Checking the Pulse of Vitamin A Metabolism and Signaling during Mammalian Spermatogenesis
Abstract
:1. Introduction
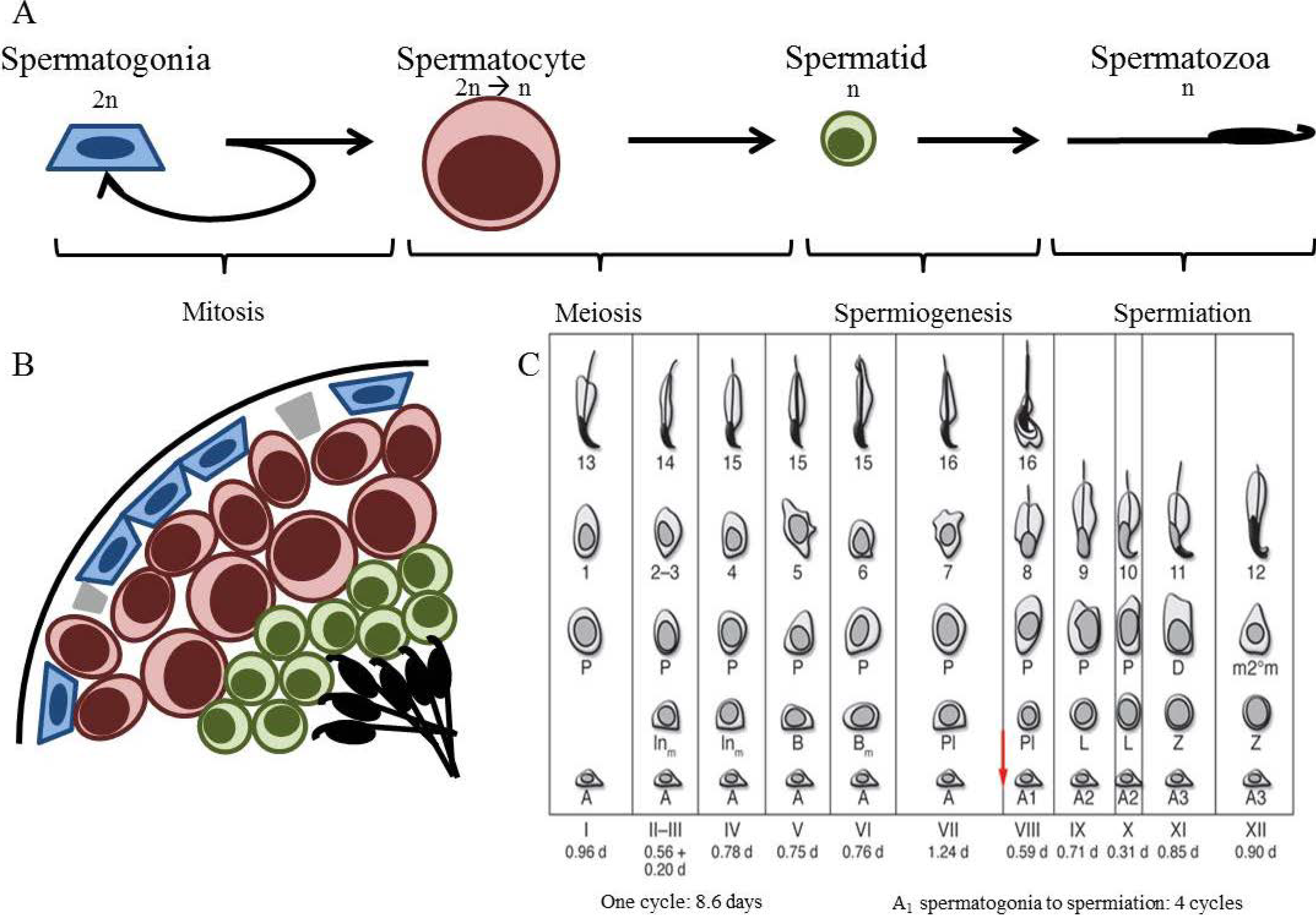
2. Spermatogenesis
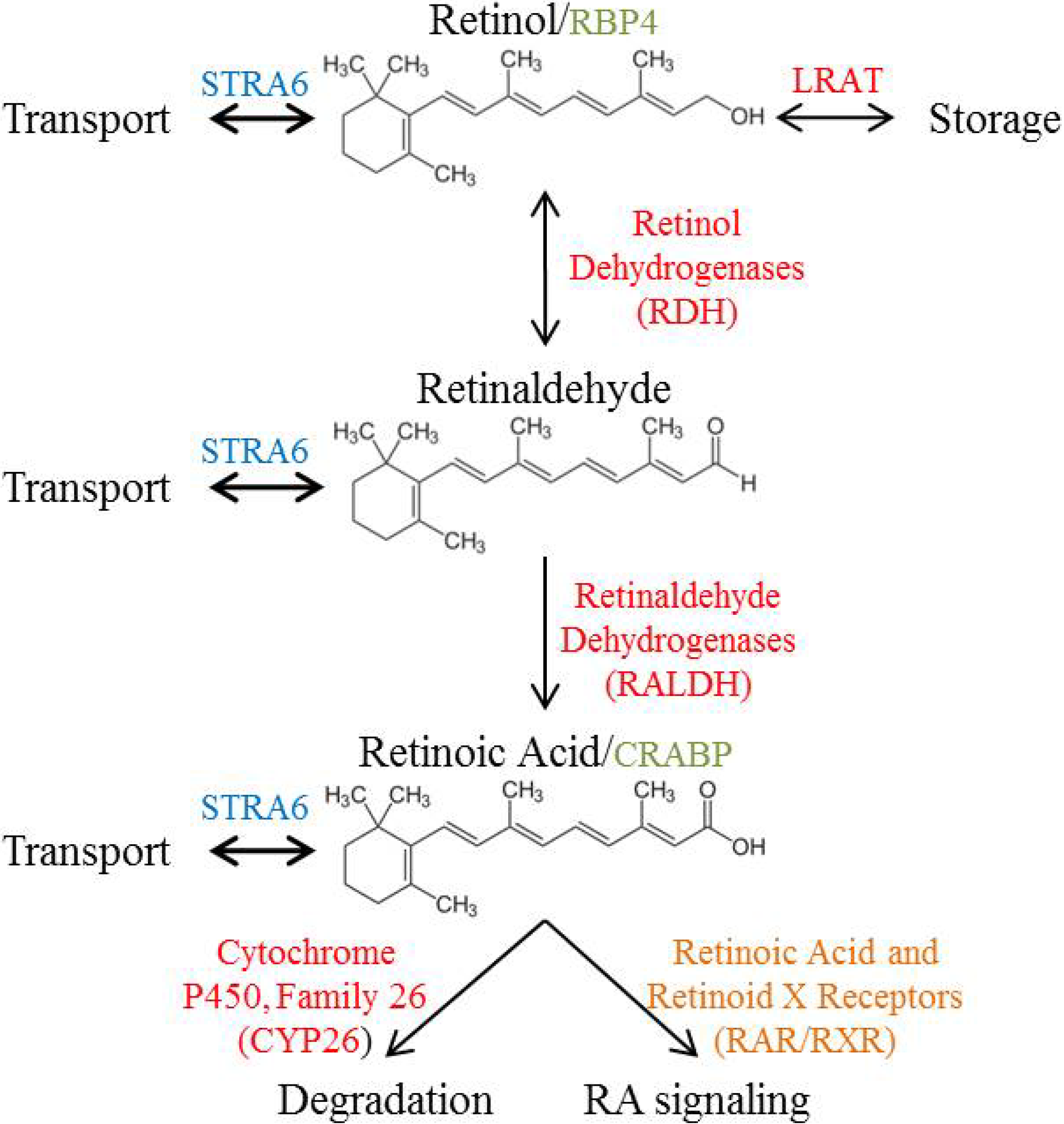
3. Vitamin A Metabolism
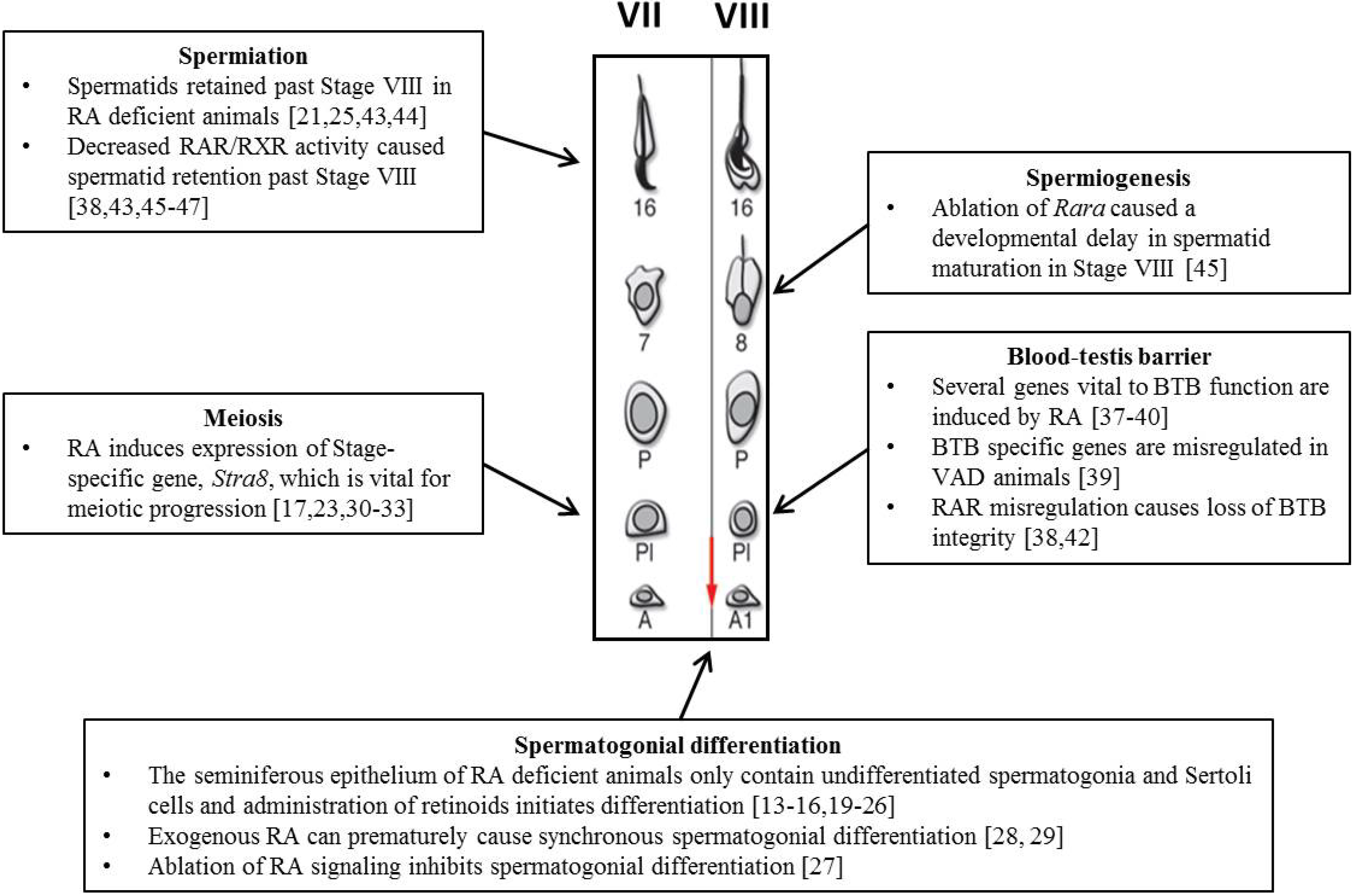
4. The Role of RA during Spermatogenesis
4.1. Spermatogonial Differentiation
4.2. Meiosis
4.3. Blood-Testes Barrier (BTB)
4.4. Spermiogenesis and Spermiation
5. What is the Cause of Stage-Specific RA Response?
5.1. Retinoid Metabolism
5.2. Retinoid Signaling
5.3. Retinoid Transport and Storage
6. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References and Notes
- Koubova, J.; Menke, D.B.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Nat. Acad. Sci. USA 2006, 103, 2474–2479. [Google Scholar]
- MacLean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar] [CrossRef]
- Kumar, S.; Chatzi, C.; Brade, T.; Cunningham, T.J.; Zhao, X.; Duester, G. Sex-specific timing of meiotic initiation is regulated by cyp26b1 independent of retinoic acid signalling. Nat. Commun. 2011, 2, 151. [Google Scholar] [CrossRef]
- Griswold, M.D.; Hogarth, C.A.; Bowles, J.; Koopman, P. Initiating meiosis: The case for retinoic acid. Biol. Reprod. 2012, 86, 35. [Google Scholar] [CrossRef]
- Anderson, J.E.; Farr, S.L.; Jamieson, D.J.; Warner, L.; Macaluso, M. Infertility services reported by men in the united states: National survey data. Fert. Steril. 2009, 91, 2466–2470. [Google Scholar] [CrossRef]
- Schulte, R.T.; Ohl, D.A.; Sigman, M.; Smith, G.D. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J. Assist. Reprod. Genet. 2010, 27, 3–12. [Google Scholar] [CrossRef]
- Nya-Ngatchou, J.J.; Arnold, S.L.; Walsh, T.J.; Muller, C.H.; Page, S.T.; Isoherranen, N.; Amory, J.K. Intratesticular 13-cis retinoic acid is lower in men with abnormal semen analyses: A pilot study. Andrology 2013, 1, 325–331. [Google Scholar] [CrossRef]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar]
- Hogarth, C.A.; Griswold, M.D. The key role of vitamin a in spermatogenesis. J. Clin. Invest. 2010, 120, 956–962. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef]
- Zechel, C.; Shen, X.Q.; Chambon, P.; Gronemeyer, H. Dimerization interfaces formed between the DNA binding domains determine the cooperative binding of rxr/rar and rxr/tr heterodimers to dr5 and dr4 elements. EMBO J. 1994, 13, 1414–1424. [Google Scholar]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: Background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. 2010, 73, 241–278. [Google Scholar] [CrossRef]
- Bishop, P.D.; Griswold, M.D. Uptake and metabolism of retinol in cultured sertoli cells: Evidence for a kinetic model. Biochemistry 1987, 26, 7511–7518. [Google Scholar] [CrossRef]
- Griswold, M.D.; Bishop, P.D.; Kim, K.H.; Ping, R.; Siiteri, J.E.; Morales, C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann. N. Y. Acad. Sci. 1989, 564, 154–172. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.H.; van de Kant, H.J.; de Rooij, D.G.; van Pelt, A.M. Differential expression of c-kit in mouse undifferentiated and differentiating type a spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.M.; Pouchnik, D.; Banasik, B.; McCarrey, J.R.; Small, C.; et al. Expression of stimulated by retinoic acid gene 8 (stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Oulad-Abdelghani, M.; Bouillet, P.; Decimo, D.; Gansmuller, A.; Heyberger, S.; Dolle, P.; Bronner, S.; Lutz, Y.; Chambon, P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by stra8, a novel retinoic acid-responsive gene. J. Cell Biol. 1996, 135, 469–477. [Google Scholar] [CrossRef]
- Pellegrini, M.; Filipponi, D.; Gori, M.; Barrios, F.; Lolicato, F.; Grimaldi, P.; Rossi, P.; Jannini, E.A.; Geremia, R.; Dolci, S. Atra and kl promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008, 7, 3878–3888. [Google Scholar] [CrossRef]
- Busada, J.T.; Kaye, E.P.; Renegar, R.H.; Geyer, C.B. Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biol. Reprod. 2014, in press. [Google Scholar]
- Morales, C.; Griswold, M.D. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 1987, 121, 432–434. [Google Scholar] [CrossRef]
- Li, H.; Palczewski, K.; Baehr, W.; Clagett-Dame, M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol. Reprod. 2011, 84, 336–341. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Vernet, N.; Dennefeld, C.; Giese, N.; Nau, H.; Chambon, P.; Viville, S.; Mark, M. Retinoids and spermatogenesis: Lessons from mutant mice lacking the plasma retinol binding protein. Develop. Dynam. 2006, 235, 1608–1622. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Evanoff, R.; Mitchell, D.; Kent, T.; Small, C.; Amory, J.K.; Griswold, M.D. Turning a spermatogenic wave into a tsunami: Synchronizing murine spermatogenesis using win 18,446. Biol. Reprod. 2013, 88, 40. [Google Scholar] [CrossRef]
- Brooks, N.L.; van der Horst, G. Short-term effects of n'n-bis(dichloroacetyl)-1,8-octamethylenediamine (win 18446) on the testes, selected sperm parameters and fertility of male cba mice. Lab. Anim. 2003, 37, 363–373. [Google Scholar] [CrossRef]
- Raverdeau, M.; Gely-Pernot, A.; Feret, B.; Dennefeld, C.; Benoit, G.; Davidson, I.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid induces sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Nat. Acad. Sci. USA 2012, 109, 16582–16587. [Google Scholar] [CrossRef]
- Tong, M.H.; Yang, Q.E.; Davis, J.C.; Griswold, M.D. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc. Nat. Acad. Sci. USA 2013, 110, 543–548. [Google Scholar] [CrossRef]
- Gely-Pernot, A.; Raverdeau, M.; Celebi, C.; Dennefeld, C.; Feret, B.; Klopfenstein, M.; Yoshida, S.; Ghyselinck, N.B.; Mark, M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology 2012, 153, 438–449. [Google Scholar] [CrossRef]
- Snyder, E.M.; Davis, J.C.; Zhou, Q.; Evanoff, R.; Griswold, M.D. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol. Reprod. 2011, 84, 886–893. [Google Scholar] [CrossRef]
- Davis, J.C.; Snyder, E.M.; Hogarth, C.A.; Small, C.; Griswold, M.D. Induction of spermatogenic synchrony by retinoic acid in neonatal mice. Spermatogenesis 2013, 3, e23180. [Google Scholar] [CrossRef]
- Bouillet, P.; Oulad-Abdelghani, M.; Vicaire, S.; Garnier, J.M.; Schuhbaur, B.; Dolle, P.; Chambon, P. Efficient cloning of cdnas of retinoic acid-responsive genes in p19 embryonal carcinoma cells and characterization of a novel mouse gene, stra1 (mouse lerk-2/eplg2). Develop. Biol. 1995, 170, 420–433. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Evanoff, R.; Snyder, E.; Kent, T.; Mitchell, D.; Small, C.; Amory, J.K.; Griswold, M.D. Suppression of stra8 expression in the mouse gonad by win 18,446. Biol. Reprod. 2011, 84, 957–965. [Google Scholar] [CrossRef]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Nat. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef]
- Mark, M.; Jacobs, H.; Oulad-Abdelghani, M.; Dennefeld, C.; Feret, B.; Vernet, N.; Codreanu, C.A.; Chambon, P.; Ghyselinck, N.B. Stra8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensatio. J. Cell Sci. 2008, 121, 3233–3242. [Google Scholar] [CrossRef]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef]
- Piprek, R.P.; Pecio, A.; Laskowska-Kaszub, K.; Kloc, M.; Kubiak, J.Z.; Szymura, J.M. Retinoic acid homeostasis regulates meiotic entry in developing anuran gonads and in bidder's organ through raldh2 and cyp26b1 proteins. Mech. Develop. 2013, 130, 613–627. [Google Scholar] [CrossRef]
- Smith, B.E.; Braun, R.E. Germ cell migration across sertoli cell tight junctions. Science 2012, 338, 798–802. [Google Scholar] [CrossRef]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef]
- Hasegawa, K.; Saga, Y. Retinoic acid signaling in sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development 2012, 139, 4347–4355. [Google Scholar] [CrossRef]
- Kubota, H.; Chiba, H.; Takakuwa, Y.; Osanai, M.; Tobioka, H.; Kohama, G.; Mori, M.; Sawada, N. Retinoid x receptor alpha and retinoic acid receptor gamma mediate expression of genes encoding tight-junction proteins and barrier function in f9 cells during visceral endodermal differentiation. Exp. Cell Res. 2001, 263, 163–172. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Harrison, C.A.; Rainczuk, K.E.; Wayne Vogl, A.; Stanton, P.G. Retinoic acid promotes sertoli cell differentiation and antagonises activin-induced proliferation. Mol. Cell. Endocrinol. 2013, 377, 33–43. [Google Scholar] [CrossRef]
- Chihara, M.; Otsuka, S.; Ichii, O.; Kon, Y. Vitamin A deprivation affects the progression of the spermatogenic wave and initial formation of the blood-testis barrier, resulting in irreversible testicular degeneration in mice. J. Reprod. Dev. 2013, 59, 525–535. [Google Scholar] [CrossRef]
- Chung, S.S.; Choi, C.; Wang, X.; Hallock, L.; Wolgemuth, D.J. Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc. Res. Tech. 2010, 73, 583–596. [Google Scholar]
- Vernet, N.; Dennefeld, C.; Klopfenstein, M.; Ruiz, A.; Bok, D.; Ghyselinck, N.B.; Mark, M. Retinoid x receptor beta (rxrb) expression in sertoli cells controls cholesterol homeostasis and spermiation. Reproduction 2008, 136, 619–626. [Google Scholar] [CrossRef]
- Huang, H.F.; Marshall, G.R. Failure of spermatid release under various vitamin A states-An indication of delayed spermiation. Biol. Reprod. 1983, 28, 1163–1172. [Google Scholar] [CrossRef]
- Chung, S.S.; Sung, W.; Wang, X.; Wolgemuth, D.J. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Develop. Dynam. 2004, 230, 754–766. [Google Scholar] [CrossRef]
- Chung, S.S.; Wang, X.; Wolgemuth, D.J. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development 2009, 136, 2091–2100. [Google Scholar] [CrossRef]
- Chung, S.S.; Wang, X.; Roberts, S.S.; Griffey, S.M.; Reczek, P.R.; Wolgemuth, D.J. Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 2011, 152, 2492–2502. [Google Scholar] [CrossRef]
- Vernet, N.; Dennefeld, C.; Rochette-Egly, C.; Oulad-Abdelghani, M.; Chambon, P.; Ghyselinck, N.B.; Mark, M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006, 147, 96–110. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, R.Y.; Guo, Q.S.; Xu, C. Expression of the retinoic acid-metabolizing enzymes raldh2 and cyp26b1 during mouse postnatal testis development. Asian J. Androl. 2008, 10, 569–576. [Google Scholar] [CrossRef]
- Kasus-Jacobi, A.; Ou, J.; Bashmakov, Y.K.; Shelton, J.M.; Richardson, J.A.; Goldstein, J.L.; Brown, M.S. Characterization of mouse short-chain aldehyde reductase (scald), an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 2003, 278, 32380–32389. [Google Scholar] [CrossRef]
- Akmal, K.M.; Dufour, J.M.; Kim, K.H. Retinoic acid receptor alpha gene expression in the rat testis: Potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol. Reprod. 1997, 56, 549–556. [Google Scholar] [CrossRef]
- Dufour, J.M.; Kim, K.H. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: Identification of potential heterodimeric receptors. Biol. Reprod. 1999, 61, 1300–1308. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Dupe, V.; Dierich, A.; Messaddeq, N.; Garnier, J.M.; Rochette-Egly, C.; Chambon, P.; Mark, M. Role of the retinoic acid receptor beta (rarbeta) during mouse development. Int. J. Develop. Biol. 1997, 41, 425–447. [Google Scholar]
- Krezel, W.; Dupe, V.; Mark, M.; Dierich, A.; Kastner, P.; Chambon, P. Rxr gamma null mice are apparently normal and compound rxr alpha +/-/rxr beta -/-/rxr gamma -/- mutant mice are viable. Proc. Nat. Acad. Sci. USA 1996, 93, 9010–9014. [Google Scholar] [CrossRef]
- Bouillet, P.; Sapin, V.; Chazaud, C.; Messaddeq, N.; Decimo, D.; Dolle, P.; Chambon, P. Developmental expression pattern of stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Develop. 1997, 63, 173–186. [Google Scholar] [CrossRef]
- Berry, D.C.; Jacobs, H.; Marwarha, G.; Gely-Pernot, A.; O'Byrne, S.M.; DeSantis, D.; Klopfenstein, M.; Feret, B.; Dennefeld, C.; Blaner, W.S.; et al. The stra6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J. Biol. Chem. 2013, 288, 24528–24539. [Google Scholar] [CrossRef]
- Rajan, N.; Kidd, G.L.; Talmage, D.A.; Blaner, W.S.; Suhara, A.; Goodman, D.S. Cellular retinoic acid-binding protein messenger RNA: Levels in rat tissues and localization in rat testis. J. Lipid Res. 1991, 32, 1195–1204. [Google Scholar]
- Zheng, W.L.; Bucco, R.A.; Schmitt, M.C.; Wardlaw, S.A.; Ong, D.E. Localization of cellular retinoic acid-binding protein (CRABP) ii and CRABP in developing rat testis. Endocrinology 1996, 137, 5028–5035. [Google Scholar]
- Hogarth, C.A.; Griswold, M.D. Retinoic acid regulation of male meiosis. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 217–223. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kent, T.; Griswold, M.D. Checking the Pulse of Vitamin A Metabolism and Signaling during Mammalian Spermatogenesis. J. Dev. Biol. 2014, 2, 34-49. https://doi.org/10.3390/jdb2010034
Kent T, Griswold MD. Checking the Pulse of Vitamin A Metabolism and Signaling during Mammalian Spermatogenesis. Journal of Developmental Biology. 2014; 2(1):34-49. https://doi.org/10.3390/jdb2010034
Chicago/Turabian StyleKent, Travis, and Michael D. Griswold. 2014. "Checking the Pulse of Vitamin A Metabolism and Signaling during Mammalian Spermatogenesis" Journal of Developmental Biology 2, no. 1: 34-49. https://doi.org/10.3390/jdb2010034



