Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges
Abstract
:1. Introduction
2. Assumption 1: There Are Algal Classes of High and Low Nutritive Quality
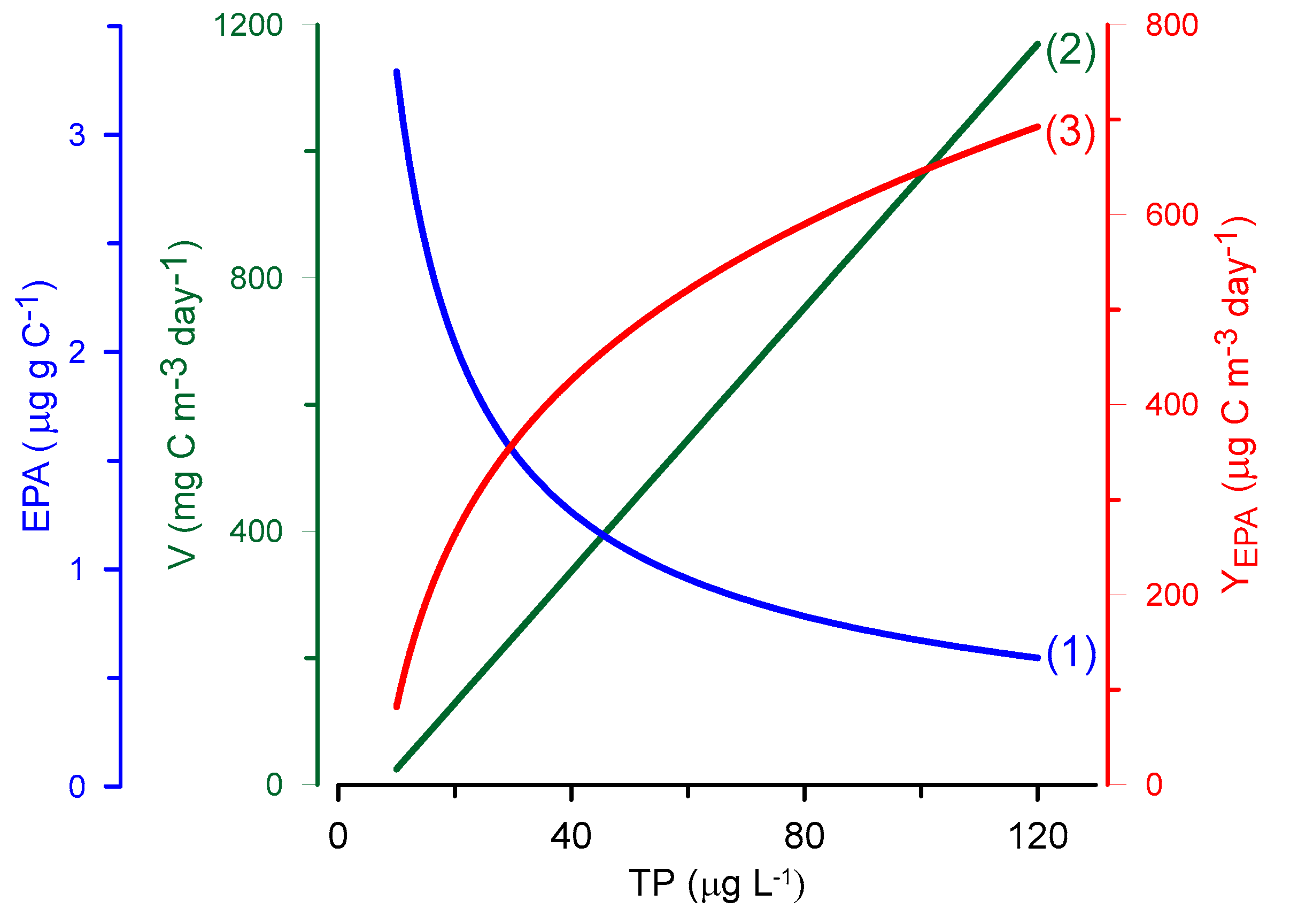
3. Assumption 2: EPA and DHA Decrease with the Increasing Eutrophication of Aquatic Ecosystems
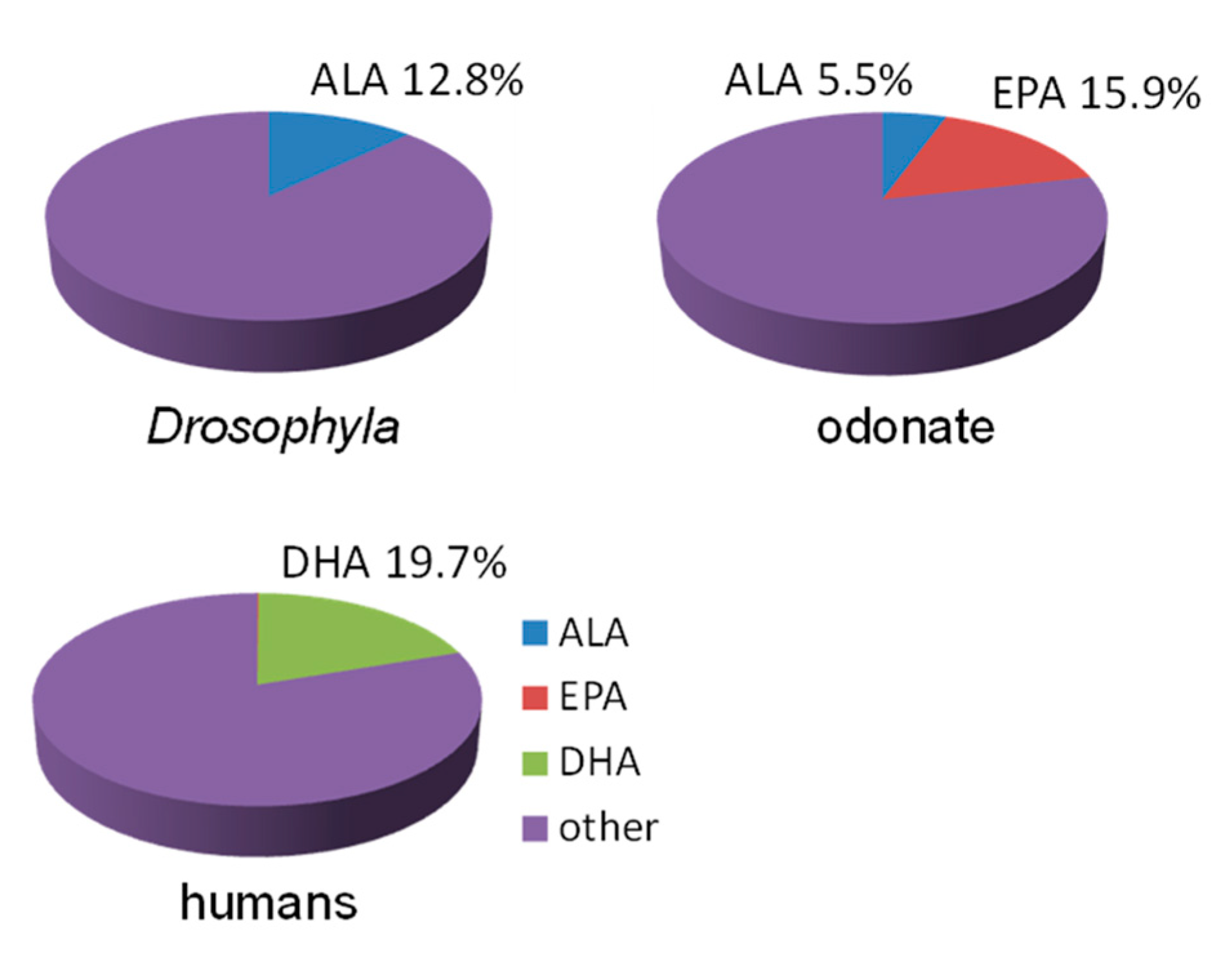
4. Assumption 3: Animals Need EPA and DHA
5. Assumption 4: Fish Are the Main Food Source of EPA and DHA for Humans
6. Assumption 5: Culinary Treatments Decrease EPA and DHA in Products
7. Conclusions
- Dividing microalgae on the basis of their LC-PUFA content into classes of high and low nutritive value appeared to be too coarse. Although there are no Chlorophyceae (green algae) that contain EPA and DHA, there are Bacillariophyceae (diatoms) with low contents of LC-PUFAs.
- The maximum LC-PUFA yield (g km−2 year−1) that can be ultimately obtained by humans occurs in mesotrophic rather than oligotrophic aquatic ecosystems.
- Many animals and terrestrial insects do not need EPA, and aquatic insects do not need DHA in any considerable quantity. Many other animals do not need LC-PUFAs in their food: some worms can obtain these biomolecules from their intestine microflora, and strictly herbivorous terrestrial mammals can synthesize required quantities of EPA and DHA from ALA obtained from the green parts of consumed plants.
- There are many fish species that are not adequate sources of EPA and DHA for humans, especially for those with a Western-type diet. In turn, there are products of terrestrial animals that can be a source of LC-PUFAs for persons who do not eat fish. In human populations with a vegetarian diet, the conversion of dietary C18-PUFAs is considered to be sufficient to meet the demands for LC-PUFAs based on the found genetic patterns; however, this statement requires further study.
- Most common culinary treatments do not decrease the EPA and DHA contents in fish and other animal products.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. Human requirement for n-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.T.; Ackman, R.G.; Holub, B.J. “Essential fatty acids” in aquatic ecosystems: A crucial link between diet and human health and evolution. Can. J. Fish. Aquat. Sci. 2001, 58, 122–137. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jorgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Casula, M.; Soranna, D.; Catapano, A.L.; Corrao, G. Longterm effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, double blind, placebo controlled trials. Atheroscler. Suppl. 2013, 14, 243–251. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, G.; Gustafsson, I.B.; Boberg, M. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 1992, 28, 37–50. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Bai, Y.; Sun, G.; Huang, Y.; Chen, Q.; Han, R.; Li, G.; Li, F. Molecular cloning, characterization, and expression analysis of chicken Δ-6 desaturase. Asian-Australas. J. Anim. Sci. 2010, 23, 116–121. [Google Scholar] [CrossRef]
- Uttaro, A.D. Biosynthesis of polyunsaturated fatty acids in lower eukaryotes. IUBMB Life 2006, 58, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-R.; Green, A.G.; Singh, S.P. Caenorhabditis elegans Δ12-desaturase FAT-2 is a bifunctional desaturase able to desaturate a diverse range of fatty acid substrates at the Δ12 and Δ15 positions. J. Biol. Chem. 2011, 286, 43644–43650. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta 1996, 1301, 7–56. [Google Scholar] [CrossRef]
- Sayanova, O.V.; Napier, J.A. Eicosapentaenoic acid: Biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 2004, 65, 147–158. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–5652. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Arts, M.T.; Sushchik, N.N. Preliminary estimates of the export of omega-3 highly unsaturated fatty acids (EPA+DHA) from aquatic to terrestrial ecosystems. In Lipids in Aquatic Ecosystems; Arts, M.T., Kainz, M., Brett, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 179–209. [Google Scholar]
- Sterner, R.W.; Schulz, K.L. Zooplankton nutrition: Recent progress and a reality check. Aquat. Ecol. 1998, 32, 261–279. [Google Scholar] [CrossRef]
- Ahlgren, G.; Lundstedt, L.; Brett, M.; Forsberg, C. Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. J. Plankton Res. 1990, 12, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Muller-Navarra, D.C. Biochemical versus mineral limitation in Daphnia. Limnol. Oceanogr. 1995, 40, 1209–1214. [Google Scholar] [CrossRef]
- Gulati, R.D.; DeMott, W.R. The role of food quality for zooplankton: Remarks on the state-of-the-art, perspectives and priorities. Freshw. Biol. 1997, 38, 753–768. [Google Scholar] [CrossRef]
- Wacker, A.; Becher, P.; Von Elert, E. Food quality effects of unsaturated fatty acids on larvae of the zebra mussel Dreissena polymorpha. Limnol. Oceanogr. 2002, 47, 1242–1248. [Google Scholar] [CrossRef]
- Chu, F.L.E.; Lund, E.D.; Podbesek, J.A. Quantitative significance of n-3 essential fatty acid contribution by heterotrophic protists in marine pelagic food webs. Mar. Ecol. Prog. Ser. 2008, 354, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Martin-Creuzburg, D.; Wacker, A.; Basena, T. Interactions between limiting nutrients: Consequences for somatic and population growth of Daphnia magna. Limnol. Oceanogr. 2010, 55, 2597–2607. [Google Scholar] [CrossRef]
- Peltomaa, E.T.; Aalto, S.L.; Vuorio, K.M.; Taipale, S.J. The Importance of phytoplankton biomolecule availability for secondary production. Front. Ecol. Evol. 2017, 5, 128. [Google Scholar] [CrossRef]
- Taipale, S.; Strandberg, U.; Peltomaa, E.; Galloway, A.W.E.; Ojala, A.; Brett, M.T. Fatty acid composition as biomarkers of freshwater microalgae: Analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquat. Microb. Ecol. 2013, 71, 165–178. [Google Scholar] [CrossRef]
- Petkov, G.; Garcia, G. Which are fatty acids of the green alga Chlorella. Biochem. Syst. Ecol. 2007, 35, 281–285. [Google Scholar] [CrossRef]
- Iliev, I.; Petkov, G.; Lukavsky, J.; Furnadzhieva, S.; Andreeva, R. Do cyanobacterial lipids contain fatty acids longer than 18 carbon atoms. Z. Naturforsch. C 2011, 66, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Weers, P.M.M.; Gulati, R.D. Growth and reproduction of Daphnia galeata response to changes in fatty acids, phosphorus, and nitrogen in Chlamydomonas reinhardtii. Limnol. Oceanogr. 1997, 42, 1584–1589. [Google Scholar] [CrossRef]
- Nanton, D.A.; Castell, J.D. The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use a live food for marine fish larvae. Aquaculture 1998, 163, 251–261. [Google Scholar] [CrossRef]
- Wacker, A.; Von Elert, E. Polyunsaturated fatty acids: Evidence for non-substitutable biochemical resources in Daphnia galeata. Ecology 2001, 82, 2507–2520. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Gladyshev, M.I.; Kalachova, G.S.; Kravchuk, E.S.; Dubovskaya, O.P.; Ivanova, E.A. Particulate fatty acids in two small Siberian reservoirs dominated by different groups of phytoplankton. Freshw. Biol. 2003, 48, 394–403. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Chen, X.; Wakeham, S.G.; Fisher, N.S. Influence of iron on fatty acid and sterol composition of marine phytoplankton and copepod consumers. Limnol. Oceanogr. 2011, 56, 716–724. [Google Scholar] [CrossRef]
- Claustre, H.; Marty, J.C.; Cassiani, L.; Dagaut, J. Fatty acid dynamics in phytoplankton and microzooplankton communities during a spring bloom in the coastal Ligurian Sea: Ecological implications. Mar. Microb. Food Webs 1989, 3, 51–66. [Google Scholar]
- Sushchik, N.N.; Gladyshev, M.I.; Makhutova, O.N.; Kalachova, G.S.; Kravchuk, E.S.; Ivanova, E.A. Associating particulate essential fatty acids of the ω3 family with phytoplankton species composition in a Siberian reservoir. Freshw. Biol. 2004, 49, 1206–1219. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Gladyshev, M.I.; Ivanova, E.A.; Kravchuk, E.S. Seasonal distribution and fatty acid composition of littoral microalgae in the Yenisei River. J. Appl. Phycol. 2010, 22, 11–24. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Kolmakova, A.A.; Kalachova, G.S.; Kravchuk, E.S.; Ivanova, E.A.; Makhutova, O.N. Seasonal correlations of elemental and ω3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Siberian Reservoir. Aquat. Ecol. 2007, 41, 9–23. [Google Scholar] [CrossRef]
- Ahlgren, G.; Sonesten, L.; Boberg, M.; Gustafsson, I.-B. Fatty acid content of some freshwater fish in lakes of different trophic levels—A bottom-up effect? Ecol. Freshw. Fish 1996, 5, 15–27. [Google Scholar] [CrossRef]
- Taipale, S.J.; Vuorioc, K.; Strandberg, U.; Kahilainen, K.K.; Jarvinen, M.; Hiltunen, M.; Peltomaa, E.; Kankaala, P. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 2016, 96, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Navarra, D.C.; Brett, M.T.; Park, S.; Chandra, S.; Ballantyne, A.P.; Zorita, E.; Goldman, C.R. Unsaturated fatty acid content in seston and tropho-dynamic coupling in lakes. Nature 2004, 427, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Razavi, N.R.; Arts, M.T.; Qua, M.; Jin, B.; Rend, W.; Wang, Y.; Campbell, L.M. Effect of eutrophication on mercury, selenium, and essential fatty acids in Bighead Carp (Hypophthalmichthys nobilis) from reservoirs of eastern China. Sci. Total Environ. 2014, 499, 36–46. [Google Scholar] [CrossRef]
- Smith, V.H. Nutrient dependence of primary productivity in lakes. Limnol. Oceanogr. 1979, 24, 1051–1064. [Google Scholar] [CrossRef]
- Gladyshev, M.I. Quality and quantity of biological production in water bodies with different concentration of phosphorus: Case study of Eurasian perch. Dokl. Biochem. Biophys. 2018, 478, 1–3. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Gladyshev, M.I.; Makhutova, O.N.; Kravchuk, E.S.; Dubovskaya, O.P.; Kalacheva, G.S. Seasonal transfer of the pool of the essential eicosapentaenoic acid along the pelagic trophic chain of a eutrophic reservoir. Dokl. Biol. Sci. 2008, 422, 355–356. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G., Jr.; Flecker, A.S. Highly unsaturated fatty acids in nature: What we know and what we need to learn. Oikos 2015, 125, 749–760. [Google Scholar] [CrossRef]
- Hixson, S.M.; Sharma, B.; Kainz, M.J.; Wacker, A.; Arts, M.T. Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: A fundamental dichotomy between freshwater and terrestrial ecosystems. Environ. Rev. 2015, 23, 414–424. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Buckner, J.S.; Hagen, M.M. Triacylglycerol and phospholipid fatty acids of the silverleaf whitefly: Composition and biosynthesis. Arch. Insect Biochem. Physiol. 2003, 53, 66–79. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, D.S.; Bolewicz, L.; Connor, W.E. The predominance of polyunsaturated fatty acids in the butterfly Morpho peleides before and after metamorphosis. J. Lipid Res. 2006, 47, 530–536. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sanchez-Muros, M.J.; Venegas, E.; Martinez-Sanchez, A.; Perez-Ban, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Sanchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Eicosanoid signaling in insects: From discovery to plant protection. Crit. Rev. Plant Sci. 2014, 33, 20–63. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Yurchenko, Y.A.; Gladyshev, M.I.; Belevich, O.E.; Kalachova, G.S.; Kolmakova, A.A. Comparison of fatty acid contents and composition in major lipid classes of larvae and adults of mosquitoes (Diptera: Culicidae) from a steppe region. Insect Sci. 2013, 20, 585–600. [Google Scholar] [CrossRef]
- Borisova, E.V.; Makhutova, O.N.; Gladyshev, M.I.; Sushchik, N.N. Fluxes of biomass and essential polyunsaturated fatty acids from water to land via chironomid emergence from a mountain lake. Contemp. Probl. Ecol. 2016, 9, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Makhutova, O.N.; Borisova, E.V.; Shulepina, S.P.; Kolmakova, A.A.; Sushchik, N.N. Fatty acid composition and content in chironomid species at various life stages dominating in a saline Siberian lake. Contemp. Probl. Ecol. 2017, 10, 230–239. [Google Scholar] [CrossRef]
- Popova, O.N.; Haritonov, A.Y.; Sushchik, N.N.; Makhutova, O.N.; Kalachova, G.S.; Kolmakova, A.A.; Gladyshev, M.I. Export of aquatic productivity, including highly unsaturated fatty acids, to terrestrial ecosystems via Odonata. Sci. Total Environ. 2017, 581, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.; Rotstein, N.; Carri, N. Effects of docosahexaenoic acid on retinal development: Cellular and molecular aspects. Lipids 2001, 36, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.B.; Ménagé, C.; Grégoire, S.; Garcia, T.; Ferveur, J.-F.; Bretillon, L.; Grosjean, Y. Lack of dietary polyunsaturated fatty acids causes synapse dysfunction in the Drosophila visual system. PLoS ONE 2015, 10, e0135353. [Google Scholar] [CrossRef] [PubMed]
- Sushchik, N.N.; Popova, O.N.; Makhutova, O.N.; Gladyshev, M.I. Fatty acid composition of odonate’s eyes. Dokl. Biochem. Biophys. 2017, 475, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, L.; Jeannotte, R.; Whalen, J.K. Trophic transfer of fatty acids from gut microbiota to the earthworm Lumbricus terrestris L. Soil Biol. Biochem. 2006, 38, 2188–2198. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Makhutova, O.N.; Kalachova, G.S.; Rechkina, E.A.; Malyshevskaya, K.K. Effect of the way of cooking on contents of essential polyunsaturated fatty acids in filets of zander. Czech. J. Food Sci. 2014, 32, 226–231. [Google Scholar] [CrossRef]
- Manukovsky, N.S.; Kovalev, V.S.; Gribovskaya, I.V. Two-stage biohumus production from inedible potato biomass. Bioresour. Technol. 2001, 78, 273–275. [Google Scholar] [CrossRef]
- Menzel, R.; von Chrzanowski, H.; Tonat, T.; van Riswyck, K.; Schliesser, P.; Ruess, L. Presence or absence? Primary structure, regioselectivity and evolution of Delta 12/omega 3 fatty acid desaturases in nematodes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef]
- Infante, J.P.; Kirwan, R.C.; Brenna, J.T. High levels of docosahexaenoic acid (22:6n-3)-containing phospholipids in high-frequency contraction muscles of hummingbirds and rattlesnakes. Comp. Biochem. Physiol. Part B 2001, 130, 291–298. [Google Scholar] [CrossRef]
- Geiser, F.; McAllan, B.M.; Kenagy, G.J.; Hiebert, S.M. Photoperiod affects daily torpor and tissue fatty acid composition in deer mice. Naturwissenschaften 2007, 94, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Klaiman, J.M.; Price, E.R.; Guglielmo, C.G. Fatty acid composition of pectoralis muscle membrane, intramuscular fat stores and adipose tissue of migrant and wintering white-throated sparrows (Zonotrichia albicollis). J. Exp. Biol. 2009, 212, 3865–3872. [Google Scholar] [CrossRef]
- Stawski, C.; Valencak, T.G.; Ruf, T.; Sadowska, E.T.; Dheyongera, G.; Rudolf, A.; Maiti, U.; Koteja, P. Effect of selection for high activity-related metabolism on membrane phospholipid fatty acid composition in bank voles. Physiol. Biochem. Zool. 2015, 88, 668–679. [Google Scholar] [CrossRef]
- Fritz, K.A.; Kirschman, L.J.; McCay, S.D.; Trushenski, J.T.; Warne, R.W.; Whiles, M.R. Subsidies of essential nutrients from aquatic environments correlate with immune function in terrestrial consumers. Freshw. Sci. 2017, 36, 893–900. [Google Scholar] [CrossRef]
- Twining, C.W.; Lawrence, P.; Winkler, D.W.; Flecker, A.S.; Brenna, J.T. Conversion efficiency of α-linolenic acid to omega-3 highly unsaturated fatty acids in aerial insectivore chicks. J. Exp. Biol. 2018, 221, jeb165373. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.S. Production of eicosapentaenoic and docosahexaenoic acid-containing oils in transgenic land plants for human and aquaculture nutrition. Mar. Biotechnol. 2006, 8, 103–109. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 2, 781–792. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Makhutova, O.N.; Gubanenko, G.A.; Rechkina, E.A.; Kalachova, G.S.; Sushchik, N.N. Livers of terrestrial production animals as a source of long-chain polyunsaturated fatty acids for humans: An alternative to fish? Eur. J. Lipid Sci. Technol. 2015, 117, 1417–1421. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kleiner, A.C.; Freiser, H.H.; Santerre, C.R. Fatty acid profiles of commercially available finfish fillets in the United States. Lipids 2014, 49, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Glushchenko, L.A.; Makhutova, O.N.; Rudchenko, A.E.; Shulepina, S.P.; Dubovskaya, O.P.; Zuev, I.V.; Kolmakov, V.I.; Sushchik, N.N. Comparative analysis of content of omega-3 polyunsaturated fatty acids in food and muscle tissue of fish from aquaculture and natural habitats. Contemp. Probl. Ecol. 2018, 11, 297–308. [Google Scholar] [CrossRef]
- Teoh, C.Y.; Ng, W.K. The implications of substituting dietary fish oilwith vegetable oils on the growth performance, fillet fatty acid profile and modulation of the fatty acid elongase, desaturase and oxidation activities of red hybrid tilapia, Oreochromis sp. Aquaculture 2016, 465, 311–322. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Gagnon, C.; Swist, E.; Rondeau, I.; Massarelli, I.; Cheung, W.; Ratnayake, W.M.N. EPA and DHA status of South Asian and white Canadians living in the National Capital Region of Canada. Lipids 2014, 49, 1057–1069. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Popova, O.N.; Makhutova, O.N.; Zinchenko, T.D.; Golovatyuk, L.V.; Yurchenko, Y.A.; Kalachova, G.S.; Krylov, A.V.; Sushchik, N.N. Comparison of fatty acid compositions in birds feeding in aquatic and terrestrial ecosystems. Contemp. Probl. Ecol. 2016, 9, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Henry, C.J. Omega-3 Polyunsaturated Fatty Acid Metabolism in Vegetarians. In Polyunsaturated Fatty Acid Metabolism; Burdge, G.C., Ed.; Elsevier Inc.: Amsterdam, The Netherland; AOCS Press: Urbana, IL, USA, 2018; pp. 193–204. [Google Scholar]
- Burdge, G.C. Is essential fatty acid interconversion an important source of PUFA in humans? Br. J. Nutr. 2019, 121, 615–624. [Google Scholar] [CrossRef]
- Kothapalli, K.S.; Ye, K.; Gadgil, M.S.; Carlson, S.E.; O’Brien, K.O.; Zhang, J.Y.; Park, H.G.; Ojukwu, K.; Zou, J.; Hyon, S.S.; et al. Positive selection on a regulatory insertion-deletion polymorphism in FADS2 influences apparent endogenous synthesis of arachidonic acid. Mol. Biol. Evol. 2016, 33, 1726–1739. [Google Scholar] [CrossRef]
- Buckley, M.T.; Racimo, F.; Allentoft, M.E.; Jensen, M.K.; Jonsson, A.; Huang, H.; Hormozdiari, F.; Sikora, M.; Marnetto, D.; Eskin, E.; et al. Selection in Europeans on fatty acid desaturases associated with dietary changes. Mol. Biol. Evol. 2017, 34, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision nutrition and omega-3 polyunsaturated fatty acids: A case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.; Ventanas, S.; Cava, R. Oxidation of lipids and proteins in frankfurters with different fatty acid compositions and tocopherol and phenolic contents. Food Chem. 2007, 100, 55–63. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Marin, F.R.; Ocana, A.; Soler-Rivas, C. Effect of domestic processing on bioactive compounds. Phytochem. Rev. 2008, 7, 345–384. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Armenteros, M.; Morcuende, D. Mediterranean berries as inhibitors of lipid oxidation in porcine burger patties subjected to cooking and chilled storage. J. Integr. Agric. 2013, 12, 1982–1992. [Google Scholar] [CrossRef]
- Ohshima, T.; Shozen, K.; Usio, H.; Koizumi, C. Effects of grilling on formation of cholesterol oxides in seafood products rich in polyunsaturated fatty acids. LWT-Food Sci. Technol. 1996, 29, 94–99. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Visentainer, J.V.; Matsushita, M.; de Souza, N.E. Proximate composition, cholesterol and fatty acids profile of canned sardines (Sardinella brasiliensis) in soybean oil and tomato sauce. Food Chem. 2004, 88, 1–6. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Bastos, D.H.M.; Soares, R.A.M.; Queiroz, Y.S.; Torres, E.A.F.S. Fatty acids and cholesterol oxidation in salted and dried shrimp. Food Chem. 2006, 95, 344–351. [Google Scholar] [CrossRef]
- De Castro, F.A.F.; Sant’Ana, H.M.P.; Campos, F.M.; Costa, N.M.B.; Silva, M.T.C.; Salaro, A.L.; Franceschini, S.D.C.C. Fatty acid composition of three freshwater fishes under different storage and cooking processes. Food Chem. 2007, 103, 1080–1090. [Google Scholar] [CrossRef]
- Saldanha, T.; Bragagnolo, N. Relation between types of packaging, frozen storage and grilling on cholesterol and fatty acids oxidation in Atlantic hake fillets (Merluccius hubbsi). Food Chem. 2008, 106, 619–627. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victorio, A.D.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Mnari, A.B.; Jrah, H.H.; Dhibi, M.; Bouhlel, I.; Hammami, M.; Chaouch, A. Nutritional fatty acid quality of raw and cooked farmed and wild sea bream (Sparus aurata). J. Agric. Food Chem. 2010, 58, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Zotos, A.; Kotaras, A.; Mikras, E. Effect of baking of sardine (Sardina pilchardus) and frying of anchovy (Engraulis encrasicholus) in olive and sunflower oil on their quality. Food Sci. Technol. Int. 2013, 19, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sampels, S.; Zajíc, T.; Mráz, J. Effects of frying fat and preparation on carp (Cyprinus carpio) fillet lipid composition and oxidation. Czech J. Food Sci. 2014, 32, 493–502. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Sampaio, G.R.; Keller, L.M.; Sawaya, A.C.H.F.; Chavez, D.W.H.; Torres, E.A.F.S.; Saldanha, T. Impact of air frying on cholesterol and fatty acids oxidation in sardines: Protective effects of aromatic herbs. J. Food Sci. 2017, 82, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Shalini, R.; Jeya Shakila, R.; Palani Kumar, M.; Jeyasekaran, G. Changes in the pattern of health beneficial omega 3 fatty acids during processing of sardine fish curry. Indian J. Fish. 2017, 64, 260–263. [Google Scholar] [CrossRef]
- Dong, X.P.; Li, D.Y.; Huang, Y.; Wu, Q.; Liu, W.T.; Qin, L.; Zhou, D.Y.; Prakash, S.; Yu, C.X. Nutritional value and flavor of turbot (Scophthalmus maximus) muscle as affected by cooking methods. Int. J. Food Prop. 2018, 21, 1972–1985. [Google Scholar] [CrossRef]
- Chaula, D.; Laswai, H.; Chove, B.; Dalsgaard, A.; Mdegela, R.; Hyldig, G. Fatty acid profiles and lipid oxidation status of sun dried, deep fried, and smoked sardine (Rastrineobola argentea) from Lake Victoria, Tanzania. J. Aquat. Food Prod. Technol. 2019, 2, 165–176. [Google Scholar] [CrossRef]
- Candela, M.; Astiasaran, I.; Bello, J. Deep-fat frying modifies high-fat fish lipid fraction. J. Agric. Food Chem. 1998, 46, 2793–2796. [Google Scholar] [CrossRef]
- Echarte, M.; Zulet, M.A.; Astiasaran, I. Oxidation process affecting fatty acids and cholesterol in fried and roasted salmon. J. Agric. Food Chem. 2001, 49, 5662–5667. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; Haak, L.; Raes, K.; Hermans, C.; De Henauw, S.; De Smet, S.; Van Camp, J. Effects of pan-frying in margarine and olive oil on the fatty acid composition of cod and salmon. Food Chem. 2006, 98, 609–617. [Google Scholar] [CrossRef]
- Stolyhwo, A.; Kolodziejska, I.; Sikorski, Z.E. Long chain polyunsaturated fatty acids in smoked Atlantic mackerel and Baltic sprats. Food Chem. 2006, 94, 589–595. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of way of cooking on content of essential polyunsaturated fatty acids in muscle tissue of humpback salmon (Oncorhynchus gorbuscha). Food Chem. 2006, 96, 446–451. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem. 2007, 101, 1694–1700. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N.; Kalachova, G.S. Content of essential polyunsaturated fatty acids in three canned fish species. Int. J. Food Sci. Nutr. 2009, 60, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Haak, L.; Sioen, I.; Raes, K.; Van Camp, J.; De Smet, S. Effect of pan-frying in different culinary fats on the fatty acid profile of pork. Food Chem. 2007, 102, 857–864. [Google Scholar] [CrossRef]
- Yanar, Y.; Büyükçapar, H.M.; Yanar, M.; Göcer, M. Effect of carotenoids from red pepper and marigold flower on pigmentation, sensory properties and fatty acid composition of rainbow trout. Food Chem. 2007, 100, 326–330. [Google Scholar] [CrossRef]
- Ansorena, D.; Guembe, A.; Mendizabal, T.; Astiasaran, I. Effect of fish and oil nature on frying process and nutritional product quality. J. Food Sci. 2010, 75, H62–H67. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.R.; Bhavsar, S.P.; Braekevelt, E.; Arts, M.T. Effects of different cooking methods on fatty acid profiles in four freshwater fishes from the Laurentian Great Lakes region. Food Chem. 2014, 164, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.K.Y.; Tomita, H.; Takemori, T. Mechanisms of docosahexaenoic and eicosapentaenoic acid loss from Pacific saury and comparison of their retention rates after various cooking methods. J. Food Sci. 2016, 81, C1899–C1907. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, S.L.; Scholljegerdes, E.J.; Maddock, R.J.; Barcelo-Coblijn, G.; Murphy, E.J. Rump and shoulder muscles from grass and linseed fed cattle as important sources of n-3 fatty acids for beef consumers. Eur. J. Lipid Sci. Technol. 2017, 119, 1600390. [Google Scholar] [CrossRef]
- Litzow, M.A.; Bailey, K.M.; Prahl, F.G.; Heintz, R. Climate regime shifts and reorganization of fish communities: The essential fatty acid limitation hypothesis. Mar. Ecol. Prog. Ser. 2006, 315, 1–11. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.B.; Fearon, A.M. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Turchini, G.M.; Hermon, K.M.; Francis, D.S. Fatty acids and beyond: Fillet nutritional characterisation of rainbow trout (Oncorhynchus mykiss) fed different dietary oil sources. Aquaculture 2018, 491, 391–397. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Tolomeev, A.P.; Dgebuadze, Y.Y. Meta-analysis of factors associated with omega-3 fatty acid contents of wild fish. Rev. Fish. Biol. Fish. 2018, 28, 277–299. [Google Scholar] [CrossRef]
- Mairesse, G.; Thomas, M.; Gardeur, J.-N.; Brun-Bellut, J. Effects of geographic source rearing system, and season on the nutritional quality of wild and farmed Perca fluviatilis. Lipids 2006, 41, 221–229. [Google Scholar] [CrossRef]
- Kiessling, A.; Pickova, J.; Johansson, L.; Esgerd, T.; Storebakken, T.; Kiessling, K.-H. Changes in fatty acid composition in muscle and adipose tissue of farmed rainbow trout (Oncorhynchus mykiss) in relation to ration and age. Food Chem. 2001, 73, 271–284. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Calduch-Giner, J.A.; Ballester-Lozano, G.F.; Pérez-Sánchez, J. Effect of ration size on fillet fatty acid composition, phospholipid allostasis and mRNA expression patterns of lipid regulatory genes in gilthead sea bream (Sparus aurata). Br. J. Nutr. 2013, 109, 1175–1187. [Google Scholar] [CrossRef]
- Moths, M.D.; Dellinger, J.A.; Holub, B.; Ripley, M.P.; McGraw, J.E.; Kinnunen, R.E. Omega-3 fatty acids in fish from the Laurentian Great Lakes tribal fisheries. Hum. Ecol. Risk Assess. 2013, 19, 1628–1643. [Google Scholar] [CrossRef]
- Huynh, M.D.; Kitts, D.D. Evaluating nutritional quality of pacific fish species from fatty acid signatures. Food Chem. 2009, 114, 912–918. [Google Scholar] [CrossRef]
- Joordens, J.C.A.; Kuipers, R.S.; Wanink, J.H.; Muskiet, F.A.J. A fish is not a fish: Patterns in fatty acid composition of aquatic food may have had implications for hominin evolution. J. Hum. Evol. 2014, 77, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Anishchenko, O.V.; Sushchik, N.N.; Makhutova, O.N.; Kalachova, G.S.; Gribovskaya, I.V.; Morgun, V.N.; Gladyshev, M.I. Benefit-risk ratio of canned pacific saury (Cololabis saira) intake: Essential fatty acids vs. heavy metals. Food Chem. Toxicol. 2017, 101, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.; Quek, S.Y.; Eyres, L. Effect of cooking method on the fatty acid profile of New Zealand King Salmon (Oncorhynchus tshawytscha). Food Chem. 2010, 119, 785–790. [Google Scholar] [CrossRef]
- Simon, S.J.G.B.; Sancho, R.A.S.; Lima, F.A.; Cabral, C.C.V.Q.; Souza, T.M.; Bragagnolo, N.; Lira, G.M. Interaction between soybean oil and the lipid fraction of fried pitu prawn. LWT-Food Sci. Technol. 2012, 48, 120–126. [Google Scholar] [CrossRef]
- Amira, M.B.; Hanene, J.H.; Madiha, D.; Imen, B.; Mohamed, H.; Abdelhamid, C. Effects of frying on the fatty acid composition in farmed and wild gilthead sea bream (Sparus aurata). Int. J. Food Sci. Technol. 2010, 45, 113–123. [Google Scholar] [CrossRef]
- Kouba, M.; Benatmane, F.; Blochet, J.E.; Mourot, J. Effect of a linseed diet on lipid oxidation, fatty acid composition of muscle, perirenal fat, and raw and cooked rabbit meat. Meat Sci. 2008, 80, 829–834. [Google Scholar] [CrossRef]
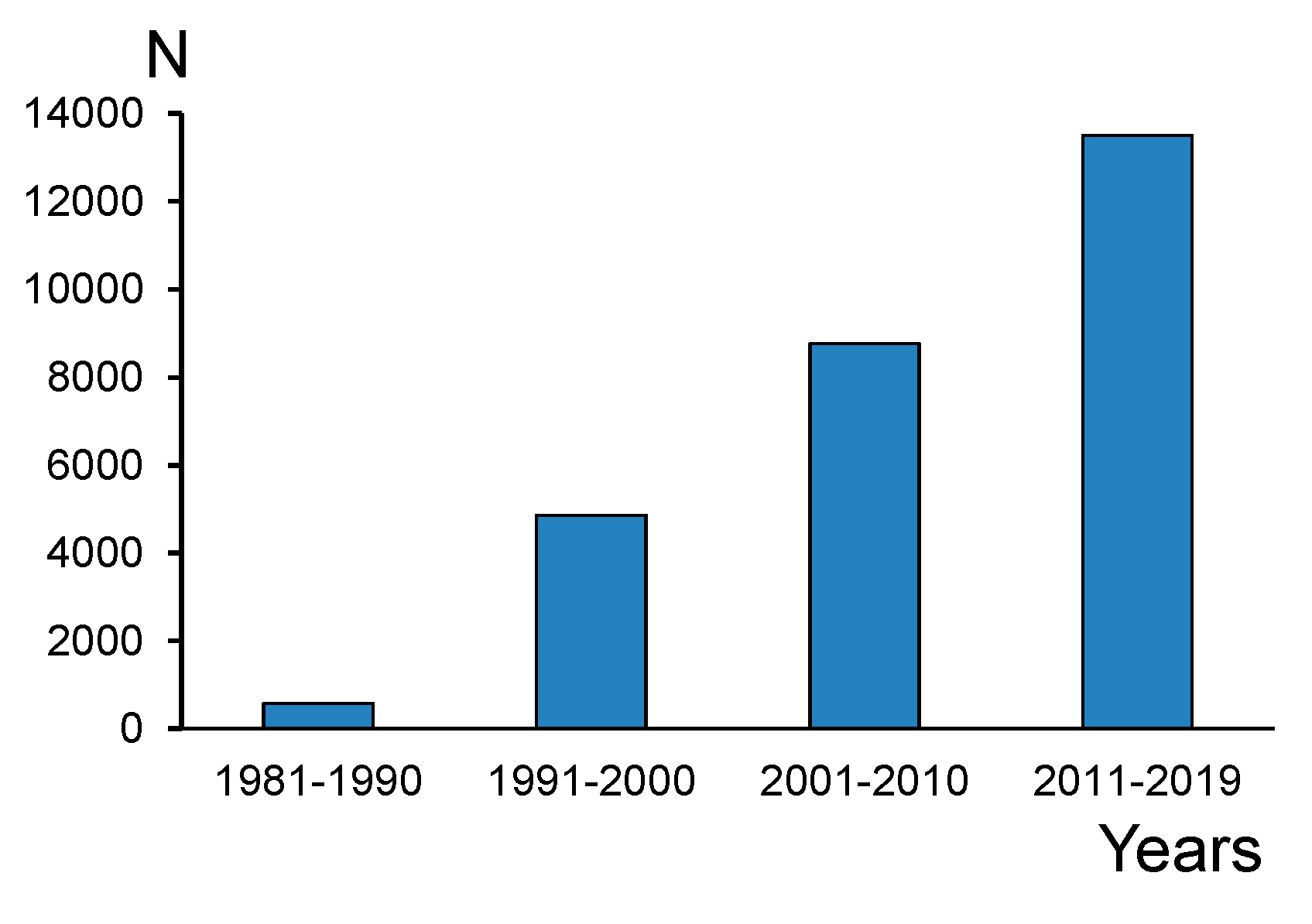
| Product | EPA + DHA | DP | Reference |
|---|---|---|---|
| Atlantic salmon Salmo salar (fried) | 40.1 | 25 | [121] |
| Pacific saury Cololabis saira (canned, brand H) | 37.9 | 26 | [136] |
| Atlantic salmon Salmo salar (fried) | 19.6 | 51 | [114] |
| Pacific herring Clupea harengus (canned) | 17.9 | 56 | [118] |
| Atlantic salmon Salmo salar (fried) | 17.0 | 59 | [112] |
| Baltic sprat Sprattus sprattus (canned) | 14.3 | 70 | [118] |
| Pacific saury Cololabis saira (canned, brand N) | 13.1 | 76 | [136] |
| King salmon Oncorhynchus tshawytscha (baked) | 12.4 | 81 | [137] |
| Lake trout Salvelinus namaycush (baked) | 12.4 | 81 | [122] |
| Lake trout Salvelinus namaycush (fried) | 12.4 | 81 | [122] |
| Lake trout Salvelinus namaycush (broiled) | 12.3 | 81 | [122] |
| King salmon Oncorhynchus tshawytscha (steamed) | 11.9 | 84 | [137] |
| King salmon Oncorhynchus tshawytscha (fried) | 11.5 | 87 | [137] |
| King salmon Oncorhynchus tshawytscha (microwaved) | 10.4 | 96 | [137] |
| King salmon Oncorhynchus tshawytscha (poached) | 10.0 | 100 | [137] |
| Sardine Sardina pilchardus (fried) | 8.8 | 114 | [112] |
| Humpback salmon Oncorhynchus gorbuscha (boiled) | 6.0 | 167 | [116] |
| Brown trout Salmo trutta (boiled) | 5.7 | 175 | [117] |
| Humpback salmon Oncorhynchus gorbuscha (stewed) | 5.3 | 189 | [116] |
| Humpback salmon Oncorhynchus gorbuscha (roasted) | 5.0 | 200 | [116] |
| Humpback salmon Oncorhynchus gorbuscha (fried) | 4.3 | 233 | [116] |
| Brown trout Salmo trutta (fried) | 4.1 | 244 | [117] |
| Cod Gadus morhua (fried) | 4.1 | 244 | [114] |
| Spanish mackerel Scomberomorus commerson (fried) | 3.9 | 256 | [112] |
| Pacific herring Clupea harengus (boiled) | 3.9 | 256 | [117] |
| Pacific herring Clupea harengus (fried) | 3.8 | 263 | [117] |
| Rock sole Lepidopsetta bilineata (boiled) | 3.6 | 278 | [117] |
| Chinook salmon Oncorhynchus tshawytscha (fried) | 3.2 | 313 | [122] |
| Rock sole Lepidopsetta bilineata (fried) | 3.1 | 323 | [117] |
| Chinook salmon Oncorhynchus tshawytscha (baked) | 3.1 | 323 | [122] |
| White sucker Catostomus commersonii (baked) | 2.3 | 435 | [122] |
| Cod Gadus morhua (boiled) | 2.4 | 417 | [117] |
| Chinook salmon Oncorhynchus tshawytscha (fried) | 2.8 | 357 | [122] |
| Cod Gadus morhua (fried) | 2.2 | 455 | [121] |
| Walleye (Sander vitreus) (baked) | 2.1 | 476 | [122] |
| White sucker Catostomus commersonii (broiled) | 2.1 | 476 | [122] |
| White sucker Catostomus commersonii (fried) | 2.0 | 500 | [122] |
| Walleye (Sander vitreus) (broiled) | 1.9 | 526 | [122] |
| Walleye (Sander vitreus) (fried) | 1.9 | 526 | [122] |
| Prawn Macrobrachium acanthurus (fried) | 1.8 | 556 | [138] |
| Beef liver (boiled) | 1.3 | 769 | [83] |
| Zander Sander lucioperca (boiled) | 1.1 | 909 | [70] |
| Pork liver (boiled) | 1.0 | 1000 | [83] |
| Zander Sander lucioperca (stewed) | 1.0 | 1000 | [70] |
| Zander Sander lucioperca (fried) | 1.0 | 1000 | [70] |
| Common carp Cyprinus carpio (fried) | 1.0 | 1000 | [122] |
| Chicken liver (boiled) | 0.7 | 1429 | [83] |
| Common carp Cyprinus carpio (baked) | 0.7 | 1429 | [122] |
| Gilthead sea bream Sparus aurata (fried) | 0.6 | 1667 | [139] |
| Common carp Cyprinus carpio (broiled) | 0.5 | 2000 | [122] |
| Pork (fried) | 0.3 | 3333 | [119] |
| White rabbit (baked) | 0.1 | 10000 | [140] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladyshev, M.I.; Sushchik, N.N. Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges. Biomolecules 2019, 9, 485. https://doi.org/10.3390/biom9090485
Gladyshev MI, Sushchik NN. Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges. Biomolecules. 2019; 9(9):485. https://doi.org/10.3390/biom9090485
Chicago/Turabian StyleGladyshev, Michail I., and Nadezhda N. Sushchik. 2019. "Long-chain Omega-3 Polyunsaturated Fatty Acids in Natural Ecosystems and the Human Diet: Assumptions and Challenges" Biomolecules 9, no. 9: 485. https://doi.org/10.3390/biom9090485






