finland. Ann. Bot. Fenn. 1969, 6, 105–111. [Google Scholar]
- Cibanova, N.A. Life cycle and age structure of the coenopopulations of Pulsatilla patens (L.) Mill. (Ranunculaceae) in the Northern Steppe. Bot. Zhurn. 1976, 61, 1272–1277. (In Russian) [Google Scholar]
- Nikitina, S.V.; Denisova, L.V.; Vakhrameeva, M.G. Pulsatilla patens. In Biological Flora of the Moscow Region; Rabotnov, T.A., Ed.; Moscow University Press: Moscow, Russia, 1978; Part 4; pp. 79–85. (In Russian) [Google Scholar]
- Rysina, G.P. On the biology of Pulsatilla patens (L.) Mill. in the environs of Moscow. Byull. Moskovsk. Obshch. Isp. Prir. Otd. Biol. 1981, 86, 129–133. (In Russian) [Google Scholar]
- Jonsson, O.; Rosquist, G.; Widén, B. Operation of dichogamy and herkogamy in five taxa of Pulsatilla. Holarc. Ecol. 1991, 14, 260–271. [Google Scholar] [CrossRef]
- Lindell, T. Breeding systems and crossing experiments in Anemone patens and in the Anemome pulsatilla group (Ranunculaceae). Nord. J. Bot. 1998, 18, 549–561. [Google Scholar] [CrossRef]
- Pilt, I.; Kukk, U. Pulsatilla patens and Pulsatilla pratensis in Estonia: Distribution and ecology. Proc. Estonian Acad. Sci. Biol. Ecol. 2002, 51, 242–256. [Google Scholar]
- Kalamees, R.; Püssa, K.; Vanha-Majamaa, I.; Zobel, K. The effects of fire and stand age on seedling establishment of Pulsatilla patens in a pine-dominated boreal forest. Can. J. Bot. 2005, 83, 688–693. [Google Scholar] [CrossRef]
- Kalamees, R.; Püssa, K.; Tamm, S.; Zobel, K. Adaptation to boreal forest wildfire in herbs: Responses to post-fire environmental cues in two Pulsatilla species. Acta Oecol. 2012, 38, 1–7. [Google Scholar] [CrossRef]
- Kalliovirta, M.; Ryttäri, T.; Heikkinen, R.K. Population structure of a threatened plant, Pulsatilla patens, in boreal forests: Modelling relationships to overgrowth and site closure. Biodiv. Conserv. 2006, 15, 3095–3108. [Google Scholar] [CrossRef]
- Röder, D.; Kiehl, K. Population structure and population dynamic of Pulsatilla patens (L.) Mill. in relation to vegetation characteristics. Flora 2006, 201, 499–507. [Google Scholar] [CrossRef]
- Kiehl, K.; Röder, D. Successful establishment of the Natura 2000 species Pulsatilla patens (L.) Mill. in newly restored calcareous grasslands. In 6th European Conference on Ecological Restoration; Research Institute for Nature and Forest (INBO): Brussel, Belgium, 2008; pp. 1–4. Available online: http://ser.semico.be/ser-pdf/051.pdf (accessed on 23 May 2014).
- Juśkiewicz-Swaczyna, B. Population structure of Pulsatilla patens in relation to the habitat quality. Tuexenia 2010, 30, 457–466. [Google Scholar]
- Juśkiewicz-Swaczyna, B.; Choszcz, D. Effect of habitat quality on the structure of populations of Pulsatilla patens (L.) Mill. (Ranunculaceae)—Rare and endangered species in European flora. Pol. J. Ecol. 2012, 60, 567–576. [Google Scholar]
- Ziman, S.N. Morphology and Phylogeny of the Battercup Family; Naukova Dumka Press: Kiev, Ukraine, 1985. (In Russian) [Google Scholar]
- Klimešová, J.; de Bello, F. CLO-PLA: The database of clonal and bud bank traits of central European flora. J. Vegetat. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Shefferson, R.P. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. J. Ecol. 2009, 97, 1000–1009. [Google Scholar] [CrossRef]
- Tuomi, J.; Crone, E.; Gremer, J.R.; Jäkäläniemi, A.; Lesica, P.A.; Pedersen, B.; Ramula, S. Prolonged dormancy interacts with senescence for two perennial herbs. J. Ecol. 2013, 101, 566–576. [Google Scholar] [CrossRef]
- Bruynooghe, J.; Macdonald, R. (Eds.) Managing Saskatchewan Rangeland; Agriculture and Agri-Food Canada, Government of Saskatchewan: Regina, SK, Canada, 2008.
- Acton, D.F.; Padbury, G.A.; Stushnoff, C.T. The Ecoregions of Saskatchewan; Canadian Plains Research Center, University of Regina Press: Regina, SK, Canada, 1998. [Google Scholar]
- Thorpe, J. Saskatchewan Rangeland Ecosystems; Saskatchewan Research Council: Regina, SK, Canada, 2007; Publications 1–11. [Google Scholar]
- Lee, H.T.; Bakowsky, W.D.; Riley, J.; Bowles, J.; Puddiester, M.; Uhlig, P.; McMurray, S. Ecological Land Classification for Southern Ontario: First Approximation and Its Application; Ontario Ministry of Natural Resources: Peterborough, ON, Canada, 1998. [Google Scholar]

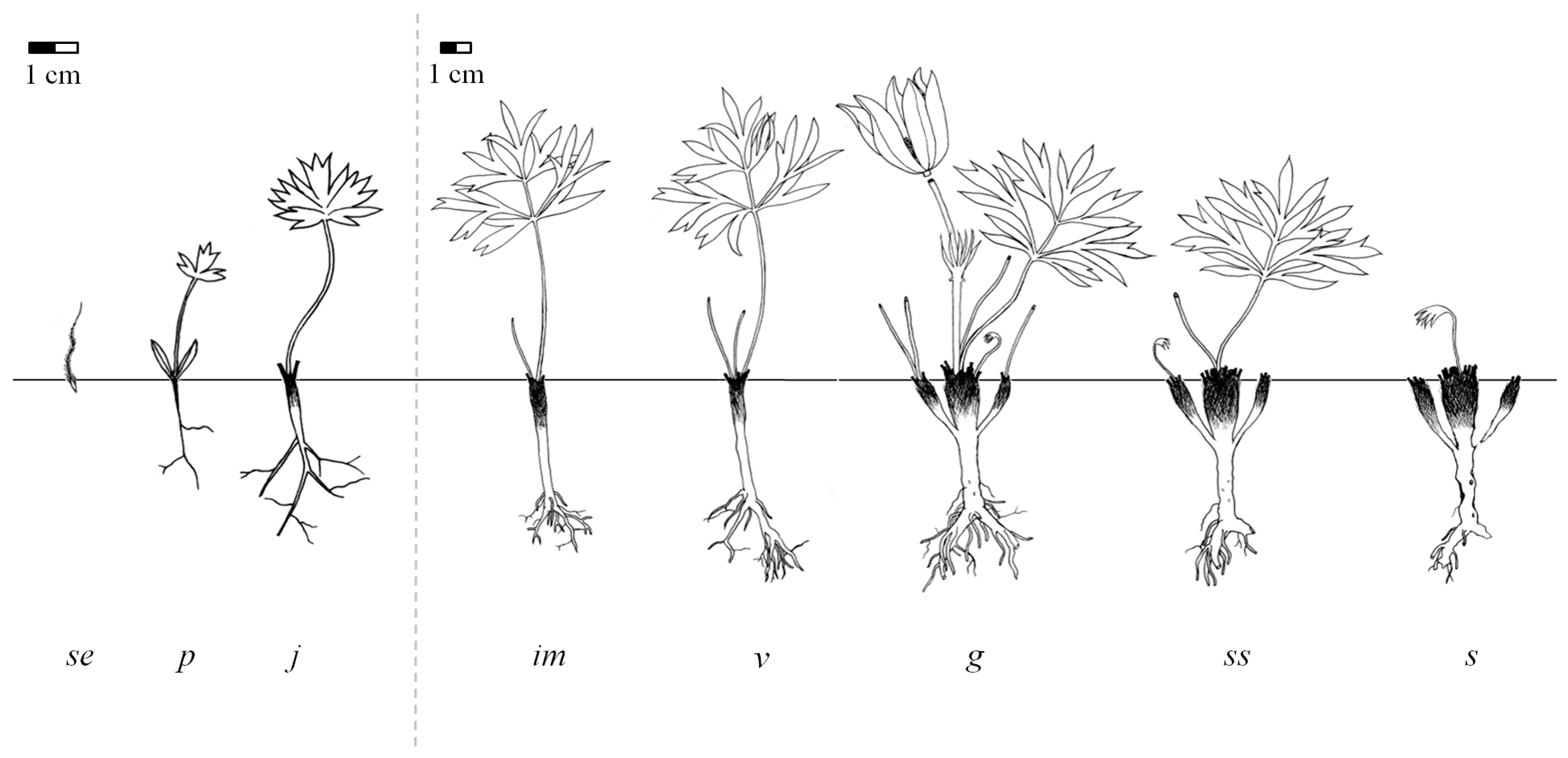
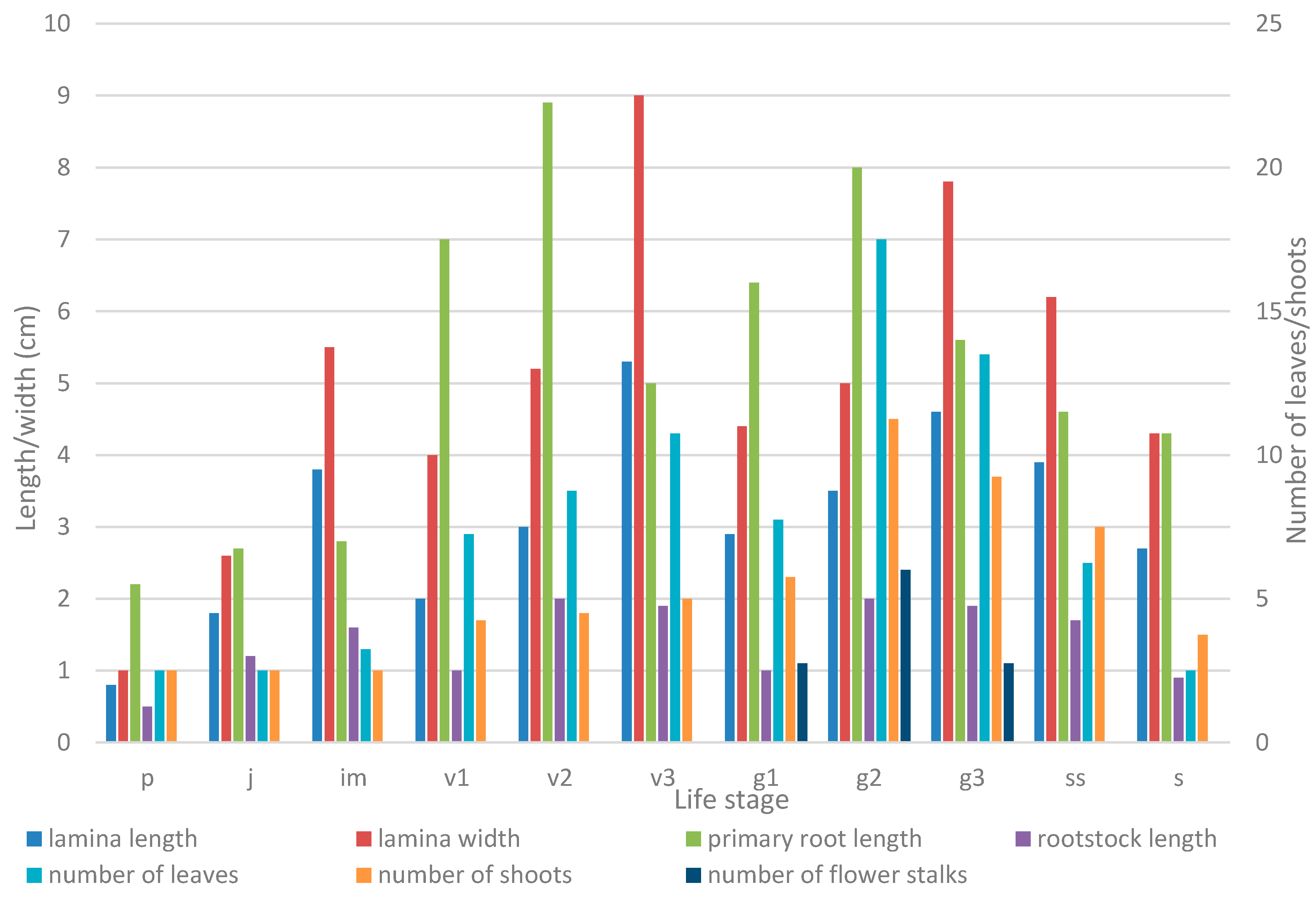


| Life Stage | Parameter | |||||
|---|---|---|---|---|---|---|
| No of Leaves | Lamina Length, cm | Lamina Width, cm | No of Vegetative Shoots | Primary Root Length, сm | Rootstock Length, cm | |
| Seedling (p) | 1 ± 0.00 | 0.78 ± 0.05 | 1.01 ± 0.08 | 1 ± 0.00 | 2.15 ± 0.25 | 0.54 ± 0.07 |
| 0 | 22 | 24 | 0 | 38 | 43 | |
| Juvenile plant (j) | 1 ± 0.00 | 1.82 ± 0.18 | 2.62 ± 0.30 | 1 ± 0.00 | 2.70 ± 0.24 | 1.16 ± 0.10 |
| 0 | 45 | 52 | 0 | 28 | 38 | |
| Immature plant (im) | 1.27 ± 0.21 | 3.83 ± 0.39 | 5.48 ± 0.49 | 1 ± 0.00 | 2.77 ± 0.37 | 1.63 ± 0.22 |
| 26 | 33 | 28 | 0 | 60 | 30 | |
| Life Stage | Parameter | |||||
|---|---|---|---|---|---|---|
| No of Leaves | Lamina Length, cm | Lamina Width, cm | No of Vegetative Shoots | Primary Root Length, сm | Rootstock Length, cm | |
| Young vegetative plant (v1) | 2.91 ± 0.51 | 4.01 ± 0.17 | 6.72 ± 0.37 | 1.7 ± 0.30 | 2.83 ± 0.62 | 1.72 ± 0.21 |
| 11 | 13 | 17 | 20 | 42 | 41 | |
| Mature vegetative plant (v2) | 3.53 ± 0.27 | 5.22 ± 0.19 | 8.89 ± 0.35 | 1.83 ± 0.11 | 3.53 ± 0.56 | 1.88 ± 0.30 |
| 19 | 11 | 12 | 25 | 50 | 44 | |
| Old vegetative plant (v3) | 4.30 ± 0.15 | 5.28 ± 0.48 | 8.97 ± 0.60 | 2.00 ± 0.26 | 4.97 ± 0.80 | 1.93 ± 0.19 |
| 11 | 25 | 21 | 41 | 51 | 30 | |
| Life Stage | Parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| No of Leaves | Lamina Length, cm | Lamina Width, cm | No of GenerativeShoots | Primary Root Length, сm | Rootstock Length, cm | No of Flower Stalks per Shoot | Flower Stalk Length, cm | |
| Young generative plant (g1) | 3.12 ± 0.31 | 4.38 ± 0.24 | 6.41 ± 0.62 | 2.34 ± 0.70 | 3.13 ± 0.61 | 1.78 ± 0.30 | 1.10 ± 0.01 | 20.63 ± 0.19 |
| 34 | 17 | 30 | 30 | 62 | 34 | 29 | 28 | |
| Mature generative plant (g2) | 6.89 ± 0.27 | 5.04 ± 0.38 | 7.97 ± 0.64 | 4.51± 0.32 | 6.97 ± 0.61 | 2.09 ± 0.26 | 2.40 ± 0.22 | 21.05 ± 1.37 |
| 24 | 24 | 26 | 24 | 27 | 39 | 50 | 21 | |
| Old generative plant (g3) | 5.44 ± 0.68 | 4.64 ± 0.24 | 7.76 ± 0.42 | 3.70 ± 0.30 | 5.55 ± 0.97 | 1.86 ± 0.21 | 1.10 ± 0.10 | 20.55 ± 1.86 |
| 40 | 16 | 17 | 37 | 55 | 36 | 29 | 2 | |
| Life Stage | Parameter | |||||
|---|---|---|---|---|---|---|
| No of Leaves | Lamina Length, cm | Lamina Width, cm | No of Vegetative Shoots | Primary Root Length, сm | Rootstock Length, cm | |
| Subsenile plant (ss) | 2.50 ± 0.72 | 3.92 ± 0.97 | 6.15 ± 1.37 | 3.00 ± 0.26 | 4.58 ± 0.57 | 1.65 ± 0.45 |
| 70 | 61 | 55 | 41 | 36 | 66 | |
| Senile plant (s) | 1 ± 0.00 | 2.70 ± 0.70 | 4.30 ± 1.10 | 1.5 ± 0.34 | 4.30 ± 1.56 | 0.88 ± 0.64 |
| 0 | 63 | 63 | 56 | 59 | 71 | |
| Population | Locality | Altitude (m a.s.l.) | Latitude/Longitude | Habitat Type | Ecosite | Disturbance Level/Type |
|---|---|---|---|---|---|---|
| Crocus Prairie Conservation Area | Saskatoon | 496 | 52.16505/ −106.6042 | Prairie | Mixed Grassland—Loam | Moderate/Recreation |
| Battlefords Provincial Park | Cochin | 562 | 53.11370/ −108.3708 | Prairie | Aspen Parkland—Loam | Low/Grazing |
| Redberry Lake Biosphere Reserve | Hafford | 642 | 52.84245/ −107.6607 | Prairie | Aspen Parkland—Gravelly | High/Grazing-Burning |
| Cranberry Flats Conservation Area | Saskatoon | 495 | 52.03216/ −106.7062 | Shrubland | Mixed Grassland—Sand dunes | Moderate/Recreation |
| Prince Albert National Park | Waskesiu Lake | 443 | 53.22359/ −105.7604 | Forest | Boreal Shield—Fresh sand | Low/Recreation |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kricsfalusy, V. Variations in the Life Cycle of Anemone patens L. (Ranunculaceae) in Wild Populations of Canada. Plants 2016, 5, 29. https://doi.org/10.3390/plants5030029
Kricsfalusy V. Variations in the Life Cycle of Anemone patens L. (Ranunculaceae) in Wild Populations of Canada. Plants. 2016; 5(3):29. https://doi.org/10.3390/plants5030029
Chicago/Turabian StyleKricsfalusy, Vladimir. 2016. "Variations in the Life Cycle of Anemone patens L. (Ranunculaceae) in Wild Populations of Canada" Plants 5, no. 3: 29. https://doi.org/10.3390/plants5030029





