Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin
Abstract
:1. Introduction
- (1)
- How to account for the amount of BR that is adsorbed by protein and thus not considered when applying the classical photometric extraction assay for the determination of HO activity?
- (2)
- Are the in vitro model systems suitable to investigate the effects of BR that is released following HO reaction?
- (3)
- Does the HO reaction rescue hepatic mitochondria from hemin-mediated toxicity?
- (4)
- Is the anti-oxidative property of BR involved in the protective effect of HO towards mitochondria?
- (5)
- How does BR formation relate to the metabolic activity and the proliferative response of cultured hepatocytes under conditions of accelerated HO activity?
- (6)
- Which subcellular structure in the hepatocyte is sensitive to increased levels of BR?
2. Aims of This Study
3. Results and Discussion
3.1. Protein Adsorption of BR and Subsequent Interference with the Quantification Can Be Corrected Using a Polynomial that Accounts for the Protein Amount Present in the Assay
- The corrected BR amount is: brcorr = br × f
- br = BR concentration (calculated from the calibration curve using the differential OD)
- f = −0.076 × x2 + 0.704 × x + 1.027
- x = protein content present in the assay in mg
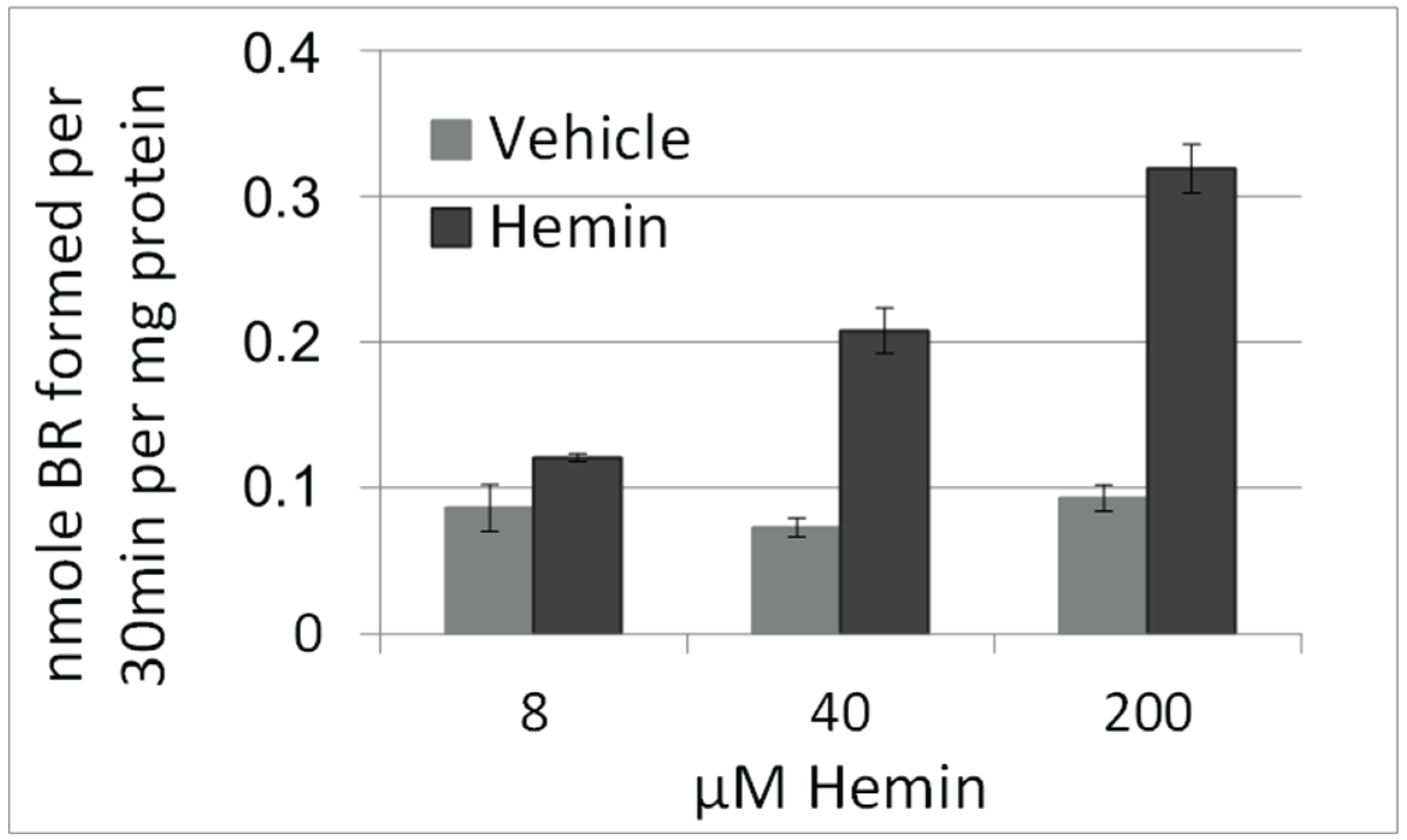
3.2. BRL3A Cells Have Similar HO Activities as Liver Tissue

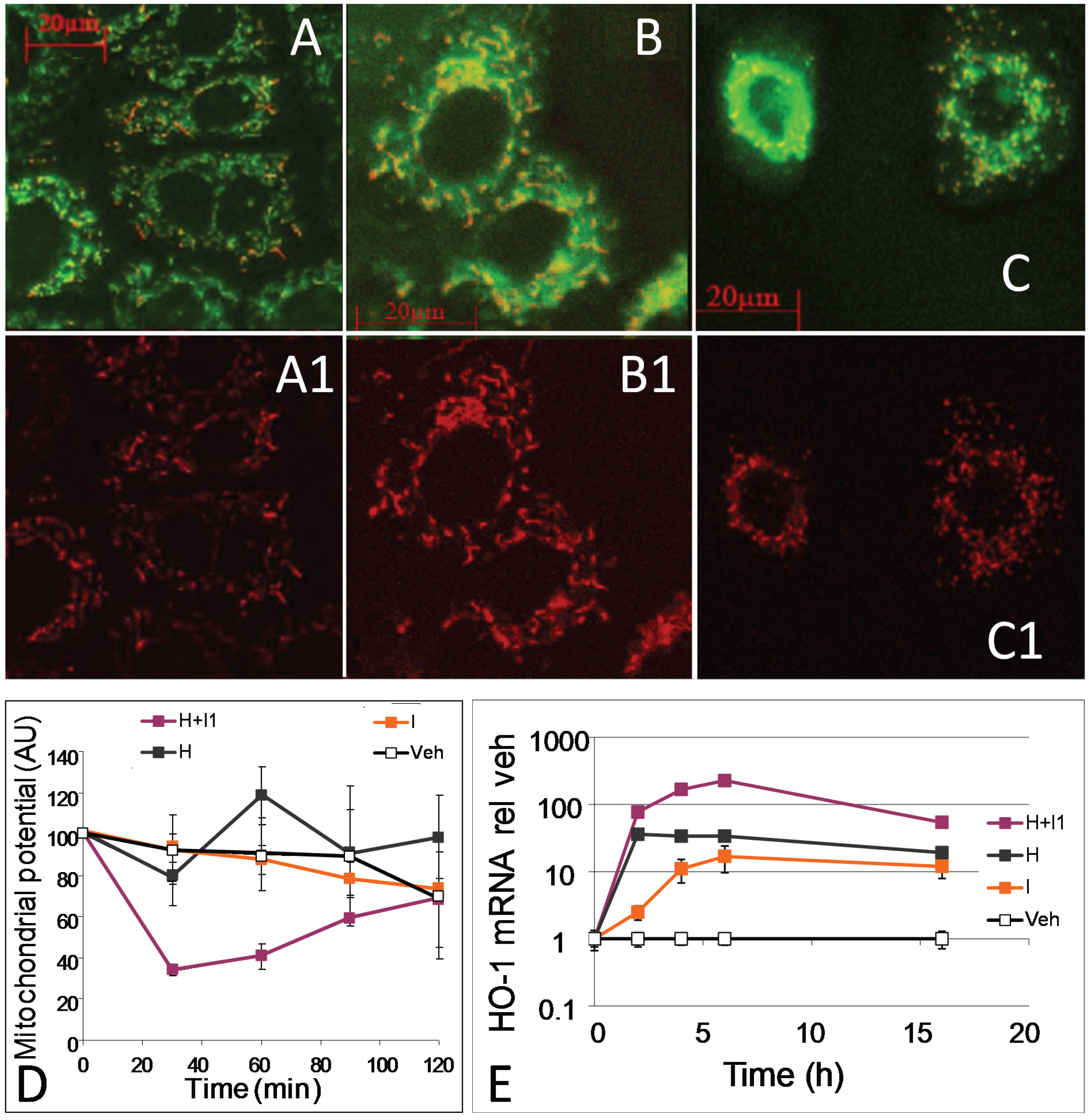
3.3. HO Reaction Rescues Mitochondria from Hemin-Mediated Impairment of Respiration and Subsequent Fragmentation
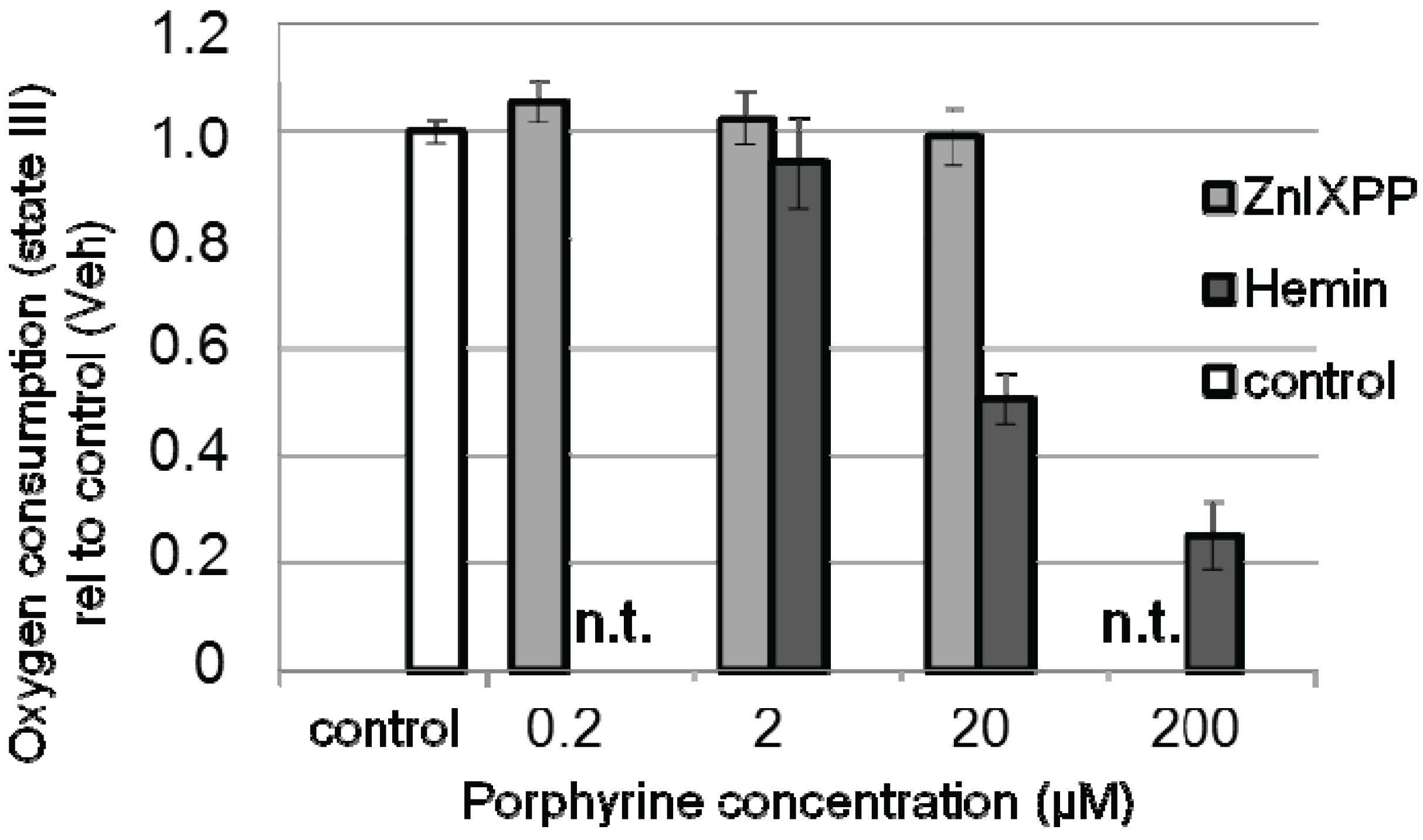
3.4. Bilirubin Does not Prevent Hemin-Induced Repression of Respiration in Liver Mitochondria

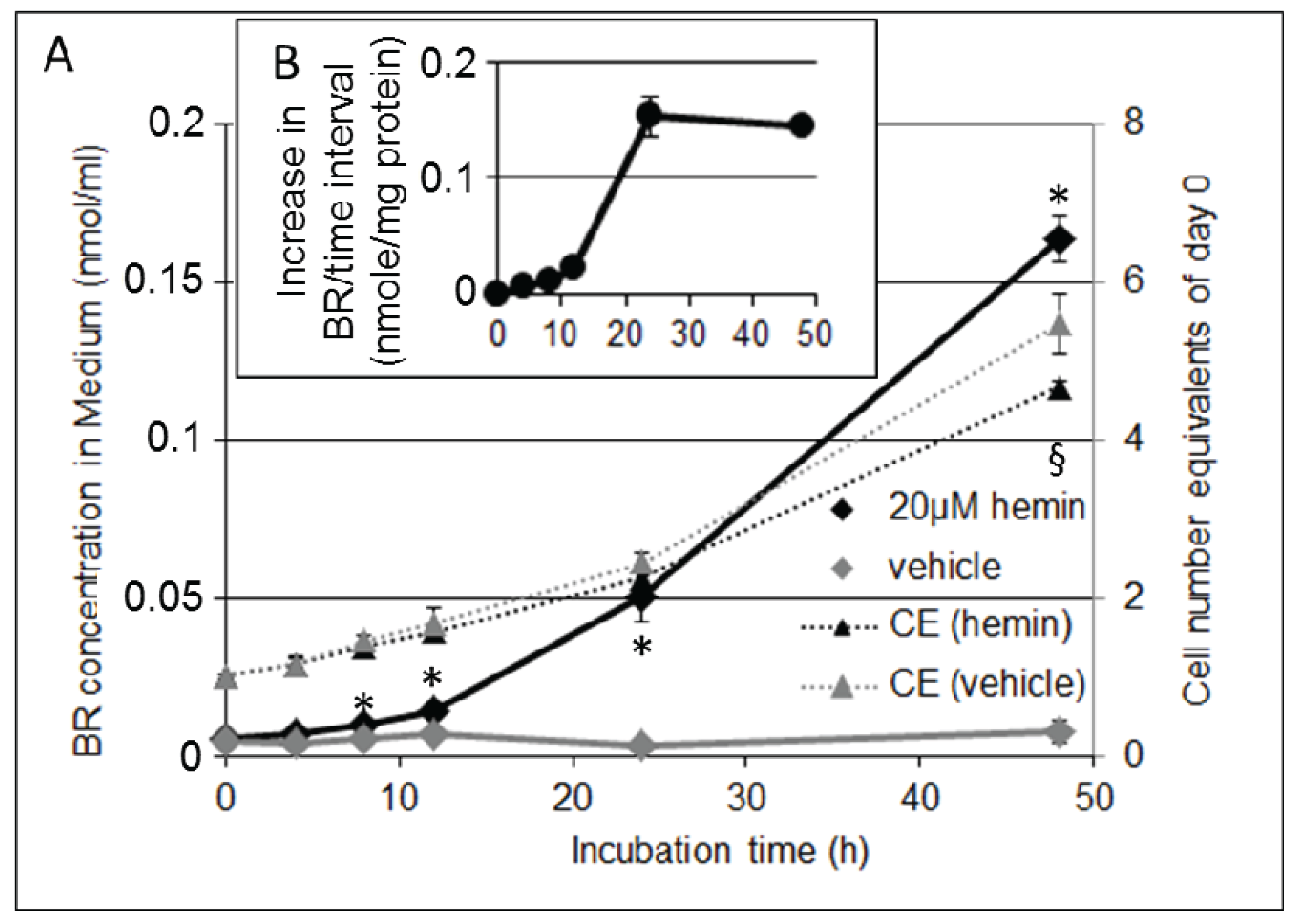
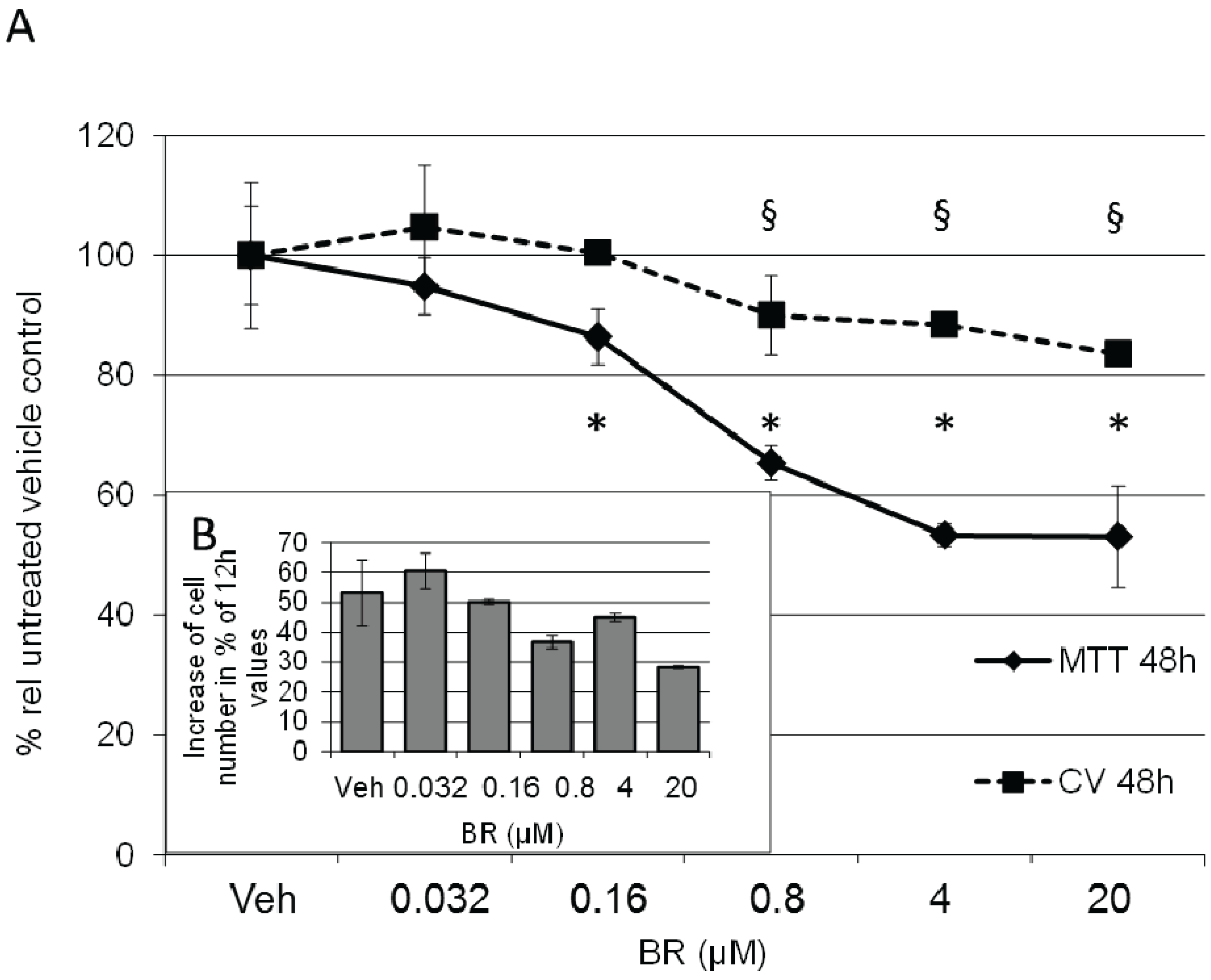
3.5. Formation of BR and Excretion to the Cell Culture Medium Is Accelerated in Response to hemin, but Decreases Cell Proliferation Rate
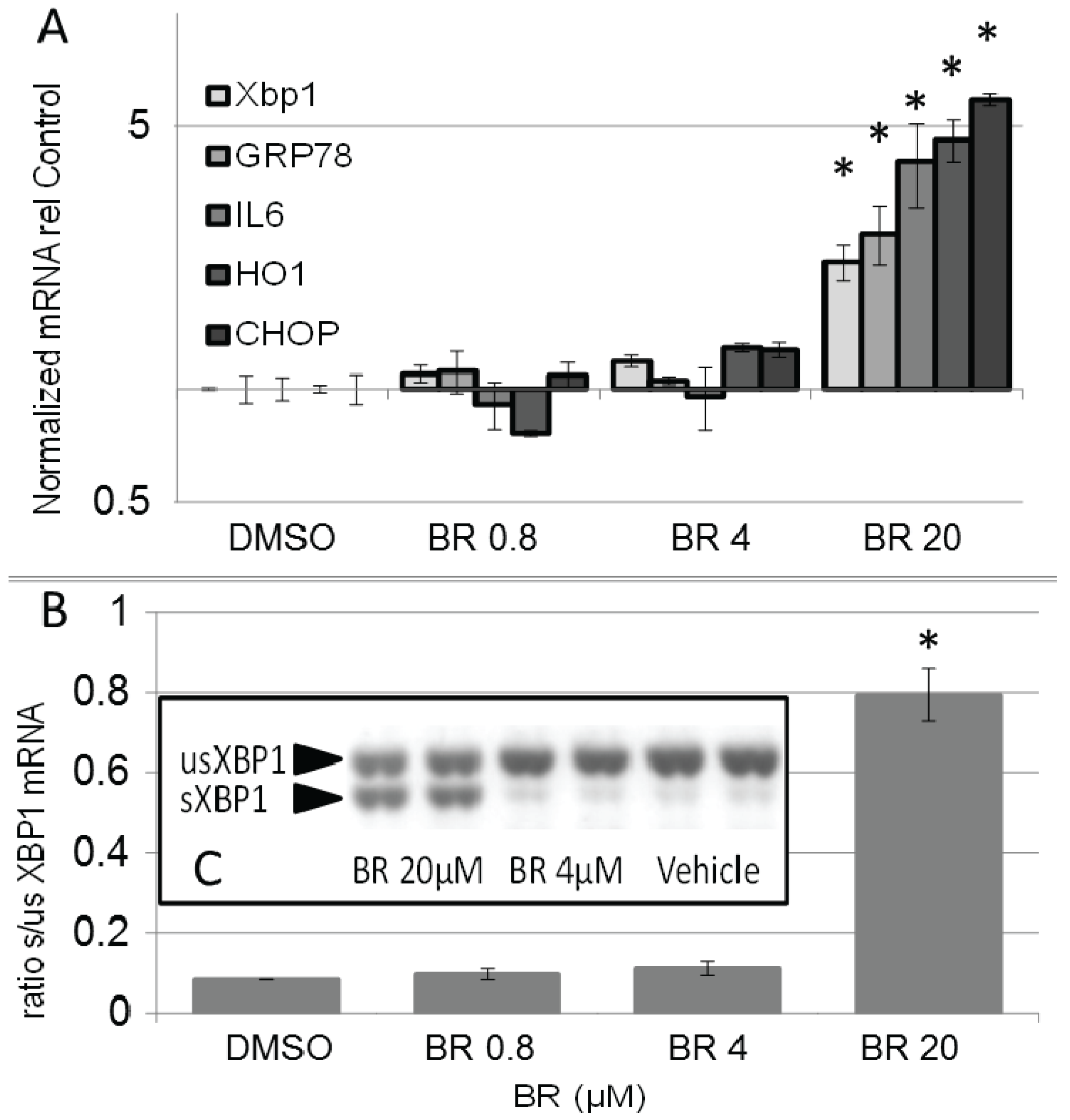
3.6. BR Increases Expression of Markers for ER Stress and Unfolded Protein Response
4. Experimental Section
4.1. Chemicals
4.2. Cell Culture
4.3. Animals
4.4. Determination of Cell Number by Crystal Violet Assay
4.5. Determination of Metabolic Activity by MTT Assay
4.6. Cellular Heme Oxygenase Activity by Determination of BR Production in Medium
4.7. Laser Scanning Microscopy
4.8. Gene Expression
| Accession | Sense Primer | Antisense Primer | Source | |
|---|---|---|---|---|
| XBP-1 | NM_001004210.2 | gag tcc aag ggg aat gga gt | aca ggg tcc aac ttg tcc ag | Designed for this study |
| GRP78 | S63521 | gtt ctg ctt gat gtg tgt cc | ttt ggt cat tgg tga tgg tg | [57] |
| IL6 | NM_012589.1 | ccg gag agg aga ctt cac ag | aca gtg cat cat cgc tgt tc | [58] |
| HO-1 | NM_012580.2 | cca gcc aca cag cac tac | gcg gtc tta gcc tct tct g | [59] |
| CHOP | NM_024134.2 | ttg ggg gca cct ata tct ca | ctc ctt cag tcg ctg ttt cc | [60] |
| GAPDH | M17701 | cat gcc gcc tgg aga aac ctg cca | tgg gct ggg tgg tcc agg ggt ttc | [61] |
| HPRT | NM_012583 | ctc atg gac tga tta tgg aca gga c | gca ggt cag caa aga act tat agc c | [62] |
| Cyc | M19533 | tat ctg cac tgc caa gac tga gtg | ctt ctt gct ggt ctt gcc att cc | [62] |
4.9. Determination of Unconventional Splicing of XBP1
4.10. Heme Oxygenase Activity of Liver Tissue
4.11. Heme Oxygenase Activity of BRL3A Cells
4.12. Preparation of Liver Mitochondria
4.13. Hepatic Mitochondrial Function
4.14. Data Analysis and Statistics
5. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| BR | Bilirubin |
| BV | Biliverdin |
| Diff. OD | background corrected optical density |
Appendix

| Target | Accession number | Start on plus strand | Stop on plus strand | Product-length (bp) | Exon junctions in | Intron size (bp) |
|---|---|---|---|---|---|---|
| XBP-1, transcript variant 1, mRNA (usXBP-1) | NM_001004210.1 | 435 | 454 | 196 | Forward Primer Product | ~300 ~740 |
| 630 | 611 | |||||
| XBP-1, transcript variant 2, mRNA (sXBP-1) | NM_001271731.1 | 454 | 473 | 170 | Forward Primer Product | ~300 ~740 |
| 623 | 604 |
| Target | Annealing temp (°C)/time (sec) | Extension temp (°C)/time (sec) | ∆Ct (RT+ to RT−) | slope | Correlation-Coefficient (Pearson) R2 | Verified dynamic range |
|---|---|---|---|---|---|---|
| XBP-1 | 65/30 | 72/20 | not detected | −3.537 | 0.997 | 105 |
Conflicts of Interest
References
- Alam, J.; Cook, J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003, 9, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Kubulus, D.; Mathes, A.; Pradarutti, S.; Raddatz, A.; Heiser, J.; Pavlidis, D.; Wolf, B.; Bauer, I.; Rensing, H. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock 2008, 29, 583–590. [Google Scholar] [PubMed]
- Wang, W.W.; Smith, D.L.; Zucker, S.D. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004, 40, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Horio, F.; Hashizume, T.; Tanaka, M.; Ikeda, S.; Kakinuma, A.; Nakajima, H. Bilirubin is oxidized in rats treated with endotoxin and acts as a physiological antioxidant synergistically with ascorbic acid in vivo. Biochem. Biophys. Res. Commun. 1995, 214, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, J.; Muchova, L.; Zelenkova, M.; Vanova, K.; Vreman, H.J.; Wong, R.J.; Vitek, L. Intracellular accumulation of bilirubin as a defense mechanism against increased oxidative stress. Biochimie 2012, 94, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef]
- Stocker, R. Antioxidant activities of bile pigments. Antioxid. Redox. Signal. 2004, 6, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Fevery, J. Bilirubin in clinical practice: A review. Liver Int. 2008, 28, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Amit, Y.; Boneh, A. Bilirubin inhibits protein kinase C activity and protein kinase C-mediated phosphorylation of endogenous substrates in human skin fibroblasts. Clin. Chim. Acta 1993, 223, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chuniaud, L.; Dessante, M.; Chantoux, F.; Blondeau, J.P.; Francon, J.; Trivin, F. Cytotoxicity of bilirubin for human fibroblasts and rat astrocytes in culture. Effect of the ratio of bilirubin to serum albumin. Clin. Chim. Acta 1996, 256, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Falcao, A.S.; Silva, R.F.; Gordo, A.C.; Gama, M.J.; Brito, M.A.; Brites, D. Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin. J. Neurochem. 2006, 96, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Ostrow, J.D. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr. Pharm. Des. 2009, 15, 2869–2883. [Google Scholar] [CrossRef] [PubMed]
- Qaisiya, M.; Coda Zabetta, C.D.; Bellarosa, C.; Tiribelli, C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell Signal. 2014, 26, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Sola, S.; Brites, D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology 2002, 35, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, W.K., Jr. Detection of heme oxygenase 1 and 2 proteins and bilirubin formation. Curr. Protoc. Toxicol. 2001. [Google Scholar] [CrossRef]
- Sunderman, F.W., Jr.; Downs, J.R.; Reid, M.C.; Bibeau, L.M. Gas-chromatographic assay for heme oxygenase activity. Clin. Chem. 1982, 28, 2026–2032. [Google Scholar] [PubMed]
- Tenhunen, R. Method for microassay of microsomal heme oxygenase activity. Anal. Biochem. 1972, 45, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Polacek, K.; Melichar, V. Competition between bilirubin and non-esterified fatty acids for binding to albumin. Biol. Neonat. 1962, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.J.; Ross, J.A.; James, G.O.; Wigmore, S.J. Optimization of the paired enzyme assay for heme oxygenase activity. Anal. Biochem. 2004, 332, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Tyrrell, R.M. An HPLC method to detect heme oxygenase activity. Curr. Protoc. Toxicol. 2001. [Google Scholar] [CrossRef]
- Vreman, H.J.; Stevenson, D.K. Heme oxygenase activity as measured by carbon monoxide production. Anal. Biochem. 1988, 168, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [PubMed]
- Converso, D.P.; Taille, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Rucker, H.; Amslinger, S. Identification of heme oxygenase-1 stimulators by a convenient ELISA-based bilirubin quantification assay. Free Radic. Biol. Med. 2015, 78, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Wanner, G.A.; Rensing, H.; Alte, C.; Miescher, E.A.; Wolf, B.; Pannen, B.H.; Clemens, M.G.; Bauer, M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology 1998, 27, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Grande, J.P.; Croatt, A.J.; Likely, S.; Hebbel, R.P.; Enright, H. Intracellular targets in heme protein-induced renal injury. Kidney Int. 1998, 53, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.G.; Irwanto, K.A.; Ostrow, J.D.; Tiribelli, C. Effect of bilirubin on cytochrome C oxidase activity of mitochondria from mouse brain and liver. BMC Res. Notes 2010. [Google Scholar] [CrossRef]
- Keshavan, P.; Schwemberger, S.J.; Smith, D.L.; Babcock, G.F.; Zucker, S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer 2004, 112, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, F.; Graziano, L. Mitochondrial swelling induced by unconjugated bilirubin in vitro. Experientia 1973, 29, 1376–1377. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.D.; Goessling, W.; Hoppin, A.G. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J. Biol. Chem. 1999, 274, 10852–10862. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, L.; Del, V.S.; Koehler, R.K.; Bayon, J.E.; Webster, C.C.; Mukerjee, P.; Ostrow, J.D.; Tiribelli, C. Albumin binding of unconjugated [3H]bilirubin and its uptake by rat liver basolateral plasma membrane vesicles. Biochem. J. 1996, 316, 999–1004. [Google Scholar] [PubMed]
- Bu-Bakar, A.; Arthur, D.M.; Aganovic, S.; Ng, J.C.; Lang, M.A. Inducible bilirubin oxidase: A novel function for the mouse cytochrome P450 2A5. Toxicol. Appl. Pharmacol. 2011, 257, 14–22. [Google Scholar] [CrossRef] [PubMed]
- De, M.F.; Lord, G.A.; Kee, L.C.; Pons, N. Bilirubin degradation by uncoupled cytochrome P450. Comparison with a chemical oxidation system and characterization of the products by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass. Spectrom. 2006, 20, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Taille, C.; Almolki, A.; Benhamed, M.; Zedda, C.; Megret, J.; Berger, P.; Leseche, G.; Fadel, E.; Yamaguchi, T.; Marthan, R.; et al. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J. Biol. Chem. 2003, 278, 27160–27168. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.; Gustincich, S. Redox state, oxidative stress, and molecular mechanisms of protective and toxic effects of bilirubin on cells. Curr. Pharm. Des. 2009, 15, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kueng, W.; Silber, E.; Eppenberger, U. Quantification of cells cultured on 96-well plates. Anal. Biochem. 1989, 182, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Tremezaygues, L.; Seifert, M.; Tilgen, W.; Reichrath, J. 1,25-dihydroxyvitamin D3 protects human keratinocytes against UV-B-induced damage: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. Dermatoendocrinology 2009, 1, 239–245. [Google Scholar] [CrossRef]
- Edwards, V.; Markovic, E.; Matisons, J.; Young, F. Development of an in vitro reproductive screening assay for novel pharmaceutical compounds. Biotechnol. Appl. Biochem. 2008, 51, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Berk, P.D.; Korenblat, K.M. Approach to the Patient with Jaundice or Abnormal Liver Test Results. In Cecil Medicine; Goldman, L., Ausiello, D., Eds.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Wolford, S.T.; Schroer, R.A.; Gohs, F.X.; Gallo, P.P.; Brodeck, M.; Falk, H.B.; Ruhren, R. Reference range data base for serum chemistry and hematology values in laboratory animals. J. Toxicol. Environ. Health 1986, 18, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Arias, I.M.; Alter, H.J.; Boyer, J.L.; Cohen, D.E.; Fausto, N.; Schafritz, D.A.; Wollkoff, A.W. The Liver, Biology and Pathobiology; Wiley: Chinchester, UK, 2009; pp. 0–1216. [Google Scholar]
- Yoshida, J.; Ishibashi, T.; Nishio, M. Antiproliferative effect of Ca2+ channel blockers on human epidermoid carcinoma A431 cells. Eur. J. Pharmacol. 2003, 472, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.A.; Schwarze, S.R. IRE1alpha controls cyclin A1 expression and promotes cell proliferation through XBP-1. Cell Stress Chaperones. 2010, 15, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, K.; Laybutt, D.R.; He, M.L.; Zhao, H.L.; Chan, J.C.; Xu, G. Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci. 2010, 87, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenbach, K.T.; Gentile, C.L.; Nivala, A.M.; Wang, D.; Wei, Y.; Pagliassotti, M.J. Linking endoplasmic reticulum stress to cell death in hepatocytes: Roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1027–E1035. [Google Scholar] [CrossRef] [PubMed]
- Van, S.A.; van’t Wout, E.F.; Stolk, J.; Hiemstra, P.S. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones 2012, 17, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shen, X.; Wu, J.; Sakaki, K.; Saunders, T.; Rutkowski, D.T.; Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 2006, 124, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, R. Bilirubin Solubility and interaction with albumin and phospholipid. J. Biol. Chem. 1979, 254, 2364–2369. [Google Scholar] [PubMed]
- Van der Veere, C.N.; Schoemaker, B.; van der, M.R.; Groen, A.K.; Jansen, P.L.; Oude Elferink, R.P. Rapid association of unconjugated bilirubin with amorphous calcium phosphate. J. Lipid. Res. 1995, 36, 1697–1707. [Google Scholar] [PubMed]
- Suzuki, N. On bilirubin-metal complex compounds in relation to black pigments of gallstones. Tohoku J. Exp. Med. 1966, 90, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, R.; Bellarosa, C.; Foti, R.; Roncaglia, P.; Giraudi, P.; Krmac, H.; Tiribelli, C.; Gustincich, S. A transcriptome analysis identifies molecular effectors of unconjugated bilirubin in human neuroblastoma SH-SY5Y cells. BMC Genomics 2009. [Google Scholar] [CrossRef]
- Dong, H.; Huang, H.; Yun, X.; Kim, D.S.; Yue, Y.; Wu, H.; Sutter, A.; Chavin, K.D.; Otterbein, L.E.; Adams, D.B.; et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology 2014, 155, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Mullebner, A.; Paier-Pourani, J.; Banerjee, A.; Miller, I.; Lauterbock, L.; Duvigneau, J.C.; Skulachev, V.P.; Redl, H.; Kozlov, A.V. Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid. Redox Signal. 2015, 22, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Althausen, S.; Paschen, W. Homocysteine-induced changes in mRNA levels of genes coding for cytoplasmic- and endoplasmic reticulum-resident stress proteins in neuronal cell cultures. Mol. Brain Res. 2000, 84, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Dungel, P.; Perlinger, M.; Singer, K.; Ghebes, C.; Duvigneau, J.C.; Mullebner, A.; Schafer, U.; Redl, H.; Kozlov, A.V. Experimental data suggesting that inflammation mediated rat liver mitochondrial dysfunction results from secondary hypoxia rather than from direct effects of inflammatory mediators. Front. Physiol. 2013. [Google Scholar] [CrossRef]
- Di, F.C.; Marfella, R.; Cuzzocrea, S.; Piegari, E.; Petronella, P.; Giugliano, D.; Rossi, F.; D’Amico, M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes 2005, 54, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Nurnberger, S.; Miller, I.; Duvigneau, J.C.; Kavanagh, E.T.; Gupta, S.; Hartl, R.T.; Hori, O.; Gesslbauer, B.; Samali, A.; Kungl, A.; et al. Impairment of endoplasmic reticulum in liver as an early consequence of the systemic inflammatory response in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1373–G1383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Altmann, S.W. mRNA and 18S-RNA coapplication-reverse transcription for quantitative gene expression analysis. Anal. Biochem. 2005, 345, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarencon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Staniek, K.; Nohl, H. H2O2 detection from intact mitochondria as a measure for one-electron reduction of dioxygen requires a non-invasive assay system. Biochim. Biophys. Acta 1999, 1413, 70–80. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müllebner, A.; Moldzio, R.; Redl, H.; Kozlov, A.V.; Duvigneau, J.C. Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin. Biomolecules 2015, 5, 679-701. https://doi.org/10.3390/biom5020679
Müllebner A, Moldzio R, Redl H, Kozlov AV, Duvigneau JC. Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin. Biomolecules. 2015; 5(2):679-701. https://doi.org/10.3390/biom5020679
Chicago/Turabian StyleMüllebner, Andrea, Rudolf Moldzio, Heinz Redl, Andrey V. Kozlov, and J. Catharina Duvigneau. 2015. "Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin" Biomolecules 5, no. 2: 679-701. https://doi.org/10.3390/biom5020679
APA StyleMüllebner, A., Moldzio, R., Redl, H., Kozlov, A. V., & Duvigneau, J. C. (2015). Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin. Biomolecules, 5(2), 679-701. https://doi.org/10.3390/biom5020679







