Design, Synthesis and Evaluation of Novel Trichloromethyl Dichlorophenyl Triazole Derivatives as Potential Safener
Abstract
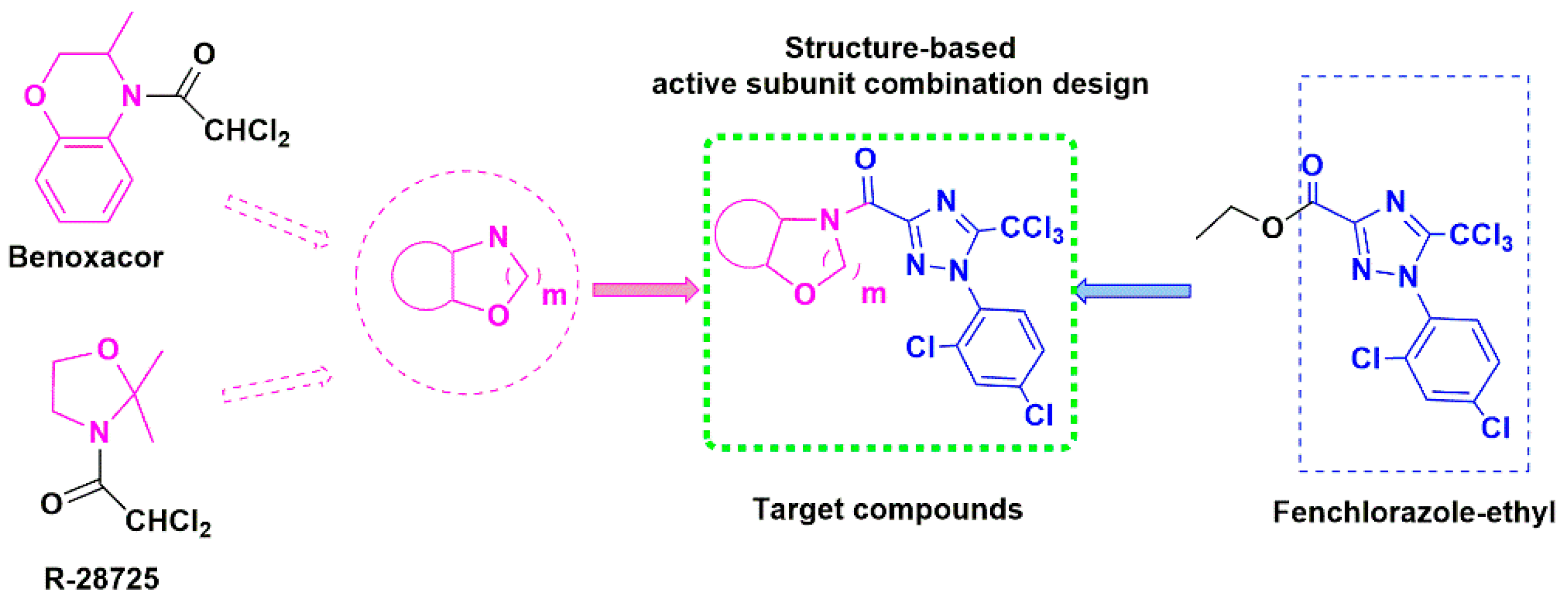
:1. Introduction
2. Materials and Methods
2.1. Chemicals
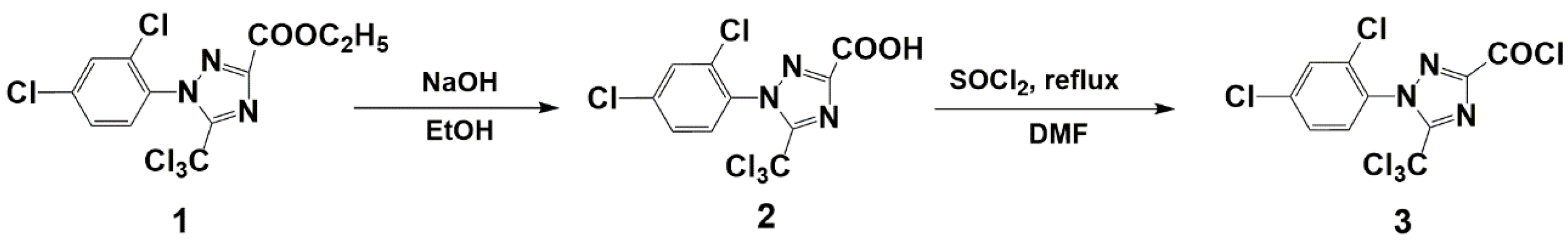
2.2. General Procedure for the Synthesis of Compound (3)
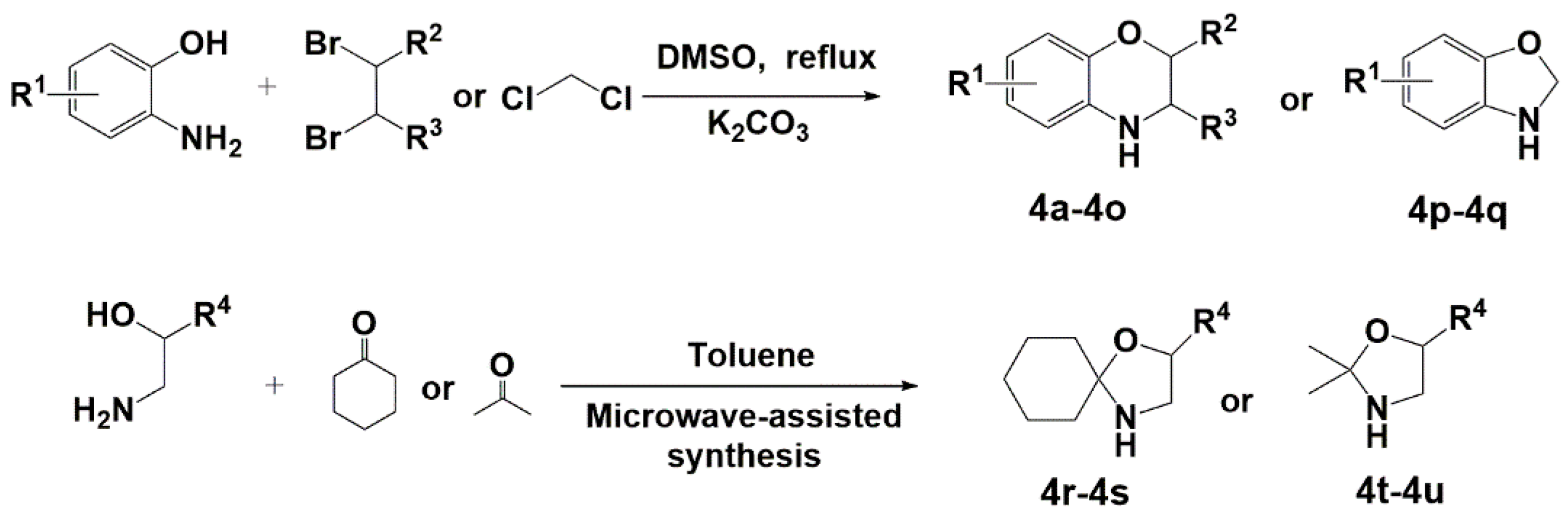
2.3. General Procedure for the Synthesis of Intermediates Compounds (4)
2.4. General Procedure for the Synthesis of Title Compounds (5)
2.5. X-ray Diffraction
2.6. Biological Assay
2.7. Computational Methods
3. Results and Discussion
3.1. Synthesis
3.2. The Structure–Activity Relationships
3.3. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the Art: I. Weed control research and safener technology: The Path to Modern Agriculture. Plant Physioly. 2014, 166, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Pesticide detox by design. J. Agric. Food Chem. 2018, 66, 9379–9383. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Ndikuryayo, F.; Moosavi, B.; Yang, W.C.; Yang, G.F. 4-Hydroxyphenylpyruvate dioxygenase inhibitors: From chemical biology to agrochemicals. J. Agric. Food Chem. 2017, 65, 8523–8537. [Google Scholar] [CrossRef] [PubMed]
- Alberto, D.; Serra, A.A.; Sulmon, C.; Gouesbet, G.; Couée, I. Herbicide-related signaling in plants reveals novel insoights for herbicide use strategies, environmental risk assessment and global change assessment challenges. Sci. Total Environ. 2016, 569, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Pornprom, T.; Mahatamnuchoke, P.; Usui, K. The role of altered acetyl-CoA carboxylase in conferring resistance to fenoxaprop-P-ethyl in Chinese sprangletop (Leptochloa Chinensis (L.) Nees). Pest Manag. Sci. 2010, 62, 1109–1115. [Google Scholar] [CrossRef]
- Guo, W.L.; Chi, Y.Y.; Feng, L.; Tian, X.S.; Liu, W.T.; Wang, J.X. Fenoxaprop-P-ethyl and mesosulfuron-methyl resistance status of shortawn foxtail (Alopecurus Aequalis Sobol.) in eastern China. Pestic. Biochem. Phys. 2018, 148, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Hatzios, K.K. Mechanisms of action of herbicide safeners: An overview. In Crop safeners for herbicides: Development, Uses and Mechanism of Action; Hatzios, K., Ed.; Elsevier Inc.: San Diego, CA, USA, 1989; pp. 65–101. [Google Scholar]
- Duhoux, A.; Pernin, F.; Desserre, D.; Délye, C. Herbicide safeners decrease sensitivity to herbicides inhibiting acetolactate-synthase and likely activate non-target-site-based resistance pathways in the Major Grass Weed Lolium sp. (Rye-Grass). Front. Plant Sci. 2017, 8, 1310. [Google Scholar] [CrossRef] [PubMed]
- Busi, R.; Nguyen, N.K.; Chauhan, B.S.; Vidotto, F.; Tabacchi, M.; Powlesa, S.B. Can herbicide safeners allow selective control of weedy rice infesting rice crops? Pest Manag. Sci. 2017, 73, 71–77. [Google Scholar] [CrossRef] [PubMed]
- John, D.S.; Hansjoachim, L.; Christopher, J.S.; Allison, N.R.; David, M.C. Environmental fate and effects of dichloroacetamide herbicide safeners: “Inert” yet biologically active agrochemical ingredients. Environ. Sci. Technol. Let. 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Taylor, V.L.; Cummins, I.; Brazier-Hicks, M.; Edwardsa, R. Protective responses induced by herbicide safeners in wheat. Environ. Exp. Bot. 2013, 88, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, M.L.; Stephenson, G.R.; Tal, A.; Hall, J.C. The effect of monooxygenase and glutathione-s-transferase inhibitors on the metabolism of diclofop-methyl and fenoxaprop-ethyl in barley and wheat. Pestic. Biochem. Phys. 1993, 46, 181–189. [Google Scholar] [CrossRef]
- Da Silva, J.R.V.; Martins, C.C.; Da Silva Junior, A.C.; Martins, D. Fluxofenim used as safener on sorghum seed for s-metolachlor herbicide. Biosci. J. 2014, 30, 158–167. [Google Scholar]
- Bolyard, K.; Gresens, S.E.; Ricko, A.N.; Sivey, J.D.; Salice, C.J. Assessing the toxicity of the “inert” safener benoxacor toward, Chironomus riparius: Effects of agrochemical mixtures. Environ. Toxicol. Chem. 2017, 36, 2660–2670. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Bonnemoy, F.; Charvy, J.C.; Bohatier, J.; Mallet, C. Toxicity assessment of the maize herbicides S-metolachlor, benoxacor, mesotrione and nicosulfuron, and their corresponding commercial formulations, alone and in mixtures, using the Microtox® test. Chemosphere 2013, 93, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Sheremetyev, V.; Kudryashova, A.; Dubinskiy, S.; Galkin, S.; Prokoshkin, S.; Brailovski, V. Structure and functional properties of metastable beta Ti-18Zr-14Nb (at.%) alloy for biomedical applications subjected to radial shear rolling and thermomechanical treatment. J. Alloy. Compd. 2018, 737, 678–683. [Google Scholar] [CrossRef]
- Miao, L.; Liu, C.L.; Yang, J.C.; Zhang, J.B.; Li, Z.N.; Zhang, H.; Li, Z.M. Synthesis and biological activity of new (E)-α-(methoxyimino) benzeneacetate derivatives containing a substituted pyrazole ring. J. Agric. Food. Chem. 2010, 58, 2664–2667. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, K.; Wang, P.; Kang, J.X.; Gao, S.; Zhao, L.X.; Ye, F. Design, synthesis and herbicidal activity evaluation of novel aryl-naphthyl methanone derivatives. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Mueller, T.; Rosinger, C.; Willms, L.; Van Almsick, A. ChemInform Abstract: 4-hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: Solutions for modern and sustainable agriculture. Angew. Chew. Int. Ed. 2014, 52, 9388–9398. [Google Scholar] [CrossRef]
- Cai, X.Y.; Wen, Y.Z.; Zhong, T.X.; Liu, W.P. Effects of methyl-CD and humic acid on hydrolytic degradation of the herbicide diclofop-methyl. J Environ. Sci. 2005, 17, 67–71. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, S.Q.; Liu, Y.X.; Wang, J.Y.; Gao, S.; Zhao, L.X.; Ye, F. Design, synthesis, SAR and molecular docking of novel green niacin-triketone HPPD inhibitor. Ind. Crops Prod. 2019, 137, 566–575. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.Y.; Zhang, D.; Chen, Y.F.; Gao, S.; Zhao, L.X.; Ye, F. Solvent-free synthesis and safener activity of sulfonylurea benzothiazolines. Molecules 2017, 22, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Ma, P.; Zhang, Y.Y.; Li, P.; Yang, F.; Fu, Y. Herbicidal activity and molecular docking study of novel ACCase inhibitors. Front. Plant Sci. 2018, 9, 1850. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Ma, P.; Zhai, Y.; Yang, F.; Gao, S.; Zhao, L.X.; Fu, Y. Design, microwave-assisted synthesis, bioactivity and SAR of novel substituted 2-phenyl-2-cyclohexanedione enol ester derivatives. RSC Adv. 2018, 8, 19883–19893. [Google Scholar] [CrossRef] [Green Version]
- Buon, C.; Chacun-Lefevre, L.; Rabot, R.; Bouyssou, P.; Coudert, G. Synthesis of 3-substituted and 2, 3-disubstituted-4H-1, 4-benzoxazines. Tetrahedron 2000, 56, 605–614. [Google Scholar] [CrossRef]
- Morales-Nava, R.; Fernández-Zertuche, M.; Ordóñez, M. Microwave-assisted improved synthesis of oxazolidin-2-ones, oxazolidine-2-thiones and thiazolidine-2-thione chiral auxiliaries. Molecules 2011, 16, 8803–8814. [Google Scholar] [CrossRef]
- Ye, F.; Zhai, Y.; Kang, T.; Wu, S.L.; Li, J.J.; Gao, S.; Fu, Y. Rational design, synthesis and structure-activity relationship of novel substituted oxazole isoxazole carboxamides as herbicide safener. Pestic. Biochem. Physiol. 2019, 157, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, L.; Ye, F. Microwave-assisted synthesis and bioactivity of novel 2, 2, 4, 5-tetrasubstituted 3-dichloroacetyl-1, 3-oxazolidines. Heterocycles 2011, 83, 2607–2613. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, D.; Kang, T.; Guo, Y.Y.; Chen, W.G.; Ye, F. Fragment splicing-based design, synthesis and safener activity of novel substituted phenyl oxazole derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 570–576. [Google Scholar] [CrossRef]
- Morrison, C.S.; Lampe, J.B.; Kolodziejczyk, T.C.; Cavazos, R.J.; Peteros, R.A. ChemInform Abstract: Rapid, quantitative, solvent-free synthesis of medium-ring diaza heterocycles from diketene-acetone adduct and diamines. Tetrahedron Lett. 2015, 55, 6547–6549. [Google Scholar] [CrossRef]
- Elmore, M.T.; Brosnan, J.T.; Armel, G.R. Herbicide safeners increase creeping bentgrass (Agrostis stolonifera) tolerance to pinoxaden and affect weed control. Weed Technol. 2017, 30, 919–928. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.X.; Yi, K.H.; Li, M.Q.; Li, J.Z.; Ye, F. Quantitative structure activity relationship studies and molecular dynamics simulations of 2-(aryloxyacetyl) cyclohexane-1,3-diones derivatives as 4-hydroxyphenylpyruvate dioxygenase inhibitors. Front. Chem. 2019, 7, 556. [Google Scholar] [CrossRef]
- Cummins, I.; Bryant, D.N.; Edwards, R. Safener responsiveness and multiple herbicide resistance in the weed black-grass (Alopecurus myosuroides). Plant Biotechnol. J. 2009, 7, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yu, J.; Pan, L.; Wu, X.; Dong, L. Target-Site Resistance to Fenoxaprop-P-ethyl in Keng Stiffgrass (Sclerochloa kengiana) from China. Weed Sci. 2017, 65, 452–460. [Google Scholar] [CrossRef]
- Cataneo, A.C.; Ferreira, L.C.; Mischan, M.M.; Velini, E.D.; Corniani, N.; Cerdeira, A.L. Mefenpyr-diethyl action on fenoxaprop-p-ethyl detoxification in wheat varieties. Planta Daninha 2013, 31, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Wang, M.X.; Zhang, D.; Hou, Y.W.; Gao, S.; Zhao, L.X.; Ye, F. Design, synthesis, and herbicidal activity of pyrazole benzophenone derivatives. RSC Adv. 2017, 7, 46858–46865. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Cao, H.F.; Fu, Y.; Zhao, L.X.; Gao, S. The safener effect of chiral derivatives of 3-dichloroacetyl oxazolidine against haloxyfop-P-methyl-induced toxicity in maize. Zemdirbyste-Agriculture 2016, 103, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tweel, B.; Tong, L. Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop. PNAS 2004, 101, 5910–5915. [Google Scholar] [CrossRef] [Green Version]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. PNAS USA. 2006, 103, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzios, K.K.; Burgos, N. Metabolism-based herbicide resistance: Regulation by safeners. Weed Sci. 2004, 52, 454–467. [Google Scholar] [CrossRef]
- Abu Qare, A.W.; Duncan, H.J. Herbicide safeners: Uses, limitations, metabolism, and mechanisms of action. Chemosphere 2002, 48, 965–974. [Google Scholar] [CrossRef]

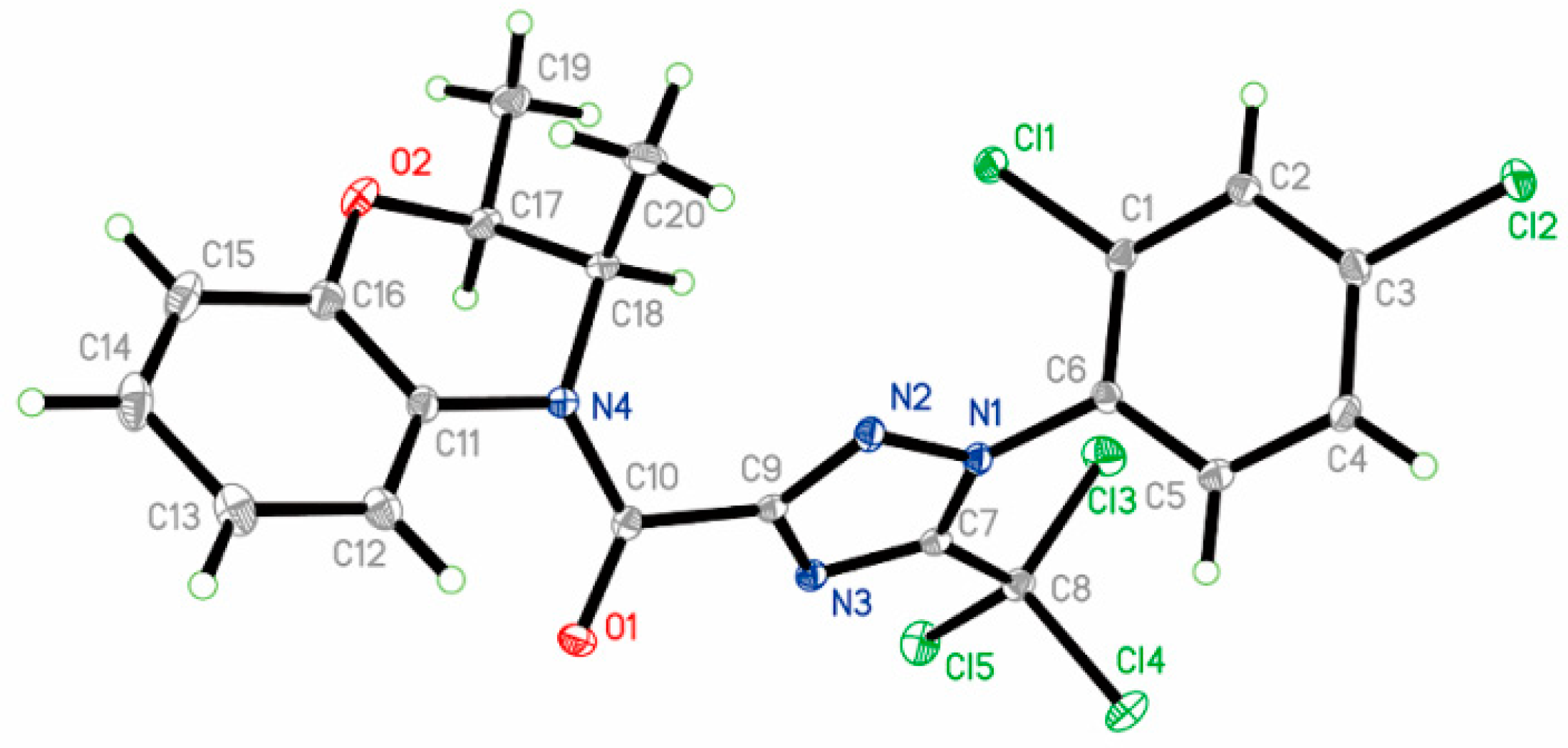
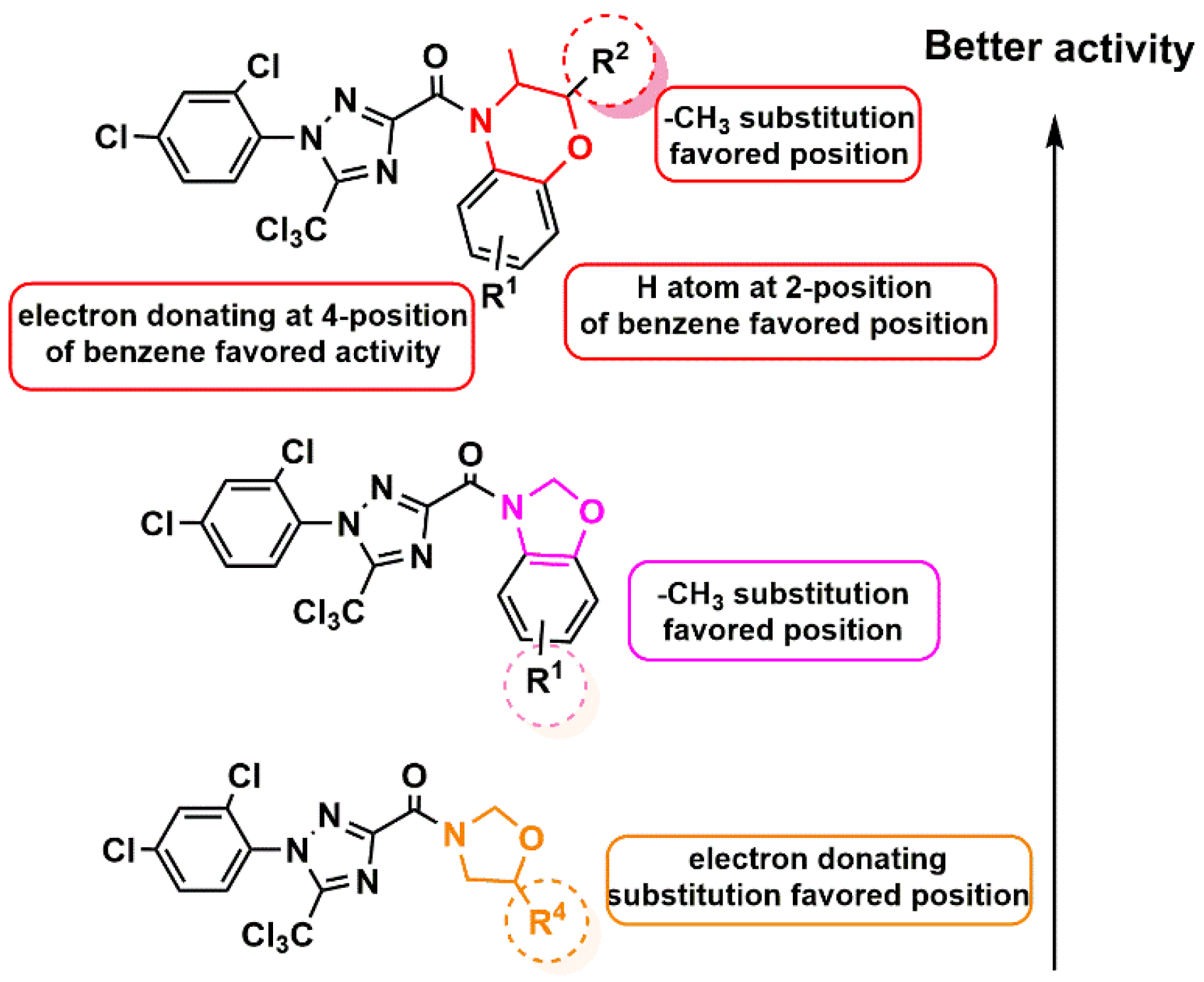

| Compound | R1 | R2 | R3 | R4 | Melting Point (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 5a | H | H | CH3 | - | 204–205 | 66 |
| 5b | 4-Cl | H | CH3 | - | 205–207 | 55 |
| 5c | 4-CH3 | H | CH3 | - | 194–196 | 75 |
| 5d | 2,4-Cl | H | CH3 | - | 195–196 | 50 |
| 5e | 4-Br | H | CH3 | - | 214–215 | 51 |
| 5f | 3-CH3 | H | CH3 | - | 203–205 | 77 |
| 5g | 4-OCH3 | H | CH3 | - | 210–212 | 70 |
| 5h | 4-C(CH3)3 | H | CH3 | - | 212–214 | 80 |
| 5i | 4-CH2CH3 | H | CH3 | - | 199–201 | 82 |
| 5j | H | CH3 | CH3 | - | 199–200 | 79 |
| 5k | 4-CH3 | CH3 | CH3 | - | 198–200 | 77 |
| 5l | 2,4-Cl | CH3 | CH3 | - | 194–196 | 55 |
| 5m | 4-CH2CH3 | CH3 | CH3 | - | 213–215 | 81 |
| 5n | 4-H | H | H | - | 189–191 | 55 |
| 5o | 4-CH3 | H | H | - | 196–198 | 78 |
| 5p | H | - | - | - | 249–250 | 51 |
| 5q | 4-CH3 | - | - | - | 202–204 | 59 |
| 5r | - | - | - | H | 185–187 | 70 |
| 5s | - | - | - | CH3 | 196–198 | 76 |
| 5t | - | - | - | H | 175–177 | 54 |
| 5u | - | - | - | CH3 | 182–183 | 67 |
| Compound | Recovery of Root Length (%) | Recovery of Plant Weight (%) | Recovery of Chlorophyll (%) |
|---|---|---|---|
| Fenchlorazole | 37.16 ± 0.63 | 130.05 ± 0.44 | 97.52 ± 0.13 |
| 5a | 20.50 ± 0.59 | 68.61 ± 0.95 | 44.90 ± 0.29 |
| 5b | 20.55 ± 0.71 | 57.84 ± 0.94 | 20.12 ± 0.25 |
| 5c | 71.72 ± 1.16 | 109.87 ± 1.57 | 55.37 ± 0.17 |
| 5d | 1.22 ± 0.84 | 51.56 ± 0.34 | 14.32 ± 0.30 |
| 5e | 13.78 ± 0.91 | 65.46 ± 0.70 | 21.23 ± 0.66 |
| 5f | 31.72 ± 0.86 | 90.59 ± 1.27 | 44.92 ± 0.61 |
| 5g | 25.59 ± 1.37 | 78.49 ± 0.63 | 35.80 ± 0.75 |
| 5h | 35.19 ± 0.42 | 65.91 ± 0.55 | 43.83 ± 0.84 |
| 5i | 47.41 ± 0.42 | 100.90 ± 1.56 | 48.75 ± 0.48 |
| 5j | 39.64 ± 1.16 | 107.63 ± 0.80 | 39.12 ± 0.80 |
| 5k | 44.48 ± 0.78 | 129.62 ± 1.00 | 46.29 ± 0.23 |
| 5l | 54.79 ± 1.02 | 70.38 ± 1.00 | 48.21 ± 0.22 |
| 5m | 48.90 ± 0.52 | 129.13 ± 1.17 | 65.84 ± 0.04 |
| 5n | 30.27 ± 1.06 | 159.18 ± 0.75 | 28.93 ± 0.35 |
| 5o | 70.76 ± 0.43 | 143.51 ± 0.82 | 99.77 ± 0.93 |
| 5p | 18.23 ± 0.59 | 78.48 ± 1.09 | 26.98 ± 0.74 |
| 5q | 34.34 ± 0.81 | 113.46 ± 0.45 | 30.31 ± 0.34 |
| 5r | 2.39 ± 0.31 | 79.84 ± 0.68 | 23.43 ± 0.36 |
| 5s | 41.22 ± 0.88 | 105.84 ± 0.82 | 55.63 ± 0.62 |
| 5t | 27.66 ± 0.54 | 71.31 ± 0.98 | 26.98 ± 0.74 |
| 5u | 24.89 ± 0.15 | 78.03 ± 0.31 | 32.51 ± 0.04 |
| Name | Log p a | ARs b | SA b | RBs b | HBAs b | Electronegativity c |
|---|---|---|---|---|---|---|
| Fenoxaprop-P-ethyl | 4.64 | 3 | 335 | 7 | 5 |  |
| Fenchlorazole | 6.07 | 2 | 344 | 5 | 4 |  |
| 5o | 6.02 | 3 | 431 | 3 | 4 |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.-L.; Zhao, L.-X.; Wang, Z.-W.; Rong, S.-Z.; Zhou, X.-L.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis and Evaluation of Novel Trichloromethyl Dichlorophenyl Triazole Derivatives as Potential Safener. Biomolecules 2019, 9, 438. https://doi.org/10.3390/biom9090438
Guo K-L, Zhao L-X, Wang Z-W, Rong S-Z, Zhou X-L, Gao S, Fu Y, Ye F. Design, Synthesis and Evaluation of Novel Trichloromethyl Dichlorophenyl Triazole Derivatives as Potential Safener. Biomolecules. 2019; 9(9):438. https://doi.org/10.3390/biom9090438
Chicago/Turabian StyleGuo, Ke-Liang, Li-Xia Zhao, Zi-Wei Wang, Shu-Zhe Rong, Xiao-Lin Zhou, Shuang Gao, Ying Fu, and Fei Ye. 2019. "Design, Synthesis and Evaluation of Novel Trichloromethyl Dichlorophenyl Triazole Derivatives as Potential Safener" Biomolecules 9, no. 9: 438. https://doi.org/10.3390/biom9090438





