Origin of Carbon and Essential Fatty Acids in Higher Trophic Level Fish in Headwater Stream Food Webs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analyses
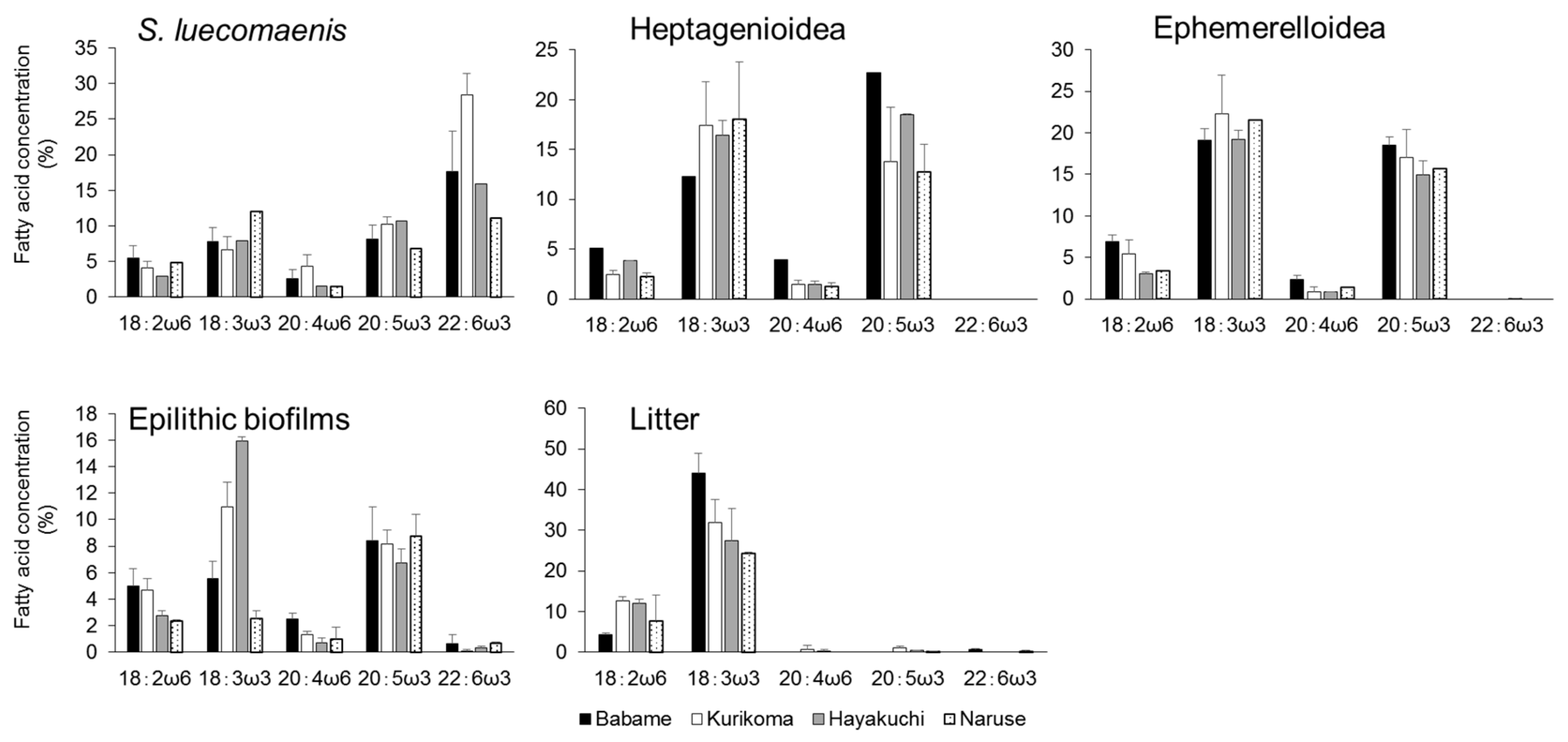
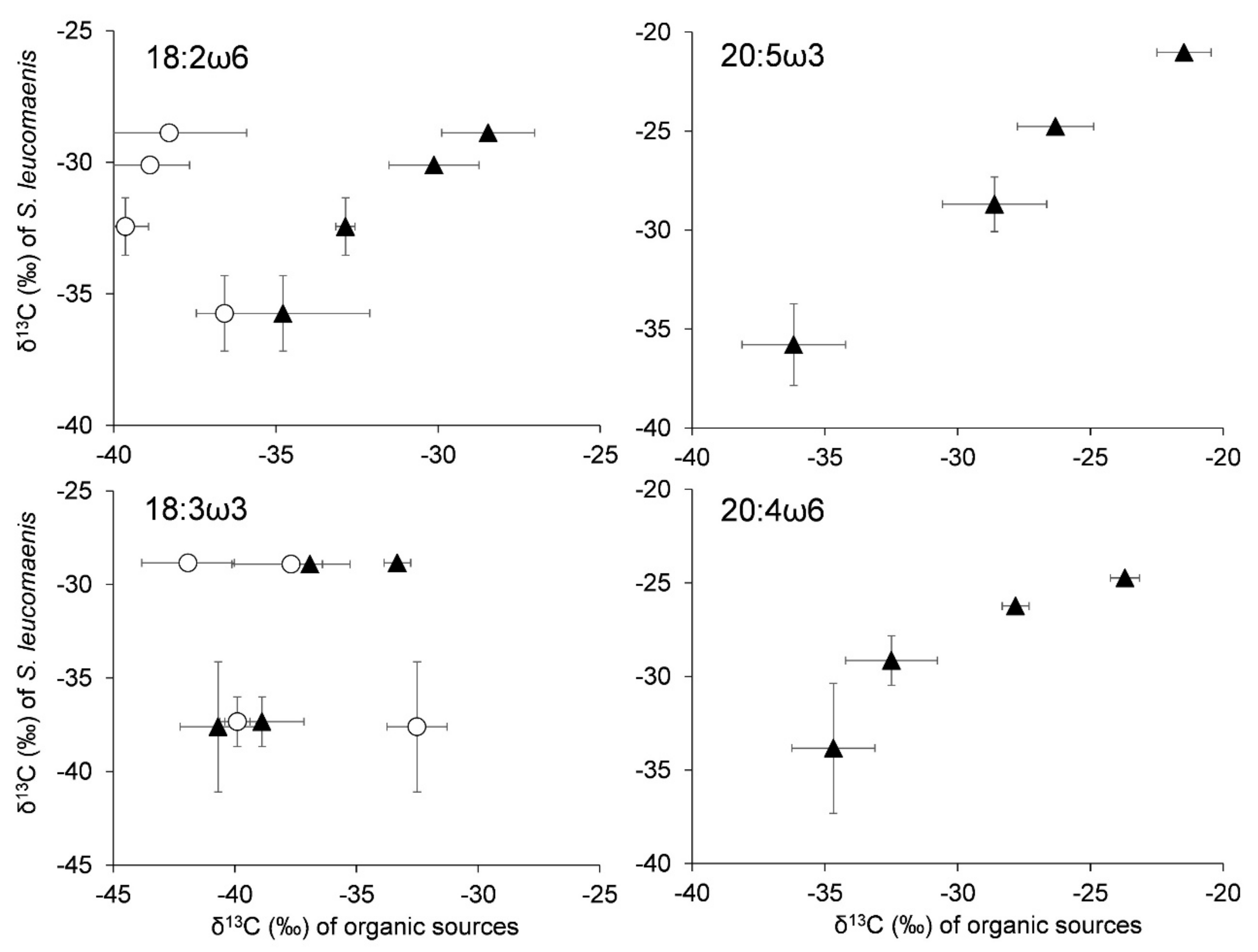
3. Results
4. Discussion
4.1. Origin of Organic Sources
4.2. Origin of Essential Fatty Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allan, J.D.; Castillo, M.M. Stream ecology: Structure and function of running waters; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Doi, H. Spatial patterns of autochthonous and allochthonous resources in aquatic food webs. Popul. Ecol. 2009, 51, 57–64. [Google Scholar] [CrossRef]
- Minshall, G.W. Autotrophy in stream ecosystems. BioScience 1978, 28, 767–771. [Google Scholar] [CrossRef]
- Lamberti, G.A.; Steinman, A.D. A comparison of primary production in stream ecosystems. J. North Am. Benthol. Soc. 1997, 16, 95–104. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Lau, D.C.P.; Leung, K.M.Y.; Dudgeon, D. Are autochthonous foods more important than allochthonous resources to benthic consumers in tropical headwater streams? J. North. Am. Benthol. Soc. 2009, 28, 426–439. [Google Scholar] [CrossRef] [Green Version]
- Hayden, B.; McWilliam-Hughes, S.M.; Cunjak, R.A. Evidence for limited trophic transfer of allochthonous energy in temperate river food webs. Freshw. Sci. 2016, 35, 544–558. [Google Scholar] [CrossRef]
- Doucett, R.R.; Power, G.; Barton, D.R.; Drimmie, R.J.; Cunjak, R.A. Stable isotope analysis of nutrient pathways leading to Atlantic salmon. Can. J. Fish. Aquat. Sci. 1996, 53, 2058–2066. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Wallace, J.B. Invertebrate food webs along a stream resource gradient. Freshw. Biol. 2002, 47, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Bunn, S.E.; Brett, M.T.; Kainz, M.J. Polyunsaturated fatty acids in stream food webs—High dissimilarity among producers and consumers. Freshw. Biol. 2017, 62, 1325–1334. [Google Scholar] [CrossRef]
- Guo, F.; Bunn, S.E.; Brett, M.T.; Fry, B.; Hager, H.; Ouyang, X.; Kainz, M.J. Feeding strategies for the acquisition of high-quality food sources in stream macroinvertebrates: Collecting, integrating, and mixed feeding. Limnol. Oceanogr. 2018, 63, 1964–1978. [Google Scholar] [CrossRef]
- Watanabe, T.; Kitajima, C.; Fujita, S. Nutritional values of live organisms used in Japan for mass propagation of fish: A review. Aquaculture 1983, 34, 115–143. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Smith, D.M.; Hunter, B.J.; Allan, G.L.; Roberts, D.C.K.; Booth, M.A.; Glencross, B.D. Essential fatty acids in the diet of silver perch (Bidyanus bidyanus): Effect of linolenic and linoleic acid on growth and survival. Aquaculture 2004, 236, 377–390. [Google Scholar] [CrossRef]
- Sargent, J.R.; McEvoy, L.A.; Estevez, A.; Bell, J.G.; Bell, M.V.; Henderson, R.J.; Tocher, D.R. Lipid nutrition of marine fish during early development: Current status and future directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Targońska, K. Enrichment of zebrafish Danio rerio (Hamilton, 1822) diet with polyunsaturated fatty acids improves fecundity and larvae quality. Zebrafish 2017, 14, 364–370. [Google Scholar] [CrossRef]
- Brett, M.T.; Bunn, S.E.; Chandra, S.; Galloway, A.W.E.; Guo, F.; Kainz, M.J.; Kankaala, P.; Lau, D.C.P.; Moulton, T.P.; Power, M.E.; et al. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 2017, 62, 833–853. [Google Scholar] [CrossRef]
- Taipale, S.; Strandberg, U.; Peltomaa, E.; Galloway, A.W.E.; Ojala, A.; Brett, M.T. Fatty acid composition as biomarkers of freshwater microalgae: Analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquat. Microb. Ecol. 2013, 71, 165–178. [Google Scholar] [CrossRef]
- Cattaneo, A.; Kerimian, T.; Roberge, M.; Marty, J. Periphyton distribution and abundance on substrata of different size along a gradient of stream trophy. Hydrobiologia 1997, 354, 101–110. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Finlay, J.C. Stable-carbon-isotope ratios of river biota: Implications for energy flow in lotic food webs. Ecology 2001, 82, 1052–1064. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Zanden, M.; Vander, J.; Rasmussen, J.B. Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumerdiet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.M.; AuCoin, L.R.; Ziegler, S.E.; Lall, S.P. Fractionation of stable carbon isotopes of tissue fatty acids in Atlantic Pollock (Pollachius virens). Ecosphere 2016, 7, e01437. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Ogino, M.; Nishimura, O. Fractionation of the stable carbon isotope ratio of essential fatty acids in zebrafish Danio rerio and mud snails Bellamya chinensis. Oecologia 2016, 180, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.M.; Wooller, M.J.; Springer, A.M.; Iverson, S.J.; McRoy, C.P.; Divoky, G.J. Tracing carbon flow in an arctic marine food web using fatty acid-stable isotope analysis. Oecologia 2008, 157, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, M.; Okano, K.; Takada, Y.; Mizutani, H.; Uchida, N.; Nishimura, O.; Miyata, N. Transfer of cyanobacterial carbon to a higher trophic-level fish community in a eutrophic lake food web: Fatty acid and stable isotope analyses. Oecologia 2018, 188, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Sushchik, N.N.; Kalachova, G.S.; Makhutova, O.N. Stable isotope composition of fatty acids in organisms of different trophic levels in the Yenisei River. PLoS One 2012, 7, e34059. [Google Scholar] [CrossRef] [PubMed]
- Bec, A.; Perga, M.E.; Koussoroplis, A.; Bardoux, G.; Desvilettes, C.; Bourdier, G.; Mariotti, A. Assessing the reliability of fatty acid-specific stable isotope analysis for trophic studies. Methods Ecol. Evol. 2011, 2, 651–659. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Makhutova, O.N.; Kravchuk, E.S.; Anishchenko, O.V.; Sushchik, N.N. Stable isotope fractionation of fatty acids of Daphnia fed laboratory cultures of microalgae. Limnologica 2016, 56, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.M.; Clare, E.L.; Hayden, B.; Brett, M.T.; Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods Ecol. Evol. 2018, 9, 278–291. [Google Scholar] [CrossRef]
- Sakamaki, T.; Richardson, J.S. Biogeochemical properties of fine particulate organic matter as an indicator of local and catchment impacts on forested streams. J. Appl. Ecol. 2011, 48, 1462–1471. [Google Scholar] [CrossRef]
- Sakamaki, T.; Shum, J.Y.T.; Richardson, J.S. Watershed effects on chemicalproperties of sediment and primary consumption in estuarine tidal flats:importance of watershed size and food selectivity by macrobenthos. Ecosystems 2010, 13, 328–337. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Sakamaki, T.; Shin, W.; Nishimura, O. Food utilization of shell-attached algae contributes to the growth of host mud snail, Bellamya chinensis: Evidence from fatty acid biomarkers and carbon stable isotope analysis. Limnologica 2016, 57, 66–72. [Google Scholar] [CrossRef]
- Takenaka, A. CanopOn 2: Hemispherical photography analysis program. Available online: http://takenaka-akio.org/etc/canopon2/index.html. (accessed on 30 June 2019).
- Tsuda, M. Consideration on the contents of alimentary canals of Salvelinus leycomaenis pluvius and Oncorhynchus masou f. masou from Ina-gawa River System. Jpn. J. Limnol. 1967, 28, 51–55. [Google Scholar] [CrossRef]
- Kato, F. Ecological and morphological notes on the charr, Salvelinus leucomaenis in the Nagara River and Ibi River systems. SUISANZOSHOKU 1992, 40, 145–152. [Google Scholar]
- Abdulkadir, S.; Tsuchiya, T. One-step method for quantitative and qualitative analysis of fatty acids in marine animal samples. J. Exp. Mar. Biol. Ecol 2008, 354, 1–8. [Google Scholar] [CrossRef]
- Sakamaki, T.; Richardson, J.S.; Arnott, S. Nonlinear variation of stream-forest linkage along a stream-size gradient: An assessment using biogeochemical proxies of in-stream fine particulate organic matter. J. Appl. Ecol. 2013, 50, 1019–1027. [Google Scholar] [CrossRef]
- Collins, S.M.; Kohler, T.J.; Thomas, S.A.; Fetzer, W.W.; Flecker, A.S. The importance of terrestrial subsidies in stream food webs varies along a stream size gradient. Oikos 2016, 125, 674–685. [Google Scholar] [CrossRef]
- Ishikawa, N.F.; Yamane, M.; Suga, H.; Ogawa, N.O.; Yokoyama, Y.; Ohkouchi, N. Chlorophyll a-specific Δ14C, δ13C and δ15N values in stream periphyton: Implications for aquatic food web studies. Biogeosciences 2015, 12, 6781–6789. [Google Scholar] [CrossRef]
- Wang, L.; Wu, F.; Xion, Y.; Fang, J. Origin and vertical variation of the bound fatty acids in core sediment of Lake Dianchi in Southwest China. Env. Sci. Poll. Res. 2013, 20, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Tall, L.; Cattaneo, A.; Cloutier, L.; Dray, S.; Legendre, P. Resource partitioning in a grazer guild feeding on a multilayer diatom mat. J. North. Am. Benthol. Soc. 2006, 25, 800–810. [Google Scholar] [CrossRef]
- Finlay, J.C. Patterns and controls of lotic algal stable carbon isotope ratios. Limnol. Oceanogr. 2004, 49, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Hill, W.R.; Middleton, R.G. Changes in carbon stable isotope ratios during periphyton development. Limnol. Oceanogr. 2006, 51, 2360–2369. [Google Scholar] [CrossRef] [Green Version]
- Huang, I.Y.; Lin, Y.S.; Chen, C.P.; Hsieh, H.L. Food web structure of a subtropical headwater stream. Mar. Freshw. Res. 2007, 58, 596–607. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.M., Jr.; Hamilton, S.K.; Rodríguez, M.A.; Saunders, J.F., III; Lasi, M.A. Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data. J. North. Am. Benthol. Soc. 2001, 20, 241–254. [Google Scholar] [CrossRef]
- Crenier, C.; Arce-Funck, J.; Bec, A.; Billoir, E.; Perrière, F.; Leflaive, J.; Guérold, F.; Felten, V.; Danger, M. Minor food sources can play a major role in secondary production in detritus-based ecosystems. Freshw. Biol. 2017, 62, 1155–1167. [Google Scholar] [CrossRef]
- Nakano, S.; Fausch, K.D.; Kitano, S. Flexible niche partitioning via a foraging mode shift: A proposed mechanism for coexistence in stream-dwelling charts. J. Anim. Ecol. 1999, 68, 1079–1092. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Arts, M.T.; Sushchik, N.N. Preliminary estimates of the export of omega-3 highly unsaturated fatty acids (EPA+DHA) from aquatic to terrestrial ecosystems. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 179–210. [Google Scholar]
- Logan, J.M.; Jardine, T.D.; Miller, T.J.; Bunn, S.E.; Cunjak, R.A.; Lutcavage, M.E. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J. Anim. Ecol. 2008, 77, 838–846. [Google Scholar] [CrossRef]
- Hastings, N.; Agaba, M.; Tocher, D.R.; Leaver, M.J.; Dick, J.R.; Sargent, J.R.; Teale, A.J. A vertebrate fatty acid desaturase with Δ5 and Δ6 activities. PNAS 2001, 98, 14304–14309. [Google Scholar] [CrossRef] [PubMed]
- Sushchik, N.N.; Gladyshev, M.I.; Moskvichova, A.V.; Makhutova, O.N.; Kalachova, G.S. Comparison of fatty acid composition in major lipid classes of the dominant benthic invertebrates of the Yenisei river. Comp. Biochem. Physiol. B 2003, 134, 111–122. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; Wehr, J.D.; Perrone, A.A. Are net-spinning caddisflies what they eat? An investigation using controlled diets and fatty acids. J. North. Am. Benthol. Soc. 2010, 29, 803–813. [Google Scholar] [CrossRef]
- Guo, F.; Kainz, M.J.; Sheldon, F.; Bunn, S.E. Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 2016, 181, 449–462. [Google Scholar] [CrossRef] [PubMed]



| Study Site | GPS | Order | Sampling Date | Canopy (%) | Water Temperature (°C) |
|---|---|---|---|---|---|
| Babame | N39.8678°, E140.2552° | 1 | 10 July | 93.5 | 15.9 |
| Hayakuchi | N40.4227°, E140.3470° | 3 | 20 July | 67.6 | 15.6 |
| Kurikoma | N38.9169°, E140.7356° | 1 | 2 September | 91.1 | 14.2 |
| Naruse | N 39.0716°, E 140.7187° | 3 | 18 September | 63.5 | 18.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujibayashi, M.; Miura, Y.; Suganuma, R.; Takahashi, S.; Sakamaki, T.; Miyata, N.; Kazama, S. Origin of Carbon and Essential Fatty Acids in Higher Trophic Level Fish in Headwater Stream Food Webs. Biomolecules 2019, 9, 487. https://doi.org/10.3390/biom9090487
Fujibayashi M, Miura Y, Suganuma R, Takahashi S, Sakamaki T, Miyata N, Kazama S. Origin of Carbon and Essential Fatty Acids in Higher Trophic Level Fish in Headwater Stream Food Webs. Biomolecules. 2019; 9(9):487. https://doi.org/10.3390/biom9090487
Chicago/Turabian StyleFujibayashi, Megumu, Yoshie Miura, Reina Suganuma, Shinji Takahashi, Takashi Sakamaki, Naoyuki Miyata, and So Kazama. 2019. "Origin of Carbon and Essential Fatty Acids in Higher Trophic Level Fish in Headwater Stream Food Webs" Biomolecules 9, no. 9: 487. https://doi.org/10.3390/biom9090487





