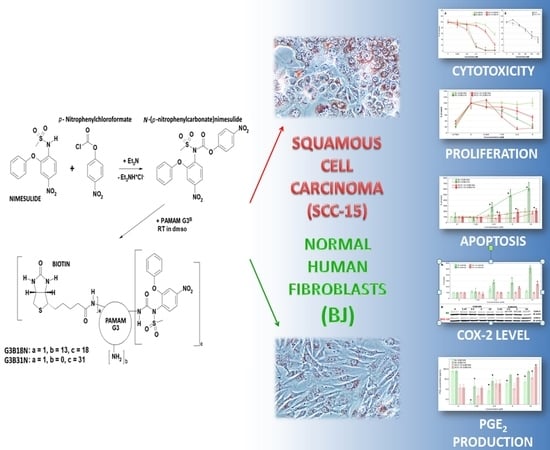
Synthesis and Different Effects of Biotinylated PAMAM G3 Dendrimer Substituted with Nimesulide in Human Normal Fibroblasts and Squamous Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Cell Cultures
2.4. Cytotoxicity
2.5. Proliferation Assay
2.6. Apoptosis
2.7. Western Blot
2.8. Prostaglandin E2 Production
2.9. Statistical Analysis
3. Results and Discussion
3.1. Bioconjugate Synthesis
3.2. Cytotoxicity of G3B18N and G3B31N Conjugates as Compared with Nimesulide Alone and PAMAM G3
3.3. Antiproliferative Activity
3.4. Apoptosis
3.5. COX-2 Expression and Prostaglandin E2 Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J. American Cancer Society Cancer statistics, 2004. CA Cancer J. Clin. 2004, 54, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, G.A.M.S.; Snow, G.B. Prospects for future studies in head and neck cancer. Eur. J. Surg. Oncol. EJSO 1997, 23, 486–491. [Google Scholar] [CrossRef]
- Gharat, S.A.; Momin, M.; Bhavsar, C. Oral Squamous Cell Carcinoma: Current Treatment Strategies and Nanotechnology-Based Approaches for Prevention and Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, P.C.; See, A.U. Cyclo-oxygenase isoenzymes: Physiological and pharmacological role. Anaesthesia 2000, 55, 442–449. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef]
- Czembirek, C.; Eder-Czembirek, C.; Erovic, B.M.; Turhani, D.; Spittler, A.; Selzer, E.; Pötter, R.; Thurnher, D. The cyclooxygenase-2 inhibitor nimesulide, a nonsteroidal analgesic, decreases the effect of radiation therapy in head-and-neck cancer cells. Strahlenther. Und Onkol. 2009, 185, 310–317. [Google Scholar] [CrossRef]
- Dang, C.T.; Shapiro, C.L.; Hudis, C.A. Potential role of selective COX-2 inhibitors in cancer management. Oncol. Williston Park N 2002, 16, 30–36. [Google Scholar]
- Fischer, S.M.; Lo, H.H.; Gordon, G.B.; Seibert, K.; Kelloff, G.; Lubet, R.A.; Conti, C.J. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 1999, 25, 231–240. [Google Scholar] [CrossRef]
- Kim, B.M.; Won, J.; Maeng, K.A.; Han, Y.S.; Yun, Y.-S.; Hong, S.H. Nimesulide, a selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int. J. Oncol. 2009, 34, 1467–1473. [Google Scholar] [PubMed]
- Rizzo, M.T. Cyclooxygenase-2 in oncogenesis. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell Biol. 2010, 2010, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 Mediated Inflammation in Oral Squamous Cell Carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef]
- Urade, M. Cyclooxygenase (COX)-2 as a potent molecular target for prevention and therapy of oral cancer. Jpn. Dent. Sci. Rev. 2008, 44, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Darby, M.V.; Brueggemeier, R.W. Synthesis and biological evaluation of novel sulfonanilide compounds as antiproliferative agents for breast cancer. J. Comb. Chem. 2008, 10, 475–483. [Google Scholar] [CrossRef]
- Zhong, B.; Cai, X.; Chennamaneni, S.; Yi, X.; Liu, L.; Pink, J.J.; Dowlati, A.; Xu, Y.; Zhou, A.; Su, B. From COX-2 inhibitor nimesulide to potent anti-cancer agent: Synthesis, in vitro, in vivo and pharmacokinetic evaluation. Eur. J. Med. Chem. 2012, 47, 432–444. [Google Scholar] [CrossRef]
- Rainsford, K.D. Nimesulide—Actions and Uses; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Suleyman, H.; Cadirci, E.; Albayrak, A.; Halici, Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr. Med. Chem. 2008, 15, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Moodley, I. Review of the cardiovascular safety of COXIBs compared to NSAIDS. Cardiovasc. J. Afr. 2008, 19, 102–107. [Google Scholar] [PubMed]
- Afzal, M.; Bhardwaj, D.P.; Khan, R.; Kazmi, I.; Saleem, S.; Al-Abbasi, F.A.; Anwar, F. Antineoplastic influence of nimesulide in chemically induced hepatocellular carcinoma by inhibition of DNA synthesis. Inflammopharmacology 2018, 27, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Khodaie, F.; Khazaei-Poul, Y.; Moini-Zanjani, T. Anti-Proliferative Effects of Piroxicam and Nimesulide on A431 Human Squamous Carcinoma Cell Line. Int. J. Cancer Manag. 2017, 10, e7565. [Google Scholar] [CrossRef]
- Vormittag, L.; Lamm, W.; Erovic, B.M.; Czembirek, C.; Kornek, G.; Thurnher, D. Expression levels of Akt in nimesulide-treated squamous carcinoma cell lines of the head and neck. Oncol. Rep. 2005, 13, 207–210. [Google Scholar] [PubMed]
- Zong, Y.; Zhang, S.-T.; Zhu, S.-T. Nicotine enhances migration and invasion of human esophageal squamous carcinoma cells which is inhibited by nimesulide. World J. Gastroenterol. 2009, 15, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Zimta, A.A.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 12, 4593–4606. [Google Scholar] [CrossRef] [PubMed]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, S.K. Nanotechnology in oral cancer: A comprehensive review. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 407–414. [Google Scholar]
- Sengel-Turk, C.T.; Hascicek, C.; Bakar, F.; Simsek, E. Comparative Evaluation of Nimesulide-Loaded Nanoparticles for Anticancer Activity Against Breast Cancer Cells. AAPS PharmSciTech 2017, 18, 393–403. [Google Scholar] [CrossRef]
- Jian, Y.-S.; Chen, C.-W.; Lin, C.-A.; Yu, H.-P.; Lin, H.-Y.; Liao, M.-Y.; Wu, S.-H.; Lin, Y.-F.; Lai, P.-S. Hyaluronic acid-nimesulide conjugates as anticancer drugs against CD44-overexpressing HT-29 colorectal cancer in vitro and in vivo. Int. J. Nanomed. 2017, 12, 2315–2333. [Google Scholar] [CrossRef]
- Satija, J.; Gupta, U.; Jain, N.K. Pharmaceutical and biomedical potential of surface engineered dendrimers. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 257–306. [Google Scholar] [CrossRef]
- De Araújo, R.V.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lee, I.; Chen, X.; Shen, M.; Xiao, S.; Zhu, M.; Baker, J.R.; Wang, S.H. Influence of dendrimer surface charge on the bioactivity of 2-methoxyestradiol complexed with dendrimers. Soft Matter 2010, 6, 2539–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Gorzkiewicz, M.; Ficker, M.; Petersen, J.F.; Paolucci, V.; Christensen, J.B.; Klajnert-Maculewicz, B. Pyrrolidone Modification Prevents PAMAM Dendrimers from Activation of Pro-Inflammatory Signaling Pathways in Human Monocytes. Mol. Pharm. 2018, 15, 12–20. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef]
- Jędrych, M.; Borowska, K.; Galus, R.; Jodłowska-Jędrych, B. The evaluation of the biomedical effectiveness of poly(amido)amine dendrimers generation 4.0 as a drug and as drug carriers: A systematic review and meta-analysis. Int. J. Pharm. 2014, 462, 38–43. [Google Scholar] [CrossRef]
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar]
- Murugan, E.; Geetha Rani, D.P.; Yogaraj, V. Drug delivery investigations of quaternised poly (propylene imine) dendrimer using nimesulide as a model drug. Colloids Surf. B Biointerfaces 2014, 114, 121–129. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Uram, Ł.; Szuster, M.; Filipowicz, A.; Zaręba, M.; Wałajtys-Rode, E.; Wołowiec, S. Cellular uptake of glucoheptoamidated poly (amidoamine) PAMAM G3 dendrimer with amide-conjugated biotin, a potential carrier of anticancer drugs. Bioorg. Med. Chem. 2017, 25, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Shadrack, D.M.; Swai, H.S.; Munissi, J.J.E.; Mubofu, E.B.; Nyandoro, S.S. Polyamidoamine Dendrimers for Enhanced Solubility of Small Molecules and Other Desirable Properties for Site Specific Delivery: Insights from Experimental and Computational Studies. Molecules. 2018, 23, 1419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, E.; Kim, W.Y.; Kang, C.; Kim, J.S. Biotin-guided anticancer drug delivery with acidity-triggered drug release. Chem. Commun. 2015, 51, 9343–9345. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Kumar, A.; Palakurthi, S. Biotinylated poly (amido) amine (PAMAM) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer. Res. 2009, 29, 2933–2943. [Google Scholar] [PubMed]
- Tomalia, D.A.; Huang, B.; Swanson, D.R.; Brothers, H.M.; Klimash, J.W. Structure control within poly(amidoamine) dendrimers: Size, shape and regio-chemical mimicry of globular proteins. Tetrahedron 2003, 59, 3799–3813. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Gargasz, K.; Filipowicz, A.; Wałajtys-Rode, E.; Wołowiec, S. In vitro cytotoxicity of the ternary PAMAM G3-pyridoxal-biotin bioconjugate. Int. J. Nanomed. 2013, 8, 4707–4720. [Google Scholar]
- Uram, Ł.; Szuster, M.; Misiorek, M.; Filipowicz, A.; Wołowiec, S.; Wałajtys-Rode, E. The effect of G3 PAMAM dendrimer conjugated with B-group vitamins on cell morphology, motility and ATP level in normal and cancer cells. Eur. J. Pharm. Sci. 2017, 102, 275–283. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Abbehausen, C.; Gomes, A.F.; Gozzo, F.C.; Lustri, W.R.; Formiga, A.L.B.; Corbi, P.P. Synthesis, spectroscopic characterization, DFT studies and antibacterial assays of a novel silver (I) complex with the anti-inflammatory nimesulide. Polyhedron 2012, 36, 112–119. [Google Scholar] [CrossRef]
- Stompor, M.; Uram, Ł.; Podgórski, R. In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef] [PubMed]
- Uram, Ł.; Filipowicz, A.; Misiorek, M.; Pieńkowska, N.; Markowicz, J.; Wałajtys-Rode, E.; Wołowiec, S. Biotinylated PAMAM G3 dendrimer conjugated with celecoxib and/or Fmoc-l-Leucine and its cytotoxicity for normal and cancer human cell lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- R&D Systems Parameter Prostaglandin E2 Assay Quant. Determ. Prostaglandin E2 PGE2 Cell Cult. Supernates Serum Plasma Urine 2019; R&D Systems: Minneapolis, MN, USA, 2019. [Google Scholar]
- Guo, N.; Jiang, D.; Wang, L.; You, X.; Teng, Y.-O.; Yu, P. Synthesis and Biological Evaluation of Novel Water-Soluble Poly-(ethylene glycol)-10-hydroxycamptothecin Conjugates. Molecules 2015, 20, 9393–9404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, R.; Gao, Y.; Tang, Y.; He, R.-R.; Liu, T.-L.; He, Y.; Sun, S.; Li, B.-Y.; Li, Y.-B.; Liu, G. PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J. 2009, 11, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; D’Emanuele, A.; Lennon, K.; Attwood, D. Synthesis and Micellization of Linear–Dendritic Copolymers and Their Solubilization Ability for Poorly Water-Soluble Drugs. Macromolecules 2009, 42, 7936–7944. [Google Scholar] [CrossRef]
- Pericherla, S.; Mareddy, J.; Rani, D.P.G.; Gollapudi, P.V.; Pal, S. Chemical modifications of nimesulide. J. Braz. Chem. Soc. 2007, 18, 384–390. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Klajnert, B.; Epand, R.M. PAMAM dendrimers and model membranes: Differential scanning calorimetry studies. Int. J. Pharm. 2005, 305, 154–166. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Bellocco, E.; Laganà, G.; Barreca, D.; Magazù, S.; Wanderlingh, U.; Kiselev, M.A. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. Biochim. Biophys. Acta 2016, 1858, 2769–2777. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—Cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Mecke, A.; Majoros, I.J.; Patri, A.K.; Baker, J.R.; Banaszak Holl, M.M.; Orr, B.G. Lipid Bilayer Disruption by Polycationic Polymers: The Roles of Size and Chemical Functional Group. Langmuir 2005, 21, 10348–10354. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Vidal, F.; Vásquez, P.; Cayumán, F.R.; Díaz, C.; Fuentealba, J.; Aguayo, L.G.; Yévenes, G.E.; Alderete, J.; Guzmán, L. Prevention of Synaptic Alterations and Neurotoxic Effects of PAMAM Dendrimers by Surface Functionalization. Nanomaterials 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.-T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, B.A.G.; Wu, Y.; Fischer, S.; Liu, W.; Weil, T.; Müllen, K. Controlling Cellular Uptake and Toxicity of Polyphenylene Dendrimers by Chemical Functionalization. ChemBioChem 2017, 18, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity of poly (amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010, 248, 259–268. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: Experimental observation and numerical simulation. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 202–211. [Google Scholar] [CrossRef]
- Periasamy, J.; Muthuswami, M.; Ramesh, V.; Muthusamy, T.; Jain, A.; Karthikeyan, C.; Trivedi, P.; Kumar, R.S.; Gunasekaran, P.; Rha, S.; et al. Nimesulide and celecoxib inhibits multiple oncogenic pathways in gastric cancer cells. J. Cancer Sci. Ther. 2013, 5, 126–136. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Cui, T.; Fei, S. The effect of nimesulide on the expression of NF-kB, Bcl-2 and bax in the human gastric cancer SGC-7901 cell line. Chin. J. Clin. Oncol. 2006, 3, 196–201. [Google Scholar] [CrossRef]
- Ko, S.-H.; Choi, G.J.; Lee, J.H.; Han, Y.A.; Lim, S.-J.; Kim, S.H. Differential effects of selective cyclooxygenase-2 inhibitors in inhibiting proliferation and induction of apoptosis in oral squamous cell carcinoma. Oncol. Rep. 2008, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Kozaki, K.; Muramatsu, H.; Masuda, A.; Shimizu, S.; Mitsudomi, T.; Sugiura, T.; Ogawa, M.; Takahashi, T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin. Cancer Res. 2000, 6, 2006–2011. [Google Scholar] [PubMed]
- Semashko, V.V.; Pudovkin, M.S.; Cefalas, A.-C.; Zelenikhin, P.V.; Gavriil, V.E.; Nizamutdinov, A.S.; Kollia, Z.; Ferraro, A.; Sarantopoulou, E. Tiny Rare-Earth Fluoride Nanoparticles Activate Tumour Cell Growth via Electrical Polar Interactions. Nanoscale Res. Lett. 2018, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J. Mechanotransduction. Compr. Physiol. 2011, 1, 1057–1073. [Google Scholar]
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 2017, 28, 3134–3155. [Google Scholar] [CrossRef]
- Chowdhury, I.; Tharakan, B.; Bhat, G.K. Caspases—An update. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 10–27. [Google Scholar] [CrossRef]
- Chu, M.; Wang, T.; Sun, A.; Chen, Y. Nimesulide inhibits proliferation and induces apoptosis of pancreatic cancer cells by enhancing expression of PTEN. Exp. Ther. Med. 2018, 16, 370–376. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Kumar, P.; Murray, D. The Growing Complexity of Cancer Cell Response to DNA-Damaging Agents: Caspase 3 Mediates Cell Death or Survival? Int. J. Mol. Sci. 2016, 17, 708. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Festjens, N.; Declercq, W.; Vanden Berghe, T.; Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007, 14, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.-F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.-C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kaakati, R.; Lee, A.K.; Liu, X.; Li, F.; Li, C.-Y. Novel roles of apoptotic caspases in tumor repopulation, epigenetic reprogramming, carcinogenesis, and beyond. Cancer Metastasis Rev. 2018, 37, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, C.; Li, M.; Fan, Y. Apoptotic Caspases in Promoting Cancer: Implications from Their Roles in Development and Tissue Homeostasis. Adv. Exp. Med. Biol. 2016, 930, 89–112. [Google Scholar] [Green Version]
- Pérez-Garijo, A. When dying is not the end: Apoptotic caspases as drivers of proliferation. Semin. Cell Dev. Biol. 2018, 82, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef]
- Sakagami, H. Apoptosis-inducing activity and tumor-specificity of antitumor agents against oral squamous cell carcinoma. Jpn. Dent. Sci. Rev. 2010, 46, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Minter, H.A.; Eveson, J.W.; Huntley, S.; Elder, D.J.E.; Hague, A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin. Cancer Res. 2003, 9, 1885–1897. [Google Scholar]
- Wada, M.; Saunders, T.L.; Morrow, J.; Milne, G.L.; Walker, K.P.; Dey, S.K.; Brock, T.G.; Opp, M.R.; Aronoff, D.M.; Smith, W.L. Two pathways for cyclooxygenase-2 protein degradation in vivo. J. Biol. Chem. 2009, 284, 30742–30753. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Saunders, M.A.; Wu, K.K. COX-2 expression and cell cycle progression in human fibroblasts. Am. J. Physiol.-Cell Physiol. 2001, 281, C188–C194. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Seyedmajidi, M.; Shafaee, S.; Siadati, S.; Khorasani, M.; Bijani, A.; Ghasemi, N. Cyclo-oxygenase-2 expression in oral squamous cell carcinoma. J. Cancer Res. Ther. 2014, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Mayer-Kuckuk, P.; Capiaux, G.; Budak-Alpdogan, T.; Gorlick, R.; Bertino, J.R. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim. Biophys. Acta 2002, 1587, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Bertino, J.R.; Cashmore, A.; Fink, M.; Calabresi, P.; Lefkowitz, E. The “induction” of leukocyte and erythrocyte dihydrofolate reductase by methotrexate. Clin. Pharmacol. Ther. 1965, 6, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.; Koeller, D.M.; Casey, J.L.; Drake, J.C.; Chabner, B.A.; Elwood, P.C.; Zinn, S.; Allegra, C.J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 8977–8981. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Ching, T.T.; Wang, D.S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397–11403. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Hatori, M.; Ito, H.; Kurihara, Y.; Ito, D.; Nagumo, M. Inhibition of cyclooxygenase-2 suppresses invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 and CD44. Clin. Exp. Metastasis 2004, 21, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yang, H.; Fu, J. Nimesulide inhibits IFN-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells by COX-2 and PGE2 independent mechanisms. Cancer Lett. 2009, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nikitakis, N.G.; Hebert, C.; Lopes, M.A.; Reynolds, M.A.; Sauk, J.J. PPARgamma-mediated antineoplastic effect of NSAID sulindac on human oral squamous carcinoma cells. Int. J. Cancer 2002, 98, 817–823. [Google Scholar] [CrossRef]
- Zhu, J.; Song, X.; Lin, H.-P.; Young, D.C.; Yan, S.; Marquez, V.E.; Chen, C.-S. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J. Natl. Cancer Inst. 2002, 94, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Dave, M.; Abramson, S.B.; Amin, A. Activation of diverse eicosanoid pathways in osteoarthritic cartilage: A lipidomic and genomic analysis. Bull. NYU Hosp. Jt. Dis. 2012, 70, 99–108. [Google Scholar] [PubMed]
- Kirtikara, K.; Morham, S.G.; Raghow, R.; Laulederkind, S.J.; Kanekura, T.; Goorha, S.; Ballou, L.R. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J. Exp. Med. 1998, 187, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mazaleuskaya, L.L.; Yuan, C.; Ballantyne, L.L.; Meng, H.; Smith, W.L.; FitzGerald, G.A.; Funk, C.D. Flipping the cyclooxygenase (Ptgs) genes reveals isoform-specific compensatory functions. J. Lipid Res. 2018, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.B.; Dave, M.; Amin, S.; Jensen, R.V.; Amin, A.R. Genomic, Lipidomic and Metabolomic Analysis of Cyclooxygenase-null Cells: Eicosanoid Storm, Cross Talk, and Compensation by COX-1. Genom. Proteom. Bioinform. 2016, 14, 81–93. [Google Scholar] [CrossRef] [Green Version]








| Compound | IC50 [µM] | Selectivity Index (SI) | |
|---|---|---|---|
| BJ | SCC-15 | ||
| PAMAM G3 | 5.64 | 12.68 | 2.25 |
| Nimesulide | 587.30 | 426.90 | 0.72 |
| G3B18N | 14.50 | 5.96 | 0.41 |
| G3B31N | 1.99 | 1.60 | 0.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uram, Ł.; Filipowicz-Rachwał, A.; Misiorek, M.; Winiarz, A.; Wałajtys-Rode, E.; Wołowiec, S. Synthesis and Different Effects of Biotinylated PAMAM G3 Dendrimer Substituted with Nimesulide in Human Normal Fibroblasts and Squamous Carcinoma Cells. Biomolecules 2019, 9, 437. https://doi.org/10.3390/biom9090437
Uram Ł, Filipowicz-Rachwał A, Misiorek M, Winiarz A, Wałajtys-Rode E, Wołowiec S. Synthesis and Different Effects of Biotinylated PAMAM G3 Dendrimer Substituted with Nimesulide in Human Normal Fibroblasts and Squamous Carcinoma Cells. Biomolecules. 2019; 9(9):437. https://doi.org/10.3390/biom9090437
Chicago/Turabian StyleUram, Łukasz, Aleksandra Filipowicz-Rachwał, Maria Misiorek, Aleksandra Winiarz, Elżbieta Wałajtys-Rode, and Stanisław Wołowiec. 2019. "Synthesis and Different Effects of Biotinylated PAMAM G3 Dendrimer Substituted with Nimesulide in Human Normal Fibroblasts and Squamous Carcinoma Cells" Biomolecules 9, no. 9: 437. https://doi.org/10.3390/biom9090437