Light Signaling in Bud Outgrowth and Branching in Plants
Abstract
:1. Introduction
2. Plant Branching and Bud Outgrowth Are Regulated by Light Intensity and Quality and by the Photoperiod
2.1. Impact of Light Intensity
2.2. Impact of Light Quality
2.3. Impact of the Photoperiod
3. Photoreceptors Involved in the Control of Branching
3.1. Phytochromes
3.2. Cryptochromes
4. Site(s) of Light Perception in the Control of Bud Outgrowth
5. Light Transduction in Bud Outgrowth and Interaction with Growth Processes
5.1. From Photoreceptors to the Early Steps of Light Signaling
5.2. Central Integrators
5.3. Interaction of Light and Hormone Signaling Pathways during Bud Outgrowth
5.3.1. Interaction with Cytokinins
5.3.2. Interaction with Auxin
5.3.3. Interaction with Strigolactones
5.3.4. Interaction with Other Hormones
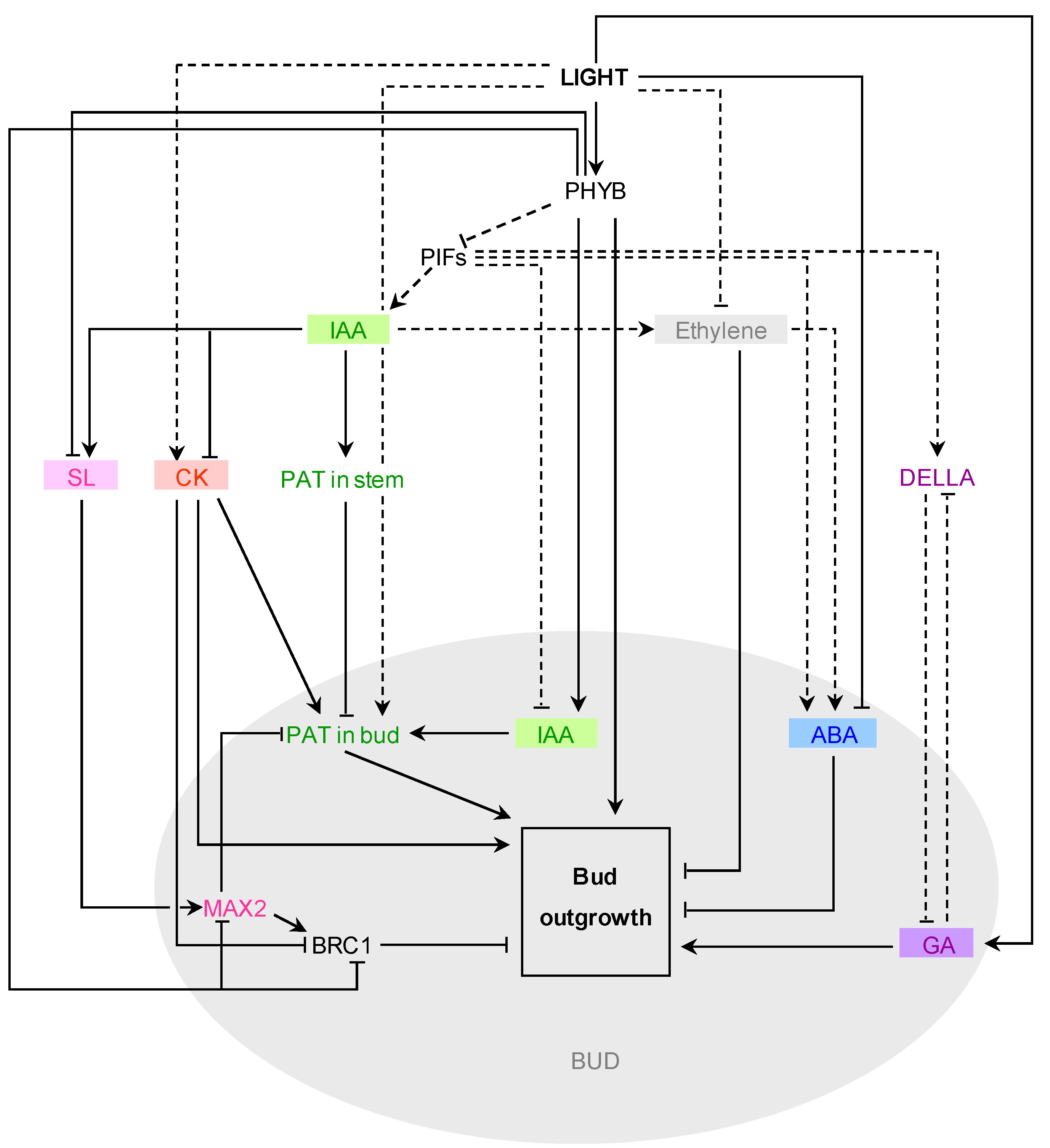
5.4. Interaction of Light Signaling and Nutrients during Bud Outgrowth
5.5. Action of Light on Cell Growth and Expansion during Bud Outgrowth
6. Response of Bud Outgrowth to Light and Photosynthetic Control
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Juenger, T.; Bergelson, J. The evolution of compensation to herbivory in Scarlet Gilia, Ipomopsis aggregata: Herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution 2000, 54, 764–777. [Google Scholar] [CrossRef]
- Lafarge, T.A.; Broad, I.J.; Hammer, G.L. Tillering in grain sorghum over a wide range of population densities: Identification of a common hierarchy for tiller emergence, leaf area development and fertility. Ann. Bot. 2002, 90, 87–98. [Google Scholar] [CrossRef]
- Moulia, B.; Loup, C.; Chartier, M.; Allirand, J.M.; Edelin, C. Dynamics of architectural development of isolated plants of maize (Zea mays L.), in a non-limiting environment: The branching potential of modern maize. Ann. Bot. 1999, 84, 645–656. [Google Scholar] [CrossRef]
- Lemerle, D.; Verbeek, B.; Cousens, R.D.; Coombes, N.E. The potential for selecting wheat varieties strongly competitive against weeds. Weed Res. 1996, 36, 505–513. [Google Scholar] [CrossRef]
- Zhao, D.L.; Atlin, G.N.; Bastiaans, L.; Spiertz, J.H.J. Developing selection protocols for weed competitiveness in aerobic rice. Field Crops Res. 2006, 97, 272–285. [Google Scholar] [CrossRef]
- Simon, S.; Morel, K.; Durand, E.; Brevalle, G.; Girard, T.; Lauri, P.-É. Aphids at crossroads: When branch architecture alters aphid infestation patterns in the apple tree. Trees 2012, 26, 273–282. [Google Scholar] [CrossRef]
- Boumaza, R.; Demotes-Mainard, S.; Huche-Thelier, L.; Guerin, V. Visual Characterization of the esthetic quality of the rosebush. J. Sens. Stud. 2009, 24, 774–796. [Google Scholar] [CrossRef]
- Burnett, S.E.; van Iersel, M. Morphology and irrigation efficiency of Gaura lindheimeri grown with capacitance sensor-controlled irrigation. HortScience 2008, 43, 1555–1560. [Google Scholar]
- Cameron, R.W.F.; Harrison-Murray, R.S.; Atkinson, C.J.; Judd, H.L. Regulated deficit irrigation: A means to control growth in woody ornamentals. J. Hortic. Sci. Biotechnol. 2006, 81, 435–443. [Google Scholar]
- Demotes-Mainard, S.; Huché-Thélier, L.; Morel, P.; Boumaza, R.; Guérin, V.; Sakr, S. Temporary water restriction or light intensity limitation promotes branching in rose bush. Sci. Hortic. 2013, 150, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Huché-Thélier, L.; Boumaza, R.; Demotes-Mainard, S.; Canet, A.; Symoneaux, R.; Douillet, O.; Guérin, V. Nitrogen deficiency increases basal branching and modifies visual quality of the rose bushes. Sci. Hortic. 2011, 130, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Morel, P.; Crespel, L.; Galopin, G.; Moulia, B. Effect of mechanical stimulation on the growth and branching of garden rose. Sci. Hortic. 2012, 135, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Martin, T.A.; Davis, J.M. Short-term physiological and developmental responses to nitrogen availability in hybrid poplar. New Phytol. 2005, 167, 41–52. [Google Scholar] [CrossRef]
- Bahmani, I.; Hazard, L.; Varlet-Grancher, C.; Betin, M.; Lemaire, G.; Matthew, C.; Thom, E.R. Differences in tillering of long- and short-leaved perennial ryegrass genetic lines under full light and shade treatments. Crop Sci. 2000, 40, 1095–1102. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Andrieu, B.; Struik, P.C. Cessation of tillering in spring wheat in relation to light interception and red: Far-red ratio. Ann. Bot. 2006, 97, 649–658. [Google Scholar] [CrossRef]
- Kawamura, K.; Takeda, H. Light environment and crown architecture of two temperate Vaccinium species: Inherent growth rules versus degree of plasticity in light response. Can. J. Bot. 2002, 80, 1063–1077. [Google Scholar] [CrossRef]
- Kawamura, K.; Takeda, H. Rules of crown development in the clonal shrub Vaccinium hirtum in a low-light understory: A quantitative analysis of architecture. Can. J. Bot. 2004, 82, 329–339. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Lukjanova, A. Total foliar area and average leaf age may be more strongly associated with branching frequency than with leaf longevity in temperate conifers. New Phytol. 2003, 158, 75–89. [Google Scholar] [CrossRef]
- Lukjanova, A. Needle longevity, shoot growth and branching frequency in relation to site fertility and within-canopy light conditions in Pinus sylvestris. Ann. For. Sci. 2003, 60, 195–208. [Google Scholar] [CrossRef]
- Takenaka, A. Shoot growth responses to light microenvironment and correlative inhibition in tree seedlings under a forest canopy. Tree Physiol. 2000, 20, 987–991. [Google Scholar] [CrossRef]
- Bartlett, G.A.; Remphrey, W.R. The effect of reduced quantities of photosynthetically active radiation on Fraxinus pennsylvanica growth and architecture. Can. J. Bot. 1998, 76, 1359–1365. [Google Scholar]
- Girault, T.; Bergougnoux, V.; Combes, D.; Viemont, J.-D.; Leduc, N. Light controls shoot meristem organogenic activity and leaf primordia growth during bud burst in Rosa sp. Plant Cell Environ. 2008, 31, 1534–1544. [Google Scholar] [CrossRef]
- Khayat, E.; Zieslin, N. Environmental factors involved in the regulation of sprouting of basal buds in rose plants. J. Exp. Bot. 1982, 33, 1286–1292. [Google Scholar] [CrossRef]
- Low, V. Effects of light and darkness on the growth of peas. Aust. J. Biol. Sci. 1970, 24, 187–195. [Google Scholar]
- Djennane, S.; Oyant, L.H.-S.; Kawamura, K.; Lalanne, D.; Laffaire, M.; Thouroude, T.; Chalain, S.; Sakr, S.; Boumaza, R.; Foucher, F.; et al. Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant Cell Environ. 2014, 37, 742–757. [Google Scholar] [CrossRef] [Green Version]
- Facella, P.; Lopez, L.; Carbone, F.; Galbraith, D.W.; Giuliano, G.; Perrotta, G. Diurnal and circadian rhythms in the tomato transcriptome and their modulation by cryptochrome photoreceptors. PLoS One 2008, 3, e2798. [Google Scholar] [CrossRef]
- Kidokoro, S.; Maruyama, K.; Nakashima, K.; Imura, Y.; Narusaka, Y.; Shinwari, Z.K.; Osakabe, Y.; Fujita, Y.; Mizoi, J.; Shinozaki, K.; et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009, 151, 2046–2057. [Google Scholar] [CrossRef]
- Nakamichi, N. Molecular mechanisms underlying the Arabidopsis circadian clock. Plant Cell Physiol. 2011, 52, 1709–1718. [Google Scholar]
- Staiger, D.; Green, R. RNA-based regulation in the plant circadian clock. Trends Plant Sci. 2011, 16, 517–523. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Trewavas, A.J. Plant behaviour special issue. Plant Cell Environ. 2009, 32. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and shade-avoidance responses in plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Pierik, R.; Millenaar, F.F.; Voesenek, L.A.C.J.; van der Straeten, D. Reaching out of the shade. Curr. Opin. Plant Biol. 2005, 8, 462–468. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Casal, J.J. Light signals perceived by crop and weed plants. Field Crops Res. 2000, 67, 149–160. [Google Scholar] [CrossRef]
- Liu, J.G.; Mahoney, K.J.; Sikkema, P.H.; Swanton, C.J. The importance of light quality in crop-weed competition. Weed Res. 2009, 49, 217–224. [Google Scholar] [CrossRef]
- Casal, J.J.; Deregibus, V.A.; Sánchez, R.A. Variations in tiller dynamics and morphology in lolium multiflorum lam. Vegetative and reproductive plants as affected by differences in red/far-red irradiation. Ann. Bot. 1985, 56, 553–559. [Google Scholar]
- Casal, J.J.; Sanchez, R.A.; Deregibus, V.A. The effect of plant density on tillering: The involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ. Exp. Bot. 1986, 26, 365–371. [Google Scholar] [CrossRef]
- Skinner, R.H.; Simmons, S.R. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant Cell Environ. 1993, 16, 555–562. [Google Scholar] [CrossRef]
- Wan, C.; Sosebee, R.E. Tillering responses to red: Far-red light ratio during different phenological stages in Eragrostis curvula. Environ. Exp. Bot. 1998, 40, 247–254. [Google Scholar] [CrossRef]
- Robin, C.; Hay, M.J.M.; Newton, P.C.D. Effect of light quality (red: far-red ratio) and defoliation treatments applied at a single phytomer on axillary bud outgrowth in Trifolium repens L. Oecologia 1994, 100, 236–242. [Google Scholar] [CrossRef]
- Marks, T.R.; Simpson, S.E. Effect of irradiance on shoot development in vitro. Plant Growth Regul. 1999, 28, 133–142. [Google Scholar] [CrossRef]
- Mor, Y.; Halevy, A.H.; Porath, D. Characterization of the light reaction in promoting the mobilizing ability of rose shoot tips. Plant Physiol. 1980, 66, 996–1000. [Google Scholar] [CrossRef]
- Barnes, C.; Bugbee, B. Morphological Responses of Wheat to Blue Light. J. Plant Physiol. 1992, 139, 339–342. [Google Scholar] [CrossRef]
- Healy, W.H.; Wilkins, H.F. Light and roses—An overview. South. Flor. Nurserym. 1980, 93, 27–40. [Google Scholar]
- Muleo, R.; Morini, S.; Casano, S. Photoregulation of growth and branching of plum shoots: Physiological action of two photosystems. In Vitro Cell. Dev. Biol. Plant 2001, 37, 609–617. [Google Scholar] [CrossRef]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ahmed, H.B.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.A.; Weigel, R.C.; Wheeler, R.M.; Sager, J.C. Light spectral quality effects on the growth of potato (Solanum tuberosum L.) nodal cuttings in vitro. In Vitro Cell. Dev. Biol. Plant 1993, 29, 5–8. [Google Scholar] [CrossRef]
- Glowacka, B. Response of the tomato (Lycopersicon esculentum Mill.) transplant to the daylight supplemented with blue spectrum. Folia Hortic. Suppl. 2006, 4, 145–149. [Google Scholar]
- Fustec, J.; Beaujard, F. Effect of photoperiod and nitrogen supply on basal shoots development in Rhododendron Catawbiense. Biol. Plant. 2000, 43, 511–515. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef]
- Jouve, L.; Greppin, H.; Agosti, R.D. Arabidopsis thaliana floral stem elongation: Evidence for an endogenous circadian rhythm. Plant Physiol. Biochem. 1998, 36, 469–472. [Google Scholar] [CrossRef]
- Jouve, L.; Charron, Y.; Couderc, C.; Greppin, H.; Agosti, R.D. Dependence of Arabidopsis thaliana floral stem growth and architecture on photoperiod. Biol. Plant. 1998, 41, 377–386. [Google Scholar] [CrossRef]
- Beveridge, C.A.; Weller, J.L.; Singer, S.R.; Hofer, J.M.I. Axillary meristem development. budding relationships between networks controlling flowering, branching, and photoperiod responsiveness. Plant Physiol. 2003, 131, 927–934. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef]
- Reed, A.J.; Canvin, D.T.; Sherrard, J.H.; Hageman, R.H. Assimilation of [N]nitrate and [N]nitrite in leaves of five plant species under light and dark conditions. Plant Physiol. 1983, 71, 291–294. [Google Scholar] [CrossRef]
- Childs, K.L.; Miller, F.R.; Cordonnier-Pratt, M.M.; Pratt, L.H.; Morgan, P.W.; Mullet, J.E. The sorghum photoperiod sensitivity gene, Ma3, Encodes a phytochrome B. Plant Physiol. 1997, 113, 611–619. [Google Scholar]
- Kebrom, T.H.; Burson, B.L.; Finlayson, S.A. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 2006, 140, 1109–1117. [Google Scholar] [CrossRef]
- Takano, M.; Kanegae, H.; Shinomura, T.; Miyao, A.; Hirochika, H.; Furuya, M. Isolation and characterization of rice phytochrome a mutants. Plant Cell Online 2001, 13, 521–534. [Google Scholar] [CrossRef]
- Weller, J.L.; Murfet, I.C.; Reid, J.B. Pea mutants with reduced sensitivity to far-red light define an important role for phytochrome A in day-length detection. Plant Physiol. 1997, 114, 1225–1236. [Google Scholar]
- Platten, J.D.; Foo, E.; Elliott, R.C.; Hecht, V.; Reid, J.B.; Weller, J.L. Cryptochrome 1 contributes to blue-light sensing in pea. Plant Physiol. 2005, 139, 1472–1482. [Google Scholar] [CrossRef]
- Weller, J.L.; Perrotta, G.; Schreuder, M.E.L.; van Tuinen, A.; Koornneef, M.; Giuliano, G.; Kendrick, R.E. Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J. 2001, 25, 427–440. [Google Scholar] [CrossRef]
- Jackson, J.A.; Jenkins, G.I. Extension-growth responses and expression of flavonoid biosynthesis genes in the Arabidopsis hy4 mutant. Planta 1995, 197, 233–239. [Google Scholar]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Lin, C.; Ahmad, M.; Gordon, D.; Cashmore, A.R. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc. Natl. Acad. Sci. USA 1995, 92, 8423–8427. [Google Scholar] [CrossRef]
- Lin, C.; Ahmad, M.; Cashmore, A.R. Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 1996, 10, 893–902. [Google Scholar]
- Lin, C.; Yang, H.; Guo, H.; Mockler, T.; Chen, J.; Cashmore, A.R. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690. [Google Scholar] [CrossRef]
- Corbesier, L.; Coupland, G. The quest for florigen: A review of recent progress. J. Exp. Bot. 2006, 57, 3395–3403. [Google Scholar] [CrossRef]
- Cordonnier, M.M.; Greppin, H.; Pratt, L.H. Phytochrome from green Avena shoots characterized with a monoclonal antibody to phytochrome from etiolated Pisum shoots. Biochemistry (Mosc.) 1986, 25, 7657–7666. [Google Scholar] [CrossRef]
- Somers, D.E.; Quail, P.H. Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J. 1995, 7, 413–427. [Google Scholar]
- Tokuhisa, J.G.; Daniels, S.M.; Quail, P.H. Phytochrome in green tissue: Spectral and immunochemical evidence for two distinct molecular species of phytochrome in light-grown Avena sativa L. Planta 1985, 164, 321–332. [Google Scholar] [CrossRef]
- Clack, T.; Mathews, S.; Sharrock, R.A. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 1994, 25, 413–427. [Google Scholar]
- Hauser, B.A.; Pratt, L.H.; Cordonnier-Pratt, M.-M. Absolute quantification of five phytochrome transcripts in seedlings and mature plants of tomato (Solanum lycopersicum L.). Planta 1997, 201, 379–387. [Google Scholar] [CrossRef]
- Sharrock, R.A.; Clack, T. Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 2002, 130, 442–456. [Google Scholar] [CrossRef]
- Sharrock, R.A.; Clack, T.; Goosey, L. Differential activities of the Arabidopsis phyB/D/E phytochromes in complementing phyB mutant phenotypes. Plant Mol. Biol. 2003, 52, 135–142. [Google Scholar] [CrossRef]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef]
- Jang, I.-C.; Henriques, R.; Seo, H.S.; Nagatani, A.; Chua, N.-H. Arabidopsis phytochrome interacting factor proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell Online 2010, 22, 2370–2383. [Google Scholar] [CrossRef]
- De Lucas, M.; Davière, J.-M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blázquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef]
- Tepperman, J.M.; Zhu, T.; Chang, H.-S.; Wang, X.; Quail, P.H. Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 9437–9442. [Google Scholar] [CrossRef]
- Tepperman, J.M.; Hudson, M.E.; Khanna, R.; Zhu, T.; Chang, S.H.; Wang, X.; Quail, P.H. Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 2004, 38, 725–739. [Google Scholar] [CrossRef]
- Galvão, R.M.; Li, M.; Kothadia, S.M.; Haskel, J.D.; Decker, P.V.; Buskirk, E.K.V.; Chen, M. Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev. 2012, 26, 1851–1863. [Google Scholar] [CrossRef]
- Hornitschek, P.; Lorrain, S.; Zoete, V.; Michielin, O.; Fankhauser, C. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009, 28, 3893–3902. [Google Scholar] [CrossRef]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef]
- Li, L.; Ljung, K.; Breton, G.; Schmitz, R.J.; Pruneda-Paz, J.; Cowing-Zitron, C.; Cole, B.J.; Ivans, L.J.; Pedmale, U.V.; Jung, H.-S.; et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012, 26, 785–790. [Google Scholar] [CrossRef]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar]
- Sun, J.; Qi, L.; Li, Y.; Zhai, Q.; Li, C. PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell Online 2013, 25, 2102–2114. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell Online 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Liu, B.; Zuo, Z.; Liu, H.; Liu, X.; Lin, C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 2011, 25, 1029–1034. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 Interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Lian, H.-L.; He, S.-B.; Zhang, Y.-C.; Zhu, D.-M.; Zhang, J.-Y.; Jia, K.-P.; Sun, S.-X.; Li, L.; Yang, H.-Q. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 2011, 25, 1023–1028. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, H.; Liu, B.; Liu, X.; Lin, C. Blue light-dependent interaction of CRY2 with SPA1 Regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 2011, 21, 841–847. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Brutnell, T.P.; Finlayson, S.A. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ. 2010, 33, 48–58. [Google Scholar]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Finlayson, S.A. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowthand is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007, 48, 667–677. [Google Scholar] [CrossRef]
- González-Grandío, E.; Poza-Carrión, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell Online 2013, 25, 834–850. [Google Scholar]
- Braun, N.; de Saint Germain, A.; Pillot, J.-P.; Boutet-Mercey, S.; Dalmais, M.; Antoniadi, I.; Li, X.; Maia-Grondard, A.; Le Signor, C.; Bouteiller, N.; et al. The pea TCP transcription factor PsBRC1 acts downstream of Strigolactones to control shoot branching. Plant Physiol. 2012, 158, 225–238. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Xi, L.; Li, J.; Zhao, R.; Ma, N.; Zhao, L. Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema × grandiflora cv. Jinba). PLoS One 2013, 8, e61717. [Google Scholar]
- Faiss, M.; Zalubìlová, J.; Strnad, M.; Schmülling, T. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J. 1997, 12, 401–415. [Google Scholar]
- Kalousek, P.; Buchtova, D.; Balla, J.; Reinoehl, V.; Prochazka, S. Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ. Agric. Silvic. Mendel. Brun. 2010, 58, 79–87. [Google Scholar]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Sachs, T.; Thimann, K.V. Release of lateral buds from apical dominance. Nature 1964, 201, 939–940. [Google Scholar] [CrossRef]
- Sachs, T.; Thimann, K.V. The role of auxins and cytokinins in the release of buds from dominance. Am. J. Bot. 1967, 54, 136–144. [Google Scholar] [CrossRef]
- Wickson, M.; Thimann, K.V. The antagonism of auxin and kinetin in apical dominance. Physiol. Plant. 1958, 11, 62–74. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Davies, C.R.; Seth, A.K.; Wareing, P.F. Auxin and kinetin interaction in apical dominance. Science 1966, 151, 468–469. [Google Scholar]
- Li, C.; Bangerth, F. Stimulatory effect of cytokinins and interaction with IAA on the release of lateral buds of pea plants from apical dominance. J. Plant Physiol. 2003, 160, 1059–1063. [Google Scholar] [CrossRef]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Tokunaga, H.; Kojima, M.; Kuroha, T.; Ishida, T.; Sugimoto, K.; Kiba, T.; Sakakibara, H. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. Plant J. Cell Mol. Biol. 2012, 69, 355–365. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Buechel, S.; Leibfried, A.; To, J.P.C.; Zhao, Z.; Andersen, S.U.; Kieber, J.J.; Lohmann, J.U. Role of A-type ARABIDOPSIS RESPONSE REGULATORS in meristem maintenance and regeneration. Eur. J. Cell Biol. 2010, 89, 279–284. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef]
- Leibfried, A.; To, J.P.C.; Busch, W.; Stehling, S.; Kehle, A.; Demar, M.; Kieber, J.J.; Lohmann, J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005, 438, 1172–1175. [Google Scholar] [CrossRef]
- Scofield, S.; Dewitte, W.; Nieuwland, J.; Murray, J.A.H. The Arabidopsis homeobox gene SHOOT MERISTEMLESS has cellular and meristem-organisational roles with differential requirements for cytokinin and CYCD3 activity. Plant J. Cell Mol. Biol. 2013, 75, 53–66. [Google Scholar]
- Yanai, O.; Shani, E.; Dolezal, K.; Tarkowski, P.; Sablowski, R.; Sandberg, G.; Samach, A.; Ori, N. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 2005, 15, 1566–1571. [Google Scholar] [CrossRef]
- Goren, R.; Galston, A.W. Phytochrome controlled C-sucrose uptake into etiolated pea buds: Effects of gibberellic acid and other substances. Plant Physiol. 1967, 42, 1087–1090. [Google Scholar] [CrossRef]
- Yoshida, S.; Mandel, T.; Kuhlemeier, C. Stem cell activation by light guides plant organogenesis. Genes Dev. 2011, 25, 1439–1450. [Google Scholar] [CrossRef]
- Snow, R. The young leaf as the inhibiting organ. New Phytol. 1929, 28, 345–358. [Google Scholar] [CrossRef]
- Ljung, K.; Bhalerao, R.P.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. Cell Mol. Biol. 2001, 28, 465–474. [Google Scholar]
- Wisniewska, J.; Xu, J.; Seifertová, D.; Brewer, P.B.; Ruzicka, K.; Blilou, I.; Rouquié, D.; Benková, E.; Scheres, B.; Friml, J. Polar PIN localization directs auxin flow in plants. Science 2006, 312. [Google Scholar] [CrossRef]
- Petrásek, J.; Friml, J. Auxin transport routes in plant development. Dev. Camb. Engl. 2009, 136, 2675–2688. [Google Scholar]
- Thimann, K.V.; Skoog, F. On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc. R. Soc. B Biol. Sci. 1934, 114, 317–339. [Google Scholar] [CrossRef]
- Hall, S.M.; Hillman, J.R. Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. timing of bud growth following decapitation. Planta 1975, 123, 137–143. [Google Scholar]
- Prasad, T.K.; Li, X.; Abdel-Rahman, A.M.; Hosokawa, Z.; Cloud, N.P.; Lamotte, C.E.; Cline, M.G. Does auxin play a role in the release of apical dominance by shoot Inversion in Ipomoea nil? Ann. Bot. 1993, 71, 223–229. [Google Scholar] [CrossRef]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef]
- Sachs, T. Polarity and the induction of organized vascular tissues. Ann. Bot. 1969, 33, 263–275. [Google Scholar]
- Li, C.; Bangerth, F. Autoinhibition of indoleacetic acid transport in the shoots of two‐branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol. Plant. 1999, 106, 415–420. [Google Scholar] [CrossRef]
- Balla, J.; Kalousek, P.; Reinöhl, V.; Friml, J.; Procházka, S. Competitive canalization of PIN‐dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 2011, 65, 571–577. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.-R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Scarpella, E.; Marcos, D.; Friml, J.; Berleth, T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006, 20, 1015–1027. [Google Scholar] [CrossRef]
- Bayer, E.M.; Smith, R.S.; Mandel, T.; Nakayama, N.; Sauer, M.; Prusinkiewicz, P.; Kuhlemeier, C. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 2009, 23, 373–384. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Lenhard, M.; Smith, R.; Kuhlemeier, C. Interaction between meristem tissue layers controls phyllotaxis. Dev. Cell 2013, 26, 616–628. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.-L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef]
- Briggs, W.R. Mediation of phototropic responses of corn coleoptiles by lateral transport of auxin. Plant Physiol. 1963, 38, 237–247. [Google Scholar] [CrossRef]
- Esmon, C.A.; Tinsley, A.G.; Ljung, K.; Sandberg, G.; Hearne, L.B.; Liscum, E. A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc. Natl. Acad. Sci. USA 2006, 103, 236–241. [Google Scholar]
- Ding, Z.; Galván-Ampudia, C.S.; Demarsy, E.; Łangowski, Ł.; Kleine-Vehn, J.; Fan, Y.; Morita, M.T.; Tasaka, M.; Fankhauser, C.; Offringa, R.; et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 2011, 13, 447–452. [Google Scholar] [CrossRef]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef]
- Zhang, K.-X.; Xu, H.-H.; Yuan, T.-T.; Zhang, L.; Lu, Y.-T. Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 2013, 76, 308–321. [Google Scholar]
- Beveridge, C.A.; Kyozuka, J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol. 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Hamiaux, C.; Drummond, R.S.M.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 Is an α/β Hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef]
- Dun, E.A.; Brewer, P.B.; Beveridge, C.A. Strigolactones: Discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009, 14, 364–372. [Google Scholar] [CrossRef]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Dynamics of strigolactone function and shoot branching responses in Pisum sativum. Mol. Plant 2013, 6, 128–140. [Google Scholar] [CrossRef]
- Shen, H.; Luong, P.; Huq, E. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol. 2007, 145, 1471–1483. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef]
- Li, J.; Nagpal, P.; Vitart, V.; McMorris, T.C.; Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 1996, 272, 398–401. [Google Scholar]
- Su, H.; Abernathy, S.D.; White, R.H.; Finlayson, S.A. Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ. 2011, 34, 1986–1998. [Google Scholar] [CrossRef]
- Choubane, D.; Rabot, A.; Mortreau, E.; Legourrierec, J.; Péron, T.; Foucher, F.; Ahcène, Y.; Pelleschi-Travier, S.; Leduc, N.; Hamama, L.; et al. Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J. Plant Physiol. 2012, 169, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Meijón, M.; Cañal, M.J.; Valledor, L.; Rodríguez, R.; Feito, I. Epigenetic and physiological effects of gibberellin inhibitors and chemical pruners on the floral transition of azalea. Physiol. Plant. 2011, 141, 276–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, L.; Zheng, S.; Xie, J.; Bi, Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J. Plant Physiol. 2010, 167, 1130–1136. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Kamiya, Y.; Bae, G.; Chung, W.-I.; Choi, G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. Cell Mol. Biol. 2006, 47, 124–139. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.-S.; Sun, T.; Kamiya, Y.; Choi, G. PIL5, a Phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell Online 2007, 19, 1192–1208. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Takeda-Kamiya, N.; Hanada, A.; Ogawa, M.; Kuwahara, A.; Seo, M.; Kamiya, Y.; Yamaguchi, S. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 2007, 48, 555–561. [Google Scholar] [CrossRef]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef]
- Eliasson, L. Effect of indoleacetic acid on the abscisic acid level in stem tissue. Physiol. Plant. 1975, 34, 117–120. [Google Scholar] [CrossRef]
- Knox, J.P.; Wareing, P.F. Apical dominance in Phaseolus vulgaris L. J. Exp. Bot. 1984, 35, 239–244. [Google Scholar] [CrossRef]
- Tucker, D.J. Effects of far-red light on the hormonal control of side shoot growth in the tomato. Ann. Bot. 1976, 40, 1033–1042. [Google Scholar]
- Gocal, G.F.; Pharis, R.P.; Yeung, E.C.; Pearce, D. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv tender green. Plant Physiol. 1991, 95, 344–350. [Google Scholar] [CrossRef]
- Chatfield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. Cell Mol. Biol. 2000, 24, 159–169. [Google Scholar] [CrossRef]
- Cline, M.G.; Oh, C. A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann. Bot. 2006, 98, 891–897. [Google Scholar] [CrossRef]
- Reddy, S.K.; Holalu, S.V.; Casal, J.J.; Finlayson, S. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 2013, 163, 1047–1058. [Google Scholar] [CrossRef]
- González, C.V.; Ibarra, S.E.; Piccoli, P.N.; Botto, J.F.; Boccalandro, H.E. Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 1958–1968. [Google Scholar]
- Blake, T.J.; Reid, D.M.; Rood, S.B. Ethylene, indoleacetic acid and apical dominance in peas: A reappraisal. Physiol. Plant. 1983, 59, 481–487. [Google Scholar]
- Burg, S.P.; Burg, E.A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic acid. Plant Physiol. 1968, 43, 1069–1074. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Lee, I.-J.; Morgan, P.W. Phytochrome B and the regulation of circadian ethylene production in Sorghum. Plant Physiol. 1998, 116, 17–25. [Google Scholar] [CrossRef]
- Beuchat, J.; Scacchi, E.; Tarkowska, D.; Ragni, L.; Strnad, M.; Hardtke, C.S. BRX promotes Arabidopsis shoot growth. New Phytol. 2010, 188, 23–29. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome signaling mechanisms. Arabidopsis Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Sun, J.; Qi, L.; Li, Y.; Chu, J.; Li, C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis Hypocotyl growth. PLoS Genet. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar]
- Grossmann, K.; Hansen, H. Ethylene-triggered abscisic acid: A principle in plant growth regulation? Physiol. Plant. 2001, 113, 9–14. [Google Scholar] [CrossRef]
- Hansen, H.; Grossmann, K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef]
- Zhong, S.; Shi, H.; Xue, C.; Wang, L.; Xi, Y.; Li, J.; Quail, P.H.; Deng, X.W.; Guo, H. A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr. Biol. 2012, 22, 1530–1535. [Google Scholar] [CrossRef]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2010, 30, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Chao, W.S.; Serpe, M.D.; Anderson, J.V.; Gesch, R.W.; Horvath, D.P. Sugars, hormones, and environment affect the dormancy status in underground adventitious buds of leafy spurge (Euphorbia esula). Weed Sci. 2006, 54, 59–68. [Google Scholar] [CrossRef]
- Decourteix, M.; Alves, G.; Bonhomme, M.; Peuch, M.; Ben Baaziz, K.; Brunel, N.; Guilliot, A.; Rageau, R.; Améglio, T.; Pétel, G.; et al. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: Their potential role in early events of growth resumption. Tree Physiol. 2008, 28, 215–224. [Google Scholar] [CrossRef]
- Henry, C.; Rabot, A.; Laloi, M.; Mortreau, E.; Sigogne, M.; Leduc, N.; Lemoine, R.; Sakr, S.; Vian, A.; Pelleschi-Travier, S. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ. 2011, 34, 1776–1789. [Google Scholar] [CrossRef] [Green Version]
- Maurel, K.; Leite, G.B.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef]
- Girault, T.; Abidi, F.; Sigogne, M.; Pelleschi-Travier, S.; Boumaza, R.; Sakr, S.; Leduc, N. Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ. 2010, 33, 1339–1350. [Google Scholar]
- Sergeeva, L.I.; Keurentjes, J.J.B.; Bentsink, L.; Vonk, J.; van der Plas, L.H.W.; Koornneef, M.; Vreugdenhil, D. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 2994–2999. [Google Scholar] [CrossRef]
- Tang, G.Q.; Lüscher, M.; Sturm, A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 1999, 11, 177–189. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.-R.; Lian, H.; Ni, D.-A.; He, Y.; Chen, X.-Y.; Ruan, Y.-L. Evidence that high activity of vacuolar invertase is required for cotton fiber and Arabidopsis root elongation through osmotic dependent and independent pathways, respectively. Plant Physiol. 2010, 154, 744–756. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S.; et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef] [Green Version]
- Guak, S.; Neilsen, D.; Millard, P.; Wendler, R.; Neilsen, G.H. Determining the role of N remobilization for growth of apple (Malus domestica Borkh) trees by measuring xylem-sap N flux. J. Exp. Bot. 2003, 54, 2121–2131. [Google Scholar] [CrossRef]
- Millard, P.; Wendler, R.; Grassi, G.; Grelet, G.-A.; Tagliavini, M. Translocation of nitrogen in the xylem of field-grown cherry and poplar trees during remobilization. Tree Physiol. 2006, 26, 527–536. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef]
- Furet, P.-M.; Vian, A.; Guérin, V.; Lothier, J.; Unité Mixte de Recherche 1345 IRHS, Angers, France. Unpublished data. 2014.
- Furet, P.-M.; Lothier, J.; Demotes-Mainard, S.; Travier, S.; Henry, C.; Guérin, V.; Vian, A. Light and nitrogen nutrition regulate apical control in Rosa hybrida L. J. Plant Physiol. 2014, 171, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.A.; Suttle, J.C.; Sell, T.W. Changes in cell cycle status and expression of p34cdc2 kinase during potato tuber meristem dormancy. Physiol. Plant. 1996, 98, 743–752. [Google Scholar] [CrossRef]
- Devitt, M.L.; Stafstrom, J.P. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol. Biol. 1995, 29, 255–265. [Google Scholar] [CrossRef]
- Le Bris, M.; Michaux-Ferrière, N.; Jacob, Y.; Poupet, A.; Barthe, P.; Guigonis, J.-M.; Page-Degivry, M.-T.L. Regulation of bud dormancy by manipulation of ABA in isolated buds of Rosa hybrida cultured in vitro. Funct. Plant Biol. 1999, 26, 273–281. [Google Scholar]
- Dewitte, W.; Murray, J.A.H. The Plant Cell Cycle. Annu. Rev. Plant Biol. 2003, 54, 235–264. [Google Scholar] [CrossRef]
- Inzé, D.; de Veylder, L. Cell cycle regulation in plant development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar]
- Komaki, S.; Sugimoto, K. Control of the Plant Cell Cycle by Developmental and environmental cues. Plant Cell Physiol. 2012, 53, 953–964. [Google Scholar] [CrossRef]
- Gaudin, V.; Lunness, P.A.; Fobert, P.R.; Towers, M.; Riou-Khamlichi, C.; Murray, J.A.; Coen, E.; Doonan, J.H. The expression of D-cyclin genes defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the Cycloidea gene. Plant Physiol. 2000, 122, 1137–1148. [Google Scholar] [CrossRef]
- Riou-Khamlichi, C.; Huntley, R.; Jacqmard, A.; Murray, J.A. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 1999, 283, 1541–1544. [Google Scholar] [CrossRef]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef]
- Murray, J.A.H.; Jones, A.; Godin, C.; Traas, J. Systems analysis of shoot apical meristem growth and development: Integrating hormonal and mechanical signaling. Plant Cell Online 2012, 24, 3907–3919. [Google Scholar] [CrossRef]
- Humphrey, T.V.; Bonetta, D.T.; Goring, D.R. Sentinels at the wall: Cell wall receptors and sensors. New Phytol. 2007, 176, 7–21. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Relaxation in a high-stress environment: The molecular bases of extensible cell walls and cell enlargement. Plant Cell 1997, 9, 1031–1041. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Li, Z.C. Role of expansin in cell enlargement of oat coleoptiles (analysis of developmental gradients and photocontrol). Plant Physiol. 1993, 103, 1321–1328. [Google Scholar]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Cosgrove, D. Expansine growth of plant cell walls. Plant Physiol. Biochem. 2000, 38, 109–124. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Darley, C.P.; Forrester, A.M.; McQueen-Mason, S.J. The molecular basis of plant cell wall extension. In Plant Cell Walls; Carpita, N.C., Campbell, M., Tierney, M., Eds.; Springer: Amsterdam Netherlands, 2001; pp. 179–195. [Google Scholar]
- Fleming, A.J.; McQueen-Mason, S.; Mandel, T.; Kuhlemeier, C. Induction of leaf primordia by the cell wall protein expansin. Science 1997, 276, 1415–1418. [Google Scholar]
- Pien, S.; Wyrzykowska, J.; Fleming, A.J. Novel marker genes for early leaf development indicate spatial regulation of carbohydrate metabolism within the apical meristem. Plant J. 2001, 25, 663–674. [Google Scholar]
- Reinhardt, D.; Wittwer, F.; Mandel, T.; Kuhlemeier, C. Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell Online 1998, 10, 1427–1437. [Google Scholar] [CrossRef]
- Vogler, H.; Caderas, D.; Mandel, T.; Kuhlemeier, C. Domains of expansin gene expression define growth regions in the shoot apex of tomato. Plant Mol. Biol. 2003, 53, 267–272. [Google Scholar]
- Cho, H.-T.; Cosgrove, D.J. Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2000, 97, 9783–9788. [Google Scholar] [CrossRef]
- Gray-Mitsumune, M.; Blomquist, K.; McQueen-Mason, S.; Teeri, T.T.; Sundberg, B.; Mellerowicz, E.J. Ectopic expression of a wood-abundant expansin PttEXPA1 promotes cell expansion in primary and secondary tissues in aspen. Plant Biotechnol. J. 2008, 6, 62–72. [Google Scholar]
- López-Juez, E.; Dillon, E.; Magyar, Z.; Khan, S.; Hazeldine, S.; de Jager, S.M.; Murray, J.A.H.; Beemster, G.T.S.; Bögre, L.; Shanahan, H. Distinct light-initiated gene expression and cell cycle programs in the shoot apex and cotyledons of Arabidopsis. Plant Cell 2008, 20, 947–968. [Google Scholar] [CrossRef] [Green Version]
- Caderas, D.; Muster, M.; Vogler, H.; Mandel, T.; Rose, J.K.C.; McQueen-Mason, S.; Kuhlemeier, C. Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol. 2000, 123, 1399–1414. [Google Scholar] [CrossRef]
- Li, Y.; Jones, L.; McQueen-Mason, S. Expansins and cell growth. Curr. Opin. Plant Biol. 2003, 6, 603–610. [Google Scholar] [CrossRef]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell Online 1992, 4, 1425–1433. [Google Scholar] [CrossRef]
- Kawamura, K.; Takeda, H. Developmentally programmed and plastic processes of growth in the multistemmed understory shrub Vaccinium hirtum (Ericaceae). Botany 2008, 86, 268–277. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- McCree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Mor, Y.; Halevy, A.H. Promotion of sink activity of developing rose shoots by light. Plant Physiol. 1980, 66, 990–995. [Google Scholar] [CrossRef]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leduc, N.; Roman, H.; Barbier, F.; Péron, T.; Huché-Thélier, L.; Lothier, J.; Demotes-Mainard, S.; Sakr, S. Light Signaling in Bud Outgrowth and Branching in Plants. Plants 2014, 3, 223-250. https://doi.org/10.3390/plants3020223
Leduc N, Roman H, Barbier F, Péron T, Huché-Thélier L, Lothier J, Demotes-Mainard S, Sakr S. Light Signaling in Bud Outgrowth and Branching in Plants. Plants. 2014; 3(2):223-250. https://doi.org/10.3390/plants3020223
Chicago/Turabian StyleLeduc, Nathalie, Hanaé Roman, François Barbier, Thomas Péron, Lydie Huché-Thélier, Jérémy Lothier, Sabine Demotes-Mainard, and Soulaiman Sakr. 2014. "Light Signaling in Bud Outgrowth and Branching in Plants" Plants 3, no. 2: 223-250. https://doi.org/10.3390/plants3020223



