Structure-Dependent Interfacial Properties of Chaplin F from Streptomyces coelicolor
Abstract
:1. Introduction
2. Results and Discussion
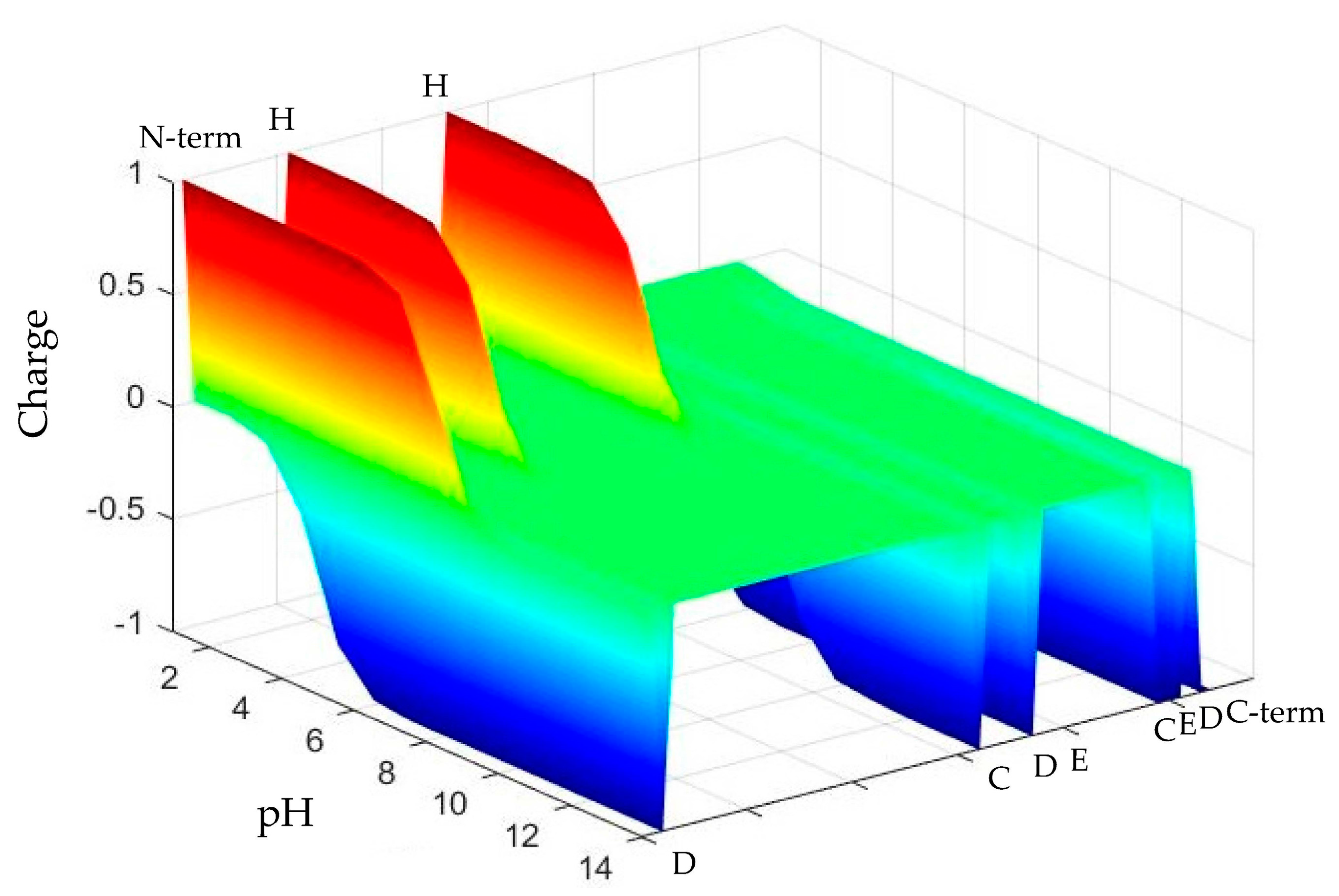
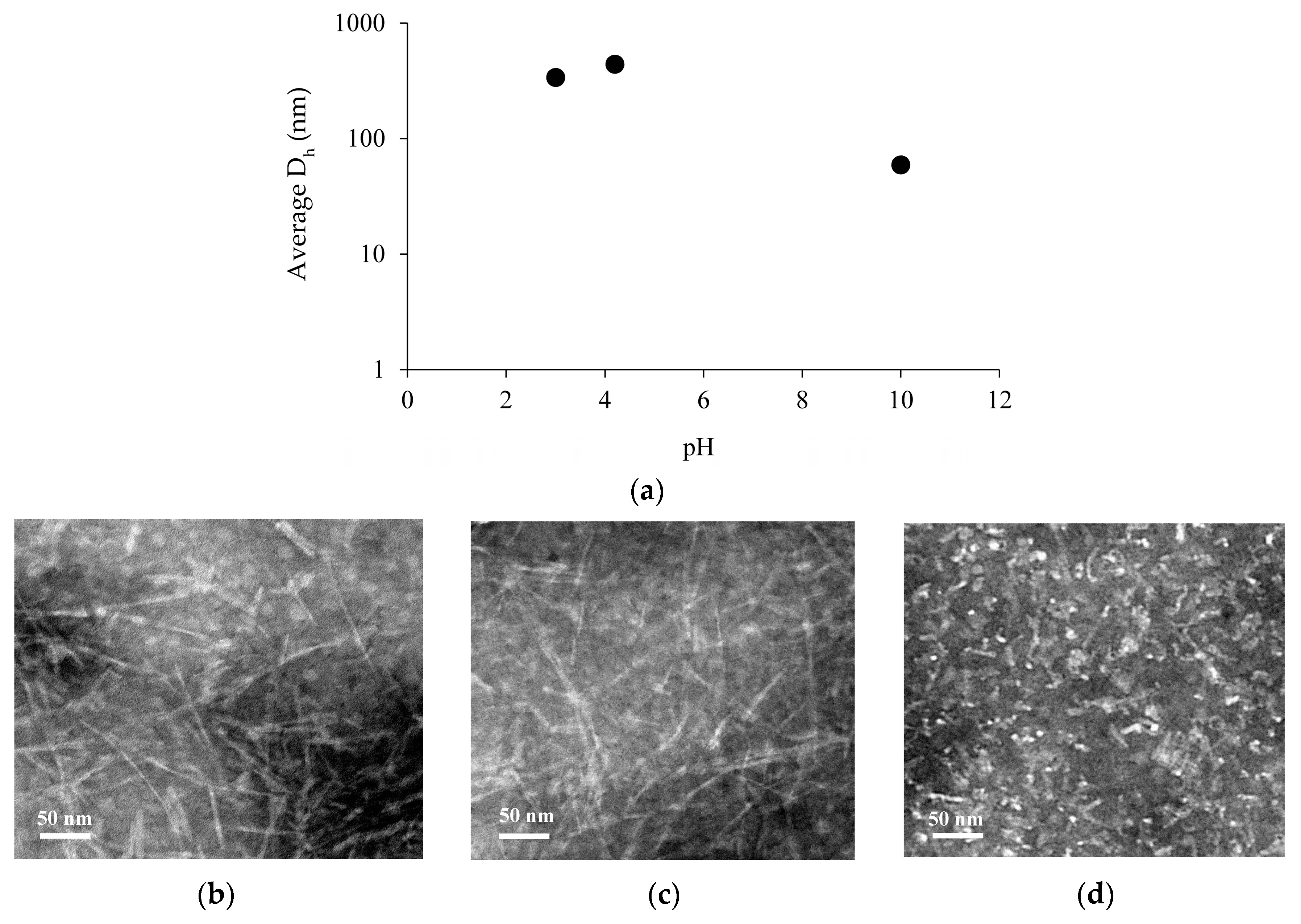
2.1. pH-Responsive Self-Assembly of Chp F in Solution
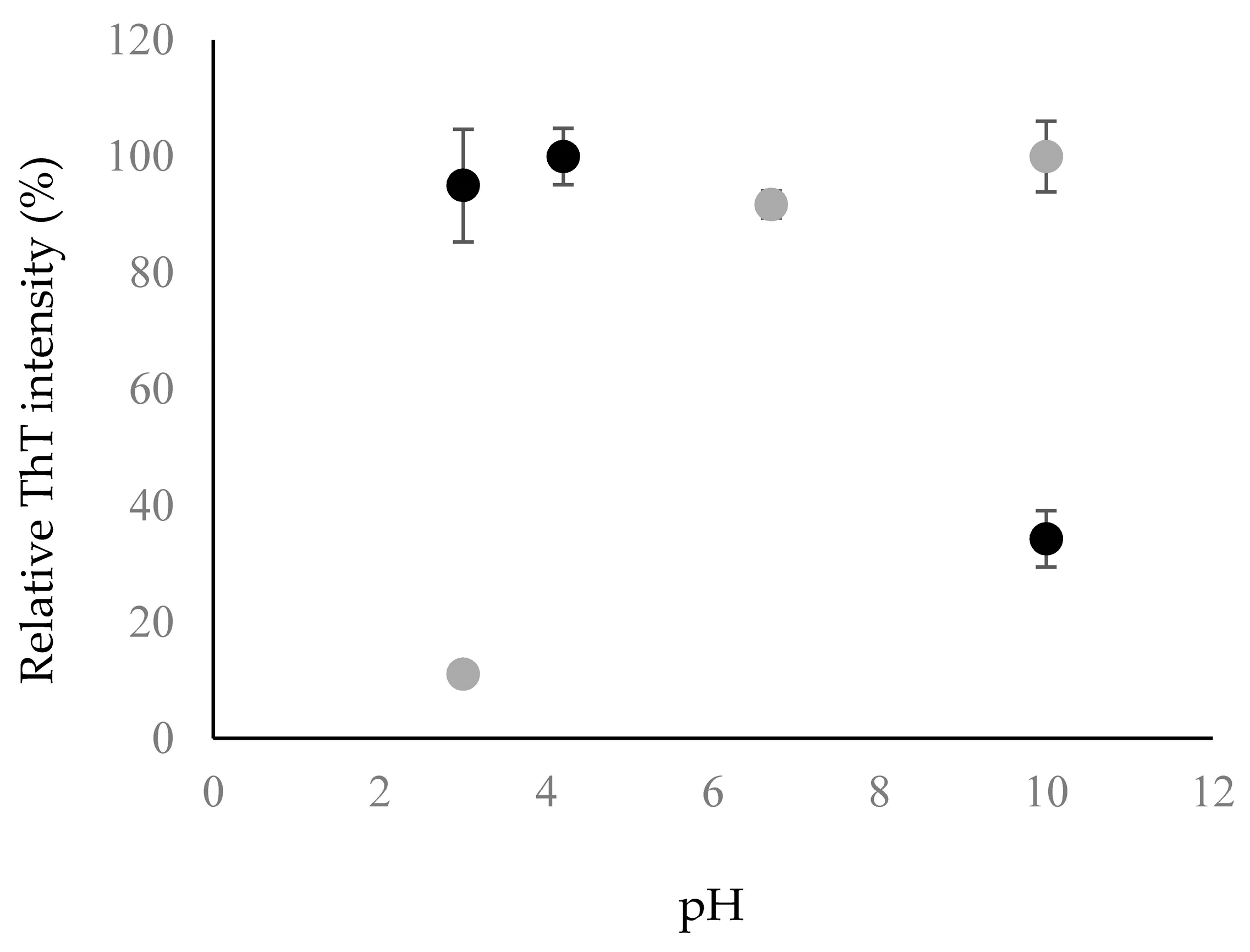
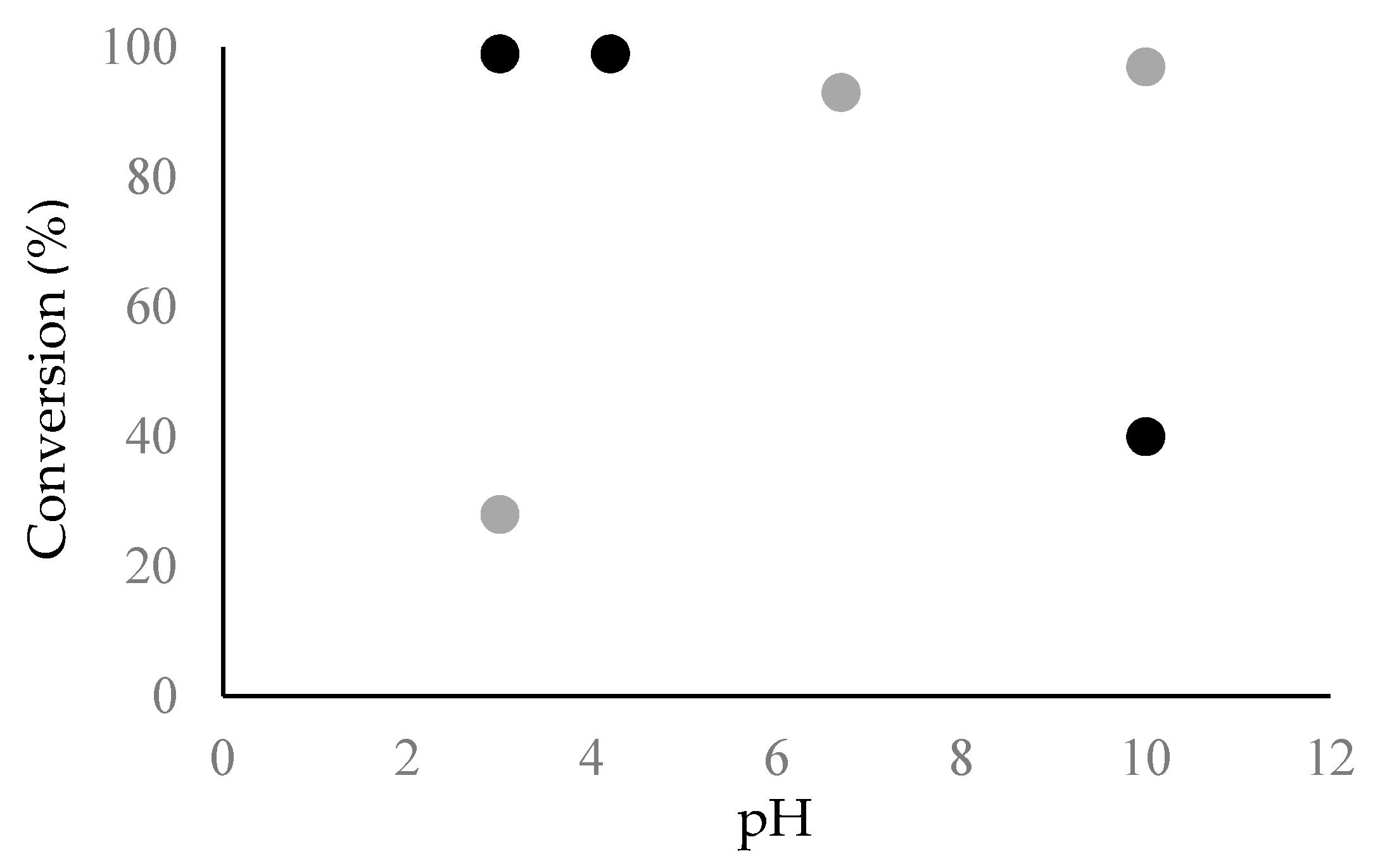
2.2. Fibril Formation of Chp F at Different pH
2.3. Cysteine-Independent Fibril Formation of Chp F
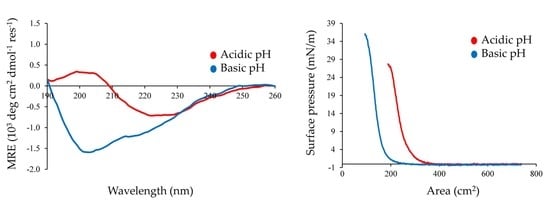
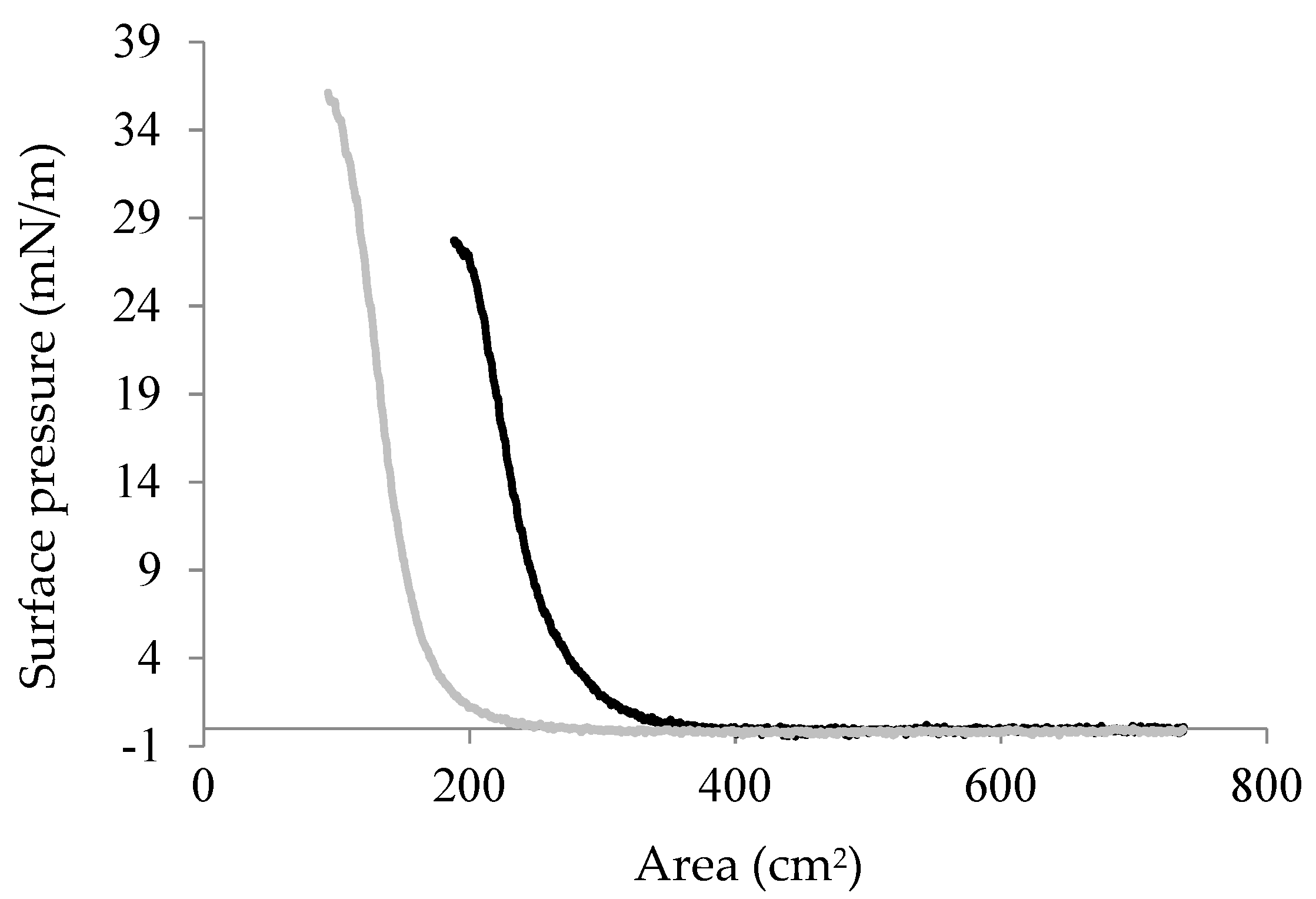
2.4. Interfacial Properties of Chp F as a Function of pH
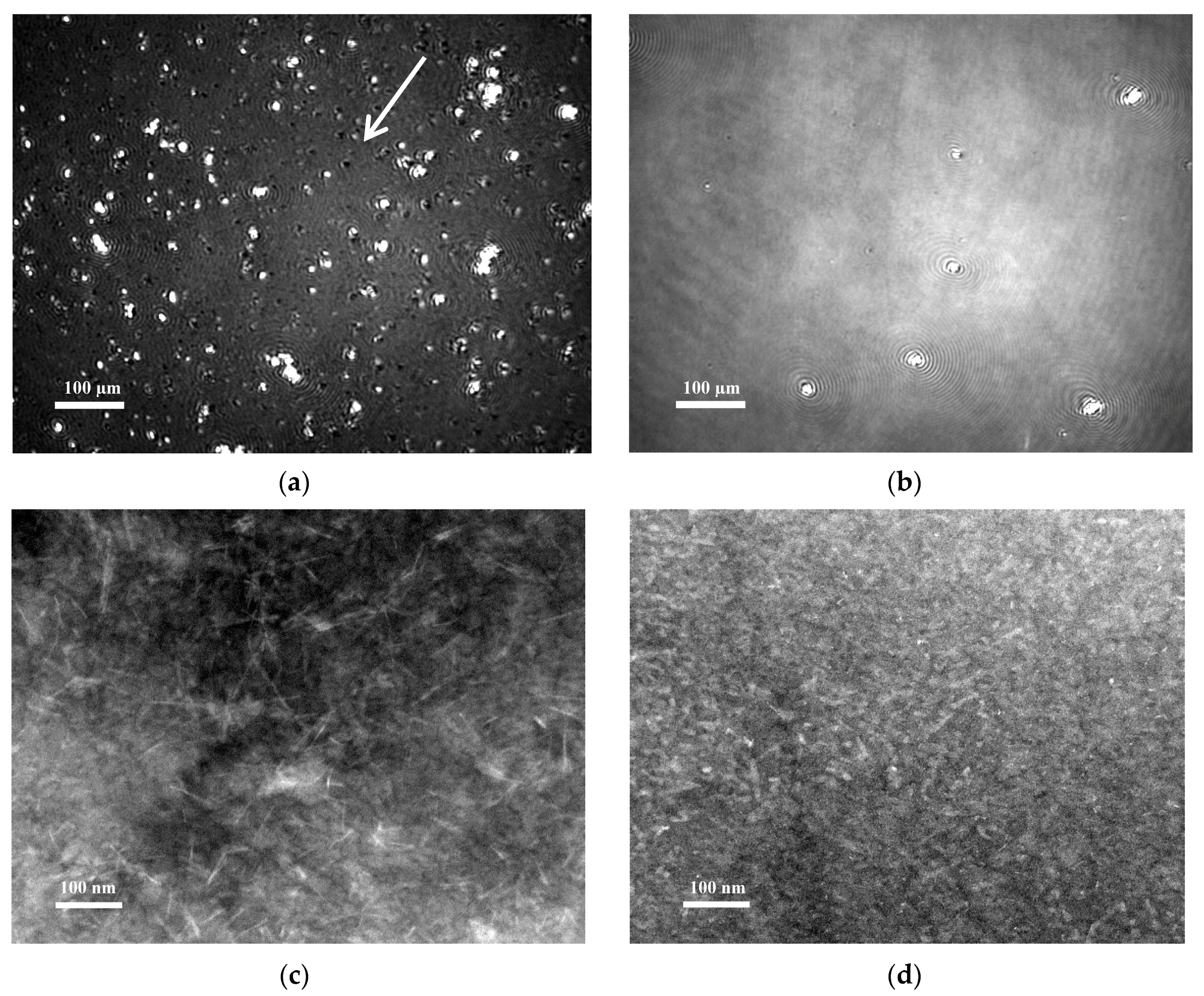
2.5. Morphology of Chp F Interfacial Films
2.6. Comparison of Chp F to Chp E at the Air/Water Interface
3. Materials and Methods
3.1. Isoelectric Focusing Gel
3.2. Preparation of Solutions
3.3. Size Measurement of Chp F as a Function of pH
3.4. Circular Dichroism Spectroscopy Measurements
3.5. Transmission Electron Microscopy
3.6. Thioflavin-T Binding
3.7. Amyloid Fibril Assembly
3.8. Apparatus
3.9. Surface Pressure/Area Isotherm
3.10. Brewster Angle Microscopy
3.11. Transmission Electron Microscopy of Langmuir Films
3.12. Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elliot, M.A.; Karoonuthaisiri, N.; Huang, J.; Bibb, M.J.; Cohen, S.N.; Kao, C.M.; Buttner, M.J. The chaplins: A family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003, 17, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S. Stabilization of bubbles and foams. Curr. Opin. Colloid Interface Sci. 2007, 12, 232–241. [Google Scholar] [CrossRef]
- Bokhove, M.; Claessen, D.; de Jong, W.; Dijkhuizen, L.; Boekema, E.J.; Oostergetel, G.T. Chaplins of Streptomyces coelicolor self-assemble into two distinct functional amyloids. J. Struct. Biol. 2013, 184, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; Rink, R.; de Jong, W.; Siebring, J.; de Vreugd, P.; Boersma, F.H.; Dijkhuizen, L.; Wösten, H.A. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003, 17, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, E.B.; Claessen, D.; Haas, M.; Hurgobin, B.; Gras, S.L. The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. PLoS ONE 2011, 6, e18839. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; Stokroos, I.; Deelstra, H.J.; Penninga, N.A.; Bormann, C.; Salas, J.A.; Dijkhuizen, L.; Wösten, H.A. The formation of the rodlet layer of Streptomycetes is the result of the interplay between rodlins and chaplins. Mol. Microbiol. 2004, 53, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ekkers, D.M.; Claessen, D.; Galli, F.; Stamhuis, E. Surface modification using interfacial assembly of the Streptomyces chaplin proteins. Appl. Microbiol. Biotechnol. 2014, 98, 4491–4501. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; De Jong, W.; Dijkhuizen, L.; Wösten, H.A. Regulation of Streptomyces development: Reach for the sky! Trends Microbiol. 2006, 14, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Di Berardo, C.; Capstick, D.S.; Bibb, M.J.; Findlay, K.C.; Buttner, M.J.; Elliot, M.A. Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor. J. Bacteriol. 2008, 190, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Dokouhaki, M.; Hung, A.; Day, L.; Gras, S.L. The pH-dependent assembly of Chaplin E from Streptomyces coelicolor. J. Struct. Biol. 2017, 198, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Dokouhaki, M.; Hung, A.; Prime, E.L.; Qiao, G.G.; Day, L.; Gras, S.L. pH-induced interfacial properties of Chaplin E from Streptomyces coelicolor. J. Struct. Biol. submitted for publication. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Ranodolph, A. Theory of Particulate Processes 2e: Analysis and Techniques of Continuous Crystallization; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Gebbink, M.F.; Claessen, D.; Bouma, B.; Dijkhuizen, L.; Wösten, H.A. Amyloids—A functional coat for microorganisms. Nat. Rev. Microbiol. 2005, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Loksztejn, A.; Dzwolak, W. Vortex-induced formation of insulin amyloid superstructures probed by time-lapse atomic force microscopy and circular dichroism spectroscopy. J. Mol. Biol. 2010, 395, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sluzky, V.; Tamada, J.A.; Klibanov, A.M.; Langer, R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. USA 1991, 88, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.; Uversky, V.N.; Fink, A.L. Effect of environmental factors on the kinetics of insulin fibril formation: Elucidation of the molecular mechanism. Biochemistry 2001, 40, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Park, J.S.; Seo, C.H.; Park, Y. Applications of circular dichroism for structural analysis of gelatin and antimicrobial peptides. Int. J. Mol. Sci. 2012, 13, 3229–3244. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Drake, A.F.; Banfield, B.A.; Bloomberg, G.B.; Palmer, M.S.; Clarke, A.R.; Collinge, J. Conformational properties of the prion octa-repeat and hydrophobic sequences. FEBS Lett. 1997, 405, 378–384. [Google Scholar] [CrossRef]
- Juban, M.M.; Javadpour, M.M.; Barkley, M.D. Circular dichroism studies of secondary structure of peptides. Methods Mol. Biol. 1997, 78, 73–78. [Google Scholar] [PubMed]
- Pastor, M.T.; de la Paz, M.L.; Lacroix, E.; Serrano, L.; Pérez-Payá, E. Combinatorial approaches: A new tool to search for highly structured β-hairpin peptides. Proc. Natl. Acad. Sci. USA 2002, 99, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Blijdenstein, T.; De Groot, P.; Stoyanov, S. On the link between foam coarsening and surface rheology: Why hydrophobins are so different. Soft Matter 2010, 6, 1799–1808. [Google Scholar] [CrossRef]
- Babchin, A.J.; Schramm, L.L. Osmotic repulsion force due to adsorbed surfactants. Colloids Surf. B 2012, 91, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hakanpää, J.; Paananen, A.; Askolin, S.; Nakari-Setälä, T.; Parkkinen, T.; Penttilä, M.; Linder, M.B.; Rouvinen, J. Atomic resolution structure of the HFBII hydrophobin, a self-assembling amphiphile. J. Biol. Chem. 2004, 279, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.B. Hydrophobins: Proteins that self assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Hörth, P.; Miller, C.A.; Preckel, T.; Wenz, C. Efficient fractionation and improved protein identification by peptide OFFGEL electrophoresis. Mol. Cell. Proteom. 2006, 5, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.A.; Butler, J.C.; Moran, J.; Whitesides, G.M. Selective reduction of disulfides by tris (2-carboxyethyl) phosphine. J. Org. Chem. 1991, 56, 2648–2650. [Google Scholar] [CrossRef]
- Cline, D.J.; Redding, S.E.; Brohawn, S.G.; Psathas, J.N.; Schneider, J.P.; Thorpe, C. New water-soluble phosphines as reductants of peptide and protein disulfide bonds: Reactivity and membrane permeability. Biochemistry 2004, 43, 15195–15203. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.; Smith, B.; Wien, F.; Miles, A.; Wallace, B. CDtool—An integrated software package for circular dichroism spectroscopic data processing, analysis, and archiving. Anal. Biochem. 2004, 332, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- AutoCAD Mechanical. Available online: http://www.autodesk.com.au/products/autocad-mechanical/free-trial (accessed on 5 January 2015).
- Srinivasan, R.; Jones, E.M.; Liu, K.; Ghiso, J.; Marchant, R.E.; Zagorski, M.G. pH-dependent amyloid and protofibril formation by the ABri peptide of familial British dementia. J. Mol. Biol. 2003, 333, 1003–1023. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.R.; MacPhee, C.E.; Miller, A.F.; Dunlop, I.E.; Dobson, C.M.; Donald, A.M. The formation of spherulites by amyloid fibrils of bovine insulin. Proc. Natl. Acad. Sci. USA 2004, 101, 14420–14424. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.N.; Scanlon, D.B.; Gras, S.L. Functional fibrils derived from the peptide TTR1-cycloRGDfK that target cell adhesion and spreading. Biomaterials 2011, 32, 6099–6110. [Google Scholar] [CrossRef] [PubMed]
- Castano, S.; Blaudez, D.; Desbat, B.; Dufourcq, J.; Wróblewski, H. Secondary structure of spiralin in solution, at the air/water interface, and in interaction with lipid monolayers. Biochim. Biophys. Acta Biomembr. 2002, 1562, 45–56. [Google Scholar] [CrossRef]
- Mackie, A.R.; Gunning, A.P.; Wilde, P.J.; Morris, V.J. Orogenic displacement of protein from the air/water interface by competitive adsorption. J. Colloid Interface Sci. 1999, 210, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Paananen, A.; Vuorimaa, E.; Torkkeli, M.; Penttilä, M.; Kauranen, M.; Ikkala, O.; Lemmetyinen, H.; Serimaa, R.; Linder, M.B. Structural hierarchy in molecular films of two class II hydrophobins. Biochemistry 2003, 42, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.H.; Prime, E.L.; Tran, D.N.; Fu, Q.; Christofferson, A.J.; Yiapanis, G.; Yarovsky, I.; Qiao, G.G.; Solomon, D.H. Dynamic performance of duolayers at the air/water interface. 1. Experimental analysis. J. Phys. Chem. B 2014, 118, 10919–10926. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Paananen, A.; Laurikainen, K.; Vuorimaa, E.; Lemmetyinen, H.; Peltonen, J.; Linder, M.B. Self-assembled hydrophobin protein films at the air-water interface: Structural analysis and molecular engineering. Biochemistry 2007, 46, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Rubinger, C.; Moreira, R.; Cury, L.; Fontes, G.; Neves, B.; Meneguzzi, A.; Ferreira, C. Langmuir–Blodgett and Langmuir–Schaefer films of poly(5-amino-1-naphthol) conjugated polymer. Appl. Surf. Sci. 2006, 253, 543–548. [Google Scholar] [CrossRef]
- Kisko, K.; Szilvay, G.R.; Vuorimaa, E.; Lemmetyinen, H.; Linder, M.B.; Torkkeli, M.; Serimaa, R. Self-assembled films of hydrophobin proteins HFBI and HFBII studied in situ at the air/water interface. Langmuir 2008, 25, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Ritva, S.; Torkkeli, M.; Paananen, A.; Linder, M.; Kisko, K.; Knaapila, M.; Ikkala, O.; Vuorimaa, E.; Lemmetyinen, H.; Seeck, O. Self-assembled structures of hydrophobins HFBI and HFBII. J. Appl. Crystallogr. 2003, 36, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.H.; van Koeverden, M.P.; Nam, E.; Guntari, S.N.; Wibowo, S.H.; Blencowe, A.; Caruso, F.; Qiao, G.G. Assembly of Nanostructured Films with Hydrophobic Subcompartments via Continuous Assembly of Polymers. Macromolecules 2013, 46, 7789–7796. [Google Scholar] [CrossRef]
- Morris, V.J.; Kirby, A.R.; Gunning, A.P. Atomic Force Microscopy for Biologists; Imperial College Press: London, UK, 1999. [Google Scholar]
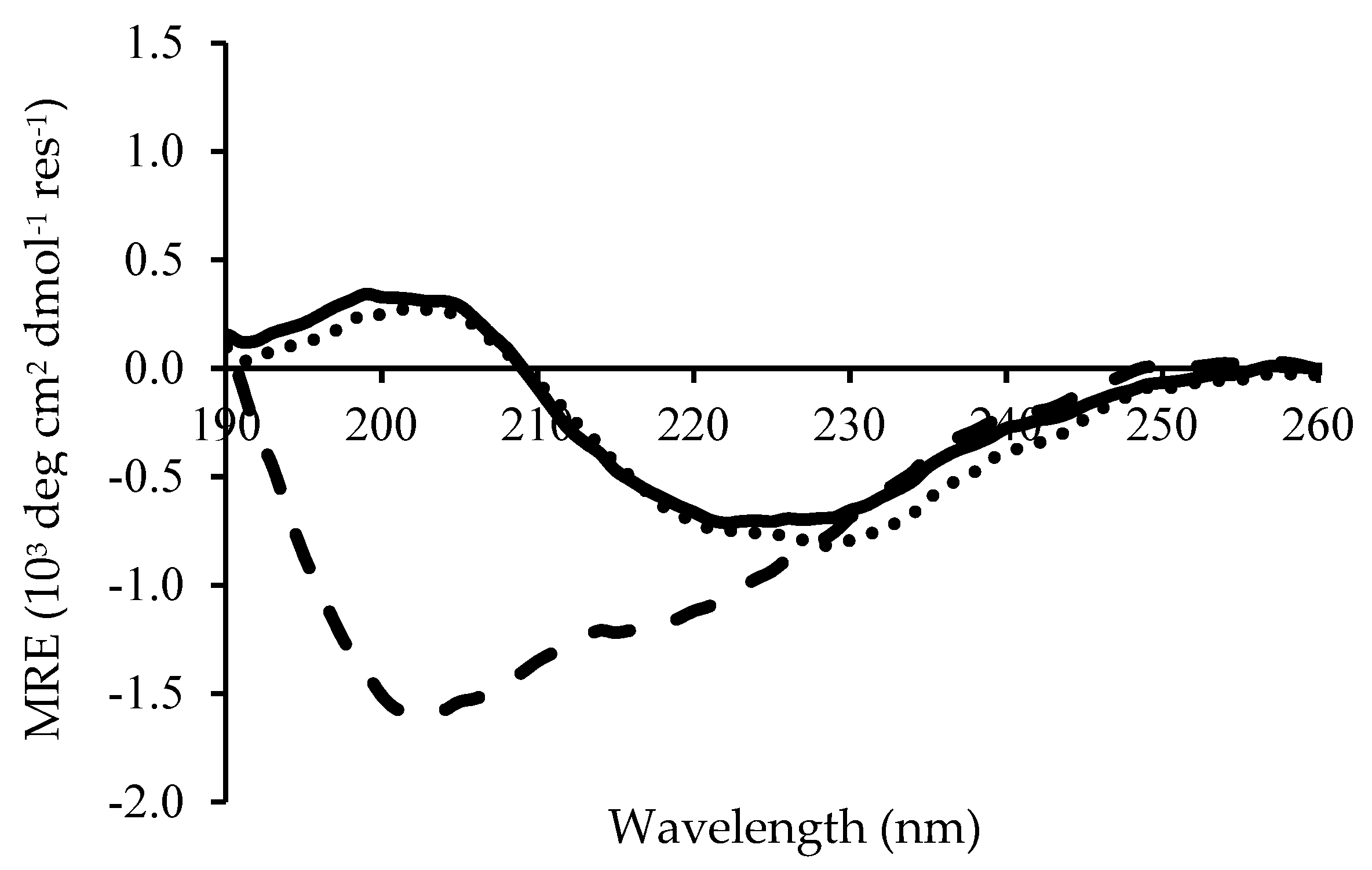
| Solution pH | β-Sheet | Random Coil |
|---|---|---|
| 3.0 | 60% | 6% |
| 4.2 | 64% | 4% |
| 10.0 | 30% | 36% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokouhaki, M.; Prime, E.L.; Hung, A.; Qiao, G.G.; Day, L.; Gras, S.L. Structure-Dependent Interfacial Properties of Chaplin F from Streptomyces coelicolor. Biomolecules 2017, 7, 68. https://doi.org/10.3390/biom7030068
Dokouhaki M, Prime EL, Hung A, Qiao GG, Day L, Gras SL. Structure-Dependent Interfacial Properties of Chaplin F from Streptomyces coelicolor. Biomolecules. 2017; 7(3):68. https://doi.org/10.3390/biom7030068
Chicago/Turabian StyleDokouhaki, Mina, Emma L. Prime, Andrew Hung, Greg G. Qiao, Li Day, and Sally L. Gras. 2017. "Structure-Dependent Interfacial Properties of Chaplin F from Streptomyces coelicolor" Biomolecules 7, no. 3: 68. https://doi.org/10.3390/biom7030068