Biotechnological Applications of Transglutaminases
Abstract
:1. Introduction
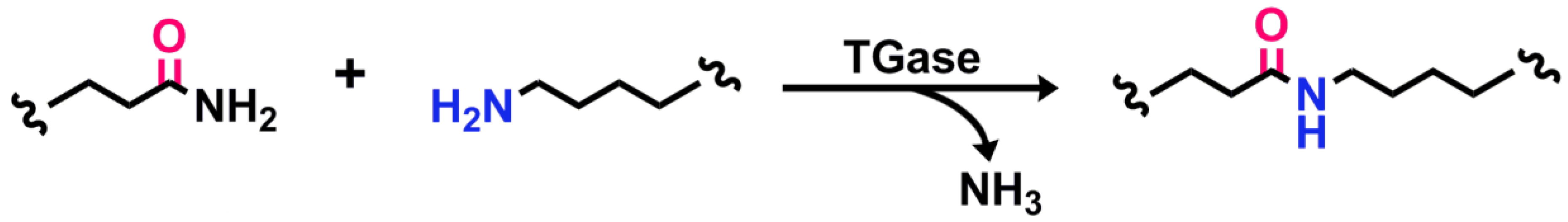
2. Production and Engineering of TGases
2.1. Transglutaminase Expression and Purification
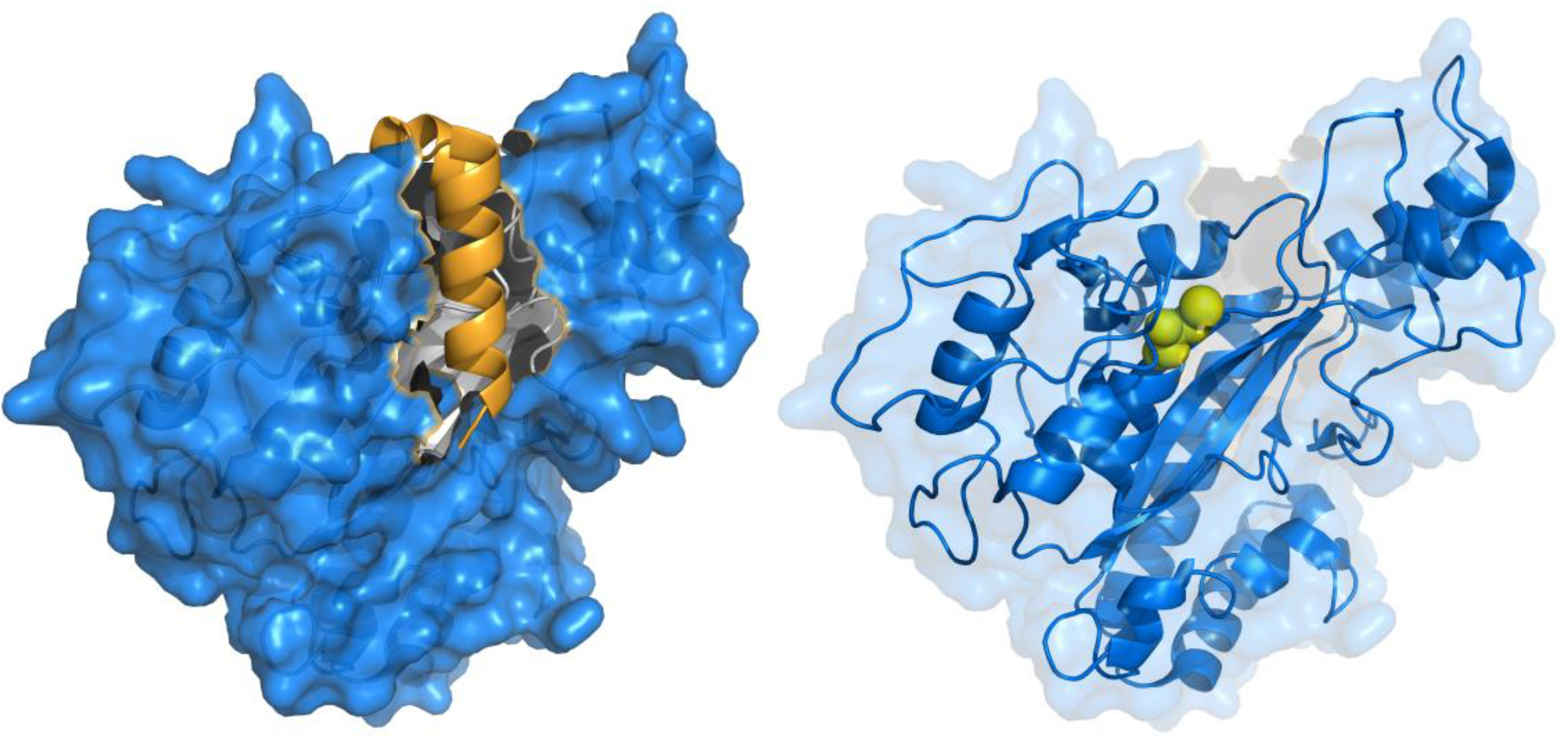
2.2. Engineering TGases for Altered Function and Properties
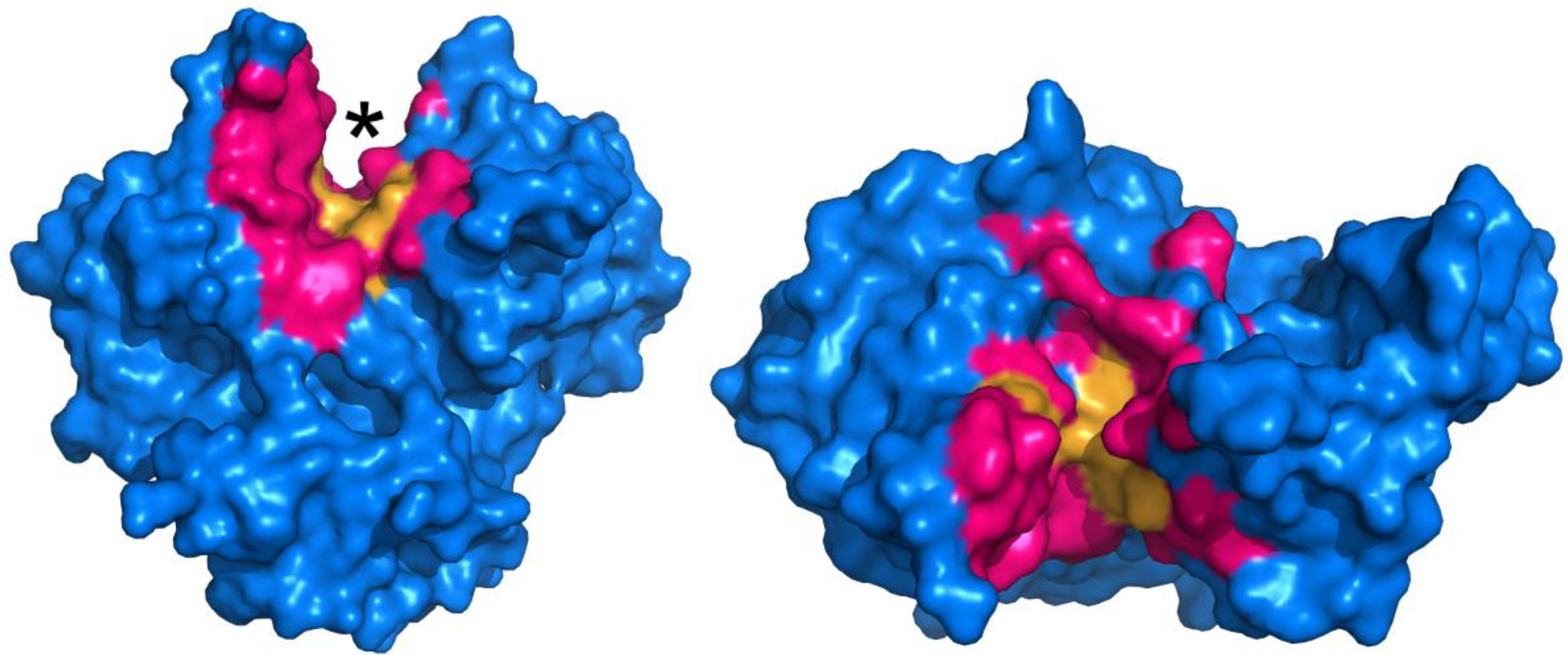
3. Substrate Specificity
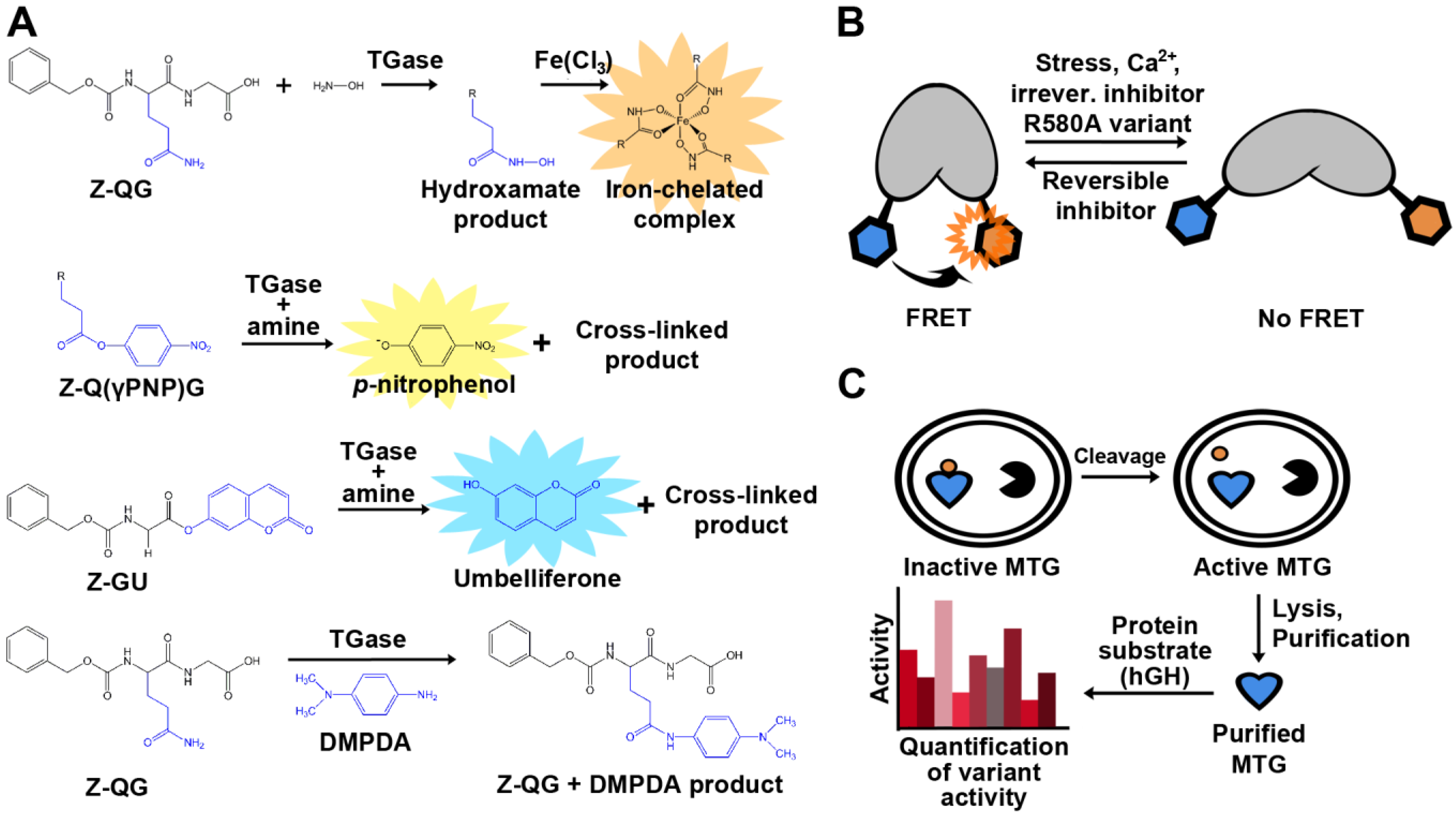
4. Assays
5. TGases as Biocatalysts for the Production of Novel Biomaterials
6. Protein Labeling
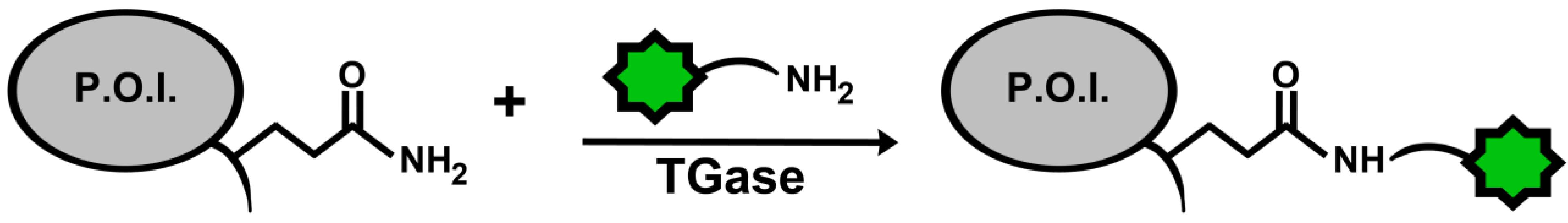
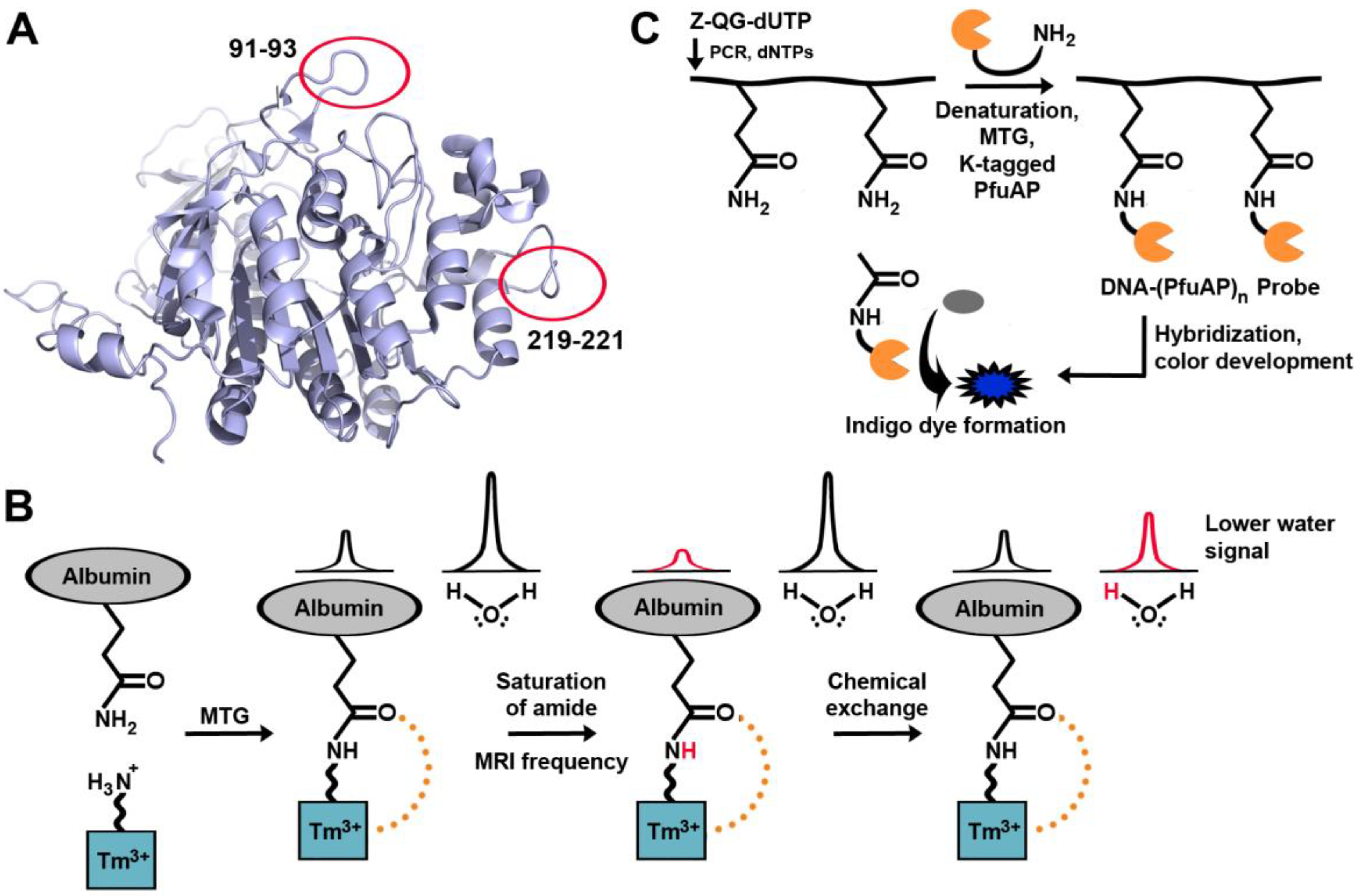
7. Conclusions
Acknowledgments
Conflict of Interest
References
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Nature’s biological glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef]
- Shleĭkin, A.G.; Danilov, N.P. Evolutionary-biological peculiarities of transglutaminase. Structure, physiological functions, application. Zh. Evol. Biokhim. Fiziol. 2011, 47, 3–14. [Google Scholar]
- Autuori, F.; Farrace, M.G.; Oliverio, S.; Piredda, L.; Piacentini, M. “Tissue” transglutaminase and apoptosis. Adv. Biochem. Eng. Biotechnol. 1998, 62, 129–136. [Google Scholar]
- Abe, S.; Yamashita, K.; Kohno, H.; Ohkubo, Y. Involvement of transglutaminase in the receptor-mediated endocytosis of mouse peritoneal macrophages. Biol. Pharm. Bull. 2000, 23, 1511–1513. [Google Scholar] [CrossRef]
- Chen, J.S.; Mehta, K. Tissue transglutaminase: an enzyme with a split personality. Int. J. Biochem. Cell Biol. 1999, 31, 817–836. [Google Scholar] [CrossRef]
- Shridas, P.; Sharma, Y.; Balasubramanian, D. Transglutaminase-mediated cross-linking of alpha-crystallin: structural and functional consequences. FEBS Lett. 2001, 499, 245–250. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Schroeder, W.T.; Thacher, S.M.; Stewart-Galetka, S.; Annarella, M.; Chema, D.; Siciliano, M.J.; Davies, P.J.; Tang, H.Y.; Sowa, B.A.; Duvic, M. Type I keratinocyte transglutaminase: expression in human skin and psoriasis. J. Invest. Dermatol. 1992, 99, 27–34. [Google Scholar]
- Ando, H.; Adachi, M.; Umeda, K.; Matsuura, A.; Nonaka, M.; Uchio, R.; Tanaka, H.; Motoki, M. Purification and characteristics of a novel transglutaminase derived from microorganisms. Agric. Biol. Chem. 1989, 53, 2613–2617. [Google Scholar] [CrossRef]
- Zhu, Y.; Rinzema, A.; Tramper, J.; Bol, J. Microbial transglutaminase - a review of its production and application in food processing. Appl. Microbiol. Biotechnol. 1995, 44, 277–282. [Google Scholar] [CrossRef]
- Suzuki, S.; Izawa, Y.; Kobayashi, K.; Eto, Y.; Yamanaka, S.; Kubota, K.; Yokozeki, K. Purification and characterization of novel transglutaminase from Bacillus subtilis spores. Biosci. Biotechnol. Biochem. 2000, 64, 2344–2351. [Google Scholar] [CrossRef]
- Pedersen, L.C.; Yee, V.C.; Bishop, P.D.; Le Trong, I.; Teller, D.C.; Stenkamp, R.E. Transglutaminase factor XIII uses proteinase-like catalytic triad to crosslink macromolecules. Protein Sci. 1994, 3, 1131–1135. [Google Scholar] [CrossRef]
- Ikura, K.; Nasu, T.; Yokota, H.; Tsuchiya, Y.; Sasaki, R.; Chiba, H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry 1988, 27, 2898–2905. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Yokoyama, K.-I.; Ishikawa, K.; Ono, K.; Ejima, D.; Matsui, H.; Suzuki, E. Crystal structure of microbial transglutaminase from Streptoverticillium mobaraense. J. Biol. Chem. 2002, 277, 44252–44560. [Google Scholar]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Panengad, P.P.; Yew, E.S.Y.; Sheppard, C.; Phan, T.T.; Raghunath, M. An in situ and in vitro investigation for the transglutaminase potential in tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 1310–1320. [Google Scholar]
- Cortez, J.; Bonner, P.L.; Griffin, M. Application of transglutaminases in the modification of wool textiles. Enzyme Microb. Technol. 2004, 34, 64–72. [Google Scholar] [CrossRef]
- Chau, D.Y.S.; Brown, S.V.; Mather, M.L.; Hutter, V.; Tint, N.L.; Dua, H.S.; Rose, F.R.A.J.; Ghaemmaghami, A.M. Tissue transglutaminase (TG-2) modified amniotic membrane: a novel scaffold for biomedical applications. Biomed. Mater. 2012, 7, 045011. [Google Scholar] [CrossRef]
- Zhu, Y.; Tramper, J. Novel applications for microbial transglutaminase beyond food processing. Trends Biotechnol. 2008, 26, 559–565. [Google Scholar] [CrossRef]
- Teixeira, L.S. M.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Gillet, S.M.F.G.; Chica, R.A.; Keillor, J.W.; Pelletier, J.N. Expression and rapid purification of highly active hexahistidine-tagged guinea pig liver transglutaminase. Protein Expr. Purif. 2004, 33, 256–264. [Google Scholar] [CrossRef]
- Yokoyama, K.-I.; Nakamura, N.; Seguro, K.; Kubota, K. Overproduction of microbial transglutaminase in Escherichia coli, in vitro refolding, and characterization of the refolded form. Biosci. Biotechnol. Biochem. 2000, 64, 1263–1270. [Google Scholar] [CrossRef]
- Shi, Q.; Kim, S.-Y.; Blass, J.P.; Cooper, A.J.L. Expression in Escherichia coli and purification of hexahistidine-tagged human tissue transglutaminase. Protein Expr. Purif. 2002, 24, 366–373. [Google Scholar] [CrossRef]
- Piper, J.L.; Gray, G.M.; Khosla, C. High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: implications for celiac sprue. Biochemistry 2002, 41, 386–393. [Google Scholar] [CrossRef]
- Roy, I.; Smith, O.; Clouthier, C.M.; Keillor, J.W. Expression, purification and kinetic characterisation of human tissue transglutaminase. Protein Expr. Purif. 2013, 87, 41–46. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hashiguchi, K.; Yokozeki, K.; Yamanaka, S. Molecular clonging of the transglutaminase gene from Bacillus subtilis and its expression in Escherichia coli. Biosci. Biotechnol. Biochem. 1998, 62, 1109–1114. [Google Scholar] [CrossRef]
- Plácido, D.; Fernandes, C.G.; Isidro, A.; Carrondo, M.A.; Henriques, A.O.; Archer, M. Auto-induction and purification of a Bacillus subtilis transglutaminase (Tgl) and its preliminary crystallographic characterization. Protein Expr. Purif. 2008, 59, 1–8. [Google Scholar]
- Marx, C.K.; Hertel, T.C.; Pietzsch, M. Soluble expression of a pro-transglutaminase from Streptomyces mobaraensis in Escherichia coli. Enzyme Microb. Technol. 2007, 40, 1543–1550. [Google Scholar] [CrossRef]
- Sommer, C.; Volk, N.; Pietzsch, M. Model based optimization of the fed-batch production of a highly active transglutaminase variant in Escherichia coli. Protein Expr. Purif. 2011, 77, 9–19. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Chen, J. Microbial transglutaminase production: understanding the mechanism. Biotechnol. Genet. Eng. Rev. 2009, 26, 205–222. [Google Scholar] [CrossRef]
- Zhao, X.; Shaw, A.C.; Wang, J.; Chang, C.-C.; Deng, J.; Su, J. A novel high-throughput screening method for microbial transglutaminases with high specificity toward Gln141 of human growth hormone. J. Biomol. Screen. 2010, 15, 206–212. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Date, M.; Yokoyama, K.; Umezawa, Y. Secretion of active-form Streptoverticillium mobaraense transglutaminase by Corynebacterium glutamicum: processing of the pro-transglutaminase by a cosecreted subtilisin-like protease from Streptomyces albogriseolus. Appl. Environ. Microbiol. 2003, 69, 358–366. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Wang, M.; Cui, W.; Chen, K.; Du, G.; Chen, J.; Zhou, Z. The order of expression is a key factor in the production of active transglutaminase in Escherichia coli by co-expression with its pro-peptide. Microb. Cell Fact. 2011, 10, 112. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.; Wang, G.; Zhang, D.; Du, G.; Chen, J.; Shi, Z. Enhancement of Streptomyces transglutaminase activity and pro-peptide cleavage efficiency by introducing linker peptide in the C-terminus of the pro-peptide. J. Ind. Microbiol. Biotechnol. 2013, 40, 317–325. [Google Scholar] [CrossRef]
- Yang, M.-T.; Chang, C.-H.; Wang, J.M.; Wu, T.K.; Wang, Y.-K.; Chang, C.-Y.; Li, T.T. Crystal structure and inhibition studies of transglutaminase from S. mobaraense. J. Biol. Chem. 2011, 286, 7301–7307. [Google Scholar]
- Eder, J.; Fersht, A.R. Pro-sequence-assisted protein folding. Mol. Microbiol. 1995, 16, 609–614. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakurai, K.; Lee, Y.-H.; Ikegami, T.; Yokoyama, K.; Goto, Y. A back hydrogen exchange procedure via the acid-unfolded state for a large protein. Biochemistry 2012, 51, 5564–5570. [Google Scholar] [CrossRef]
- Suzuki, M.; Yokoyama, K.; Lee, Y.-H.; Goto, Y. A two-step refolding of acid-denatured microbial transglutaminase escaping from the aggregation-prone intermediate. Biochemistry 2011, 50, 10390–10398. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; Devine, P.N.; Huisman, G.W.; Hughes, G.J. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Clouthier, C.M.; Pelletier, J.N. Expanding the organic toolbox: a guide to integrating biocatalysis in synthesis. Chem. Soc. Rev. 2012, 41, 1585–1605. [Google Scholar] [CrossRef]
- Brustad, E.M.; Arnold, F.H. Optimizing non-natural protein function with directed evolution. Curr. Opin. Chem. Biol. 2011, 15, 201–210. [Google Scholar] [CrossRef]
- Keillor, J.W.; Chica, R.A.; Chabot, N.; Vinci, V.; Pardin, C.; Fortin, E.; Gillet, S.M.F.G.; Nakano, Y.; Kaartinen, M.T.; Pelletier, J.N.; Lubell, W.D. The bioorganic chemistry of transglutaminase—from mechanism to inhibition and engineering. Can. J. Chem. 2008, 276, 271–276. [Google Scholar]
- Marx, C.K.; Hertel, T.C.; Pietzsch, M. Random mutagenesis of a recombinant microbial transglutaminase for the generation of thermostable and heat-sensitive variants. J. Biotechnol. 2008, 136, 156–162. [Google Scholar] [CrossRef]
- Folk, J.; Cole, P. Mechanism of action of guinea pig liver transglutaminase. J. Biol. Chem. 1966, 241, 5518–5525. [Google Scholar]
- Buettner, K.; Hertel, T.C.; Pietzsch, M. Increased thermostability of microbial transglutaminase by combination of several hot spots evolved by random and saturation mutagenesis. Amino Acids 2012, 42, 987–996. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.; Ma, J.; Zhang, D.; Shi, Z.; Du, G.; Chen, J. Deletion combined with saturation mutagenesis of N-terminal residues in transglutaminase from Streptomyces hygroscopicus results in enhanced activity and thermostability. Process Biochem. 2012, 47, 2329–2334. [Google Scholar] [CrossRef]
- Tagami, U.; Shimba, N.; Nakamura, M.; Yokoyama, K.-I.; Suzuki, E.-I.; Hirokawa, T. Substrate specificity of microbial transglutaminase as revealed by three-dimensional docking simulation and mutagenesis. Protein Eng. Des. Sel. 2009, 22, 747–752. [Google Scholar] [CrossRef]
- Coussons, P.J.; Price, N.C.; Kelly, S.M.; Smith, B.; Sawyer, L. Factors that govern the specificity of transglutaminase-catalysed modification of proteins and peptides. Biochem. J. 1992, 282, 929–930. [Google Scholar]
- Hu, B.H.; Messersmith, P.B. Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J. Am. Chem. Soc. 2003, 125, 14298–14299. [Google Scholar] [CrossRef]
- Mero, A.; Spolaore, B.; Veronese, F.M.; Fontana, A. Transglutaminase-mediated PEGylation of proteins: direct identification of the sites of protein modification by mass spectrometry using a novel monodisperse PEG. Bioconjug. Chem. 2009, 20, 384–389. [Google Scholar] [CrossRef]
- Stachel, I.; Schwarzenbolz, U.; Henle, T.; Meyer, M. Cross-linking of type I collagen with microbial transglutaminase: identification of cross-linking sites. Biomacromolecules 2010, 11, 698–705. [Google Scholar] [CrossRef]
- Spolaore, B.; Raboni, S.; Ramos Molina, A.; Satwekar, A.; Damiano, N.; Fontana, A. Local unfolding is required for the site-specific protein modification by transglutaminase. Biochemistry 2012, 51, 8679–8689. [Google Scholar] [CrossRef]
- Sugimura, Y.; Hosono, M.; Wada, F.; Yoshimura, T.; Maki, M.; Hitomi, K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGase 2 and Factor XIIIA. J. Biol. Chem. 2006, 281, 17699–17706. [Google Scholar]
- Sugimura, Y.; Yokoyama, K.; Nio, N.; Maki, M.; Hitomi, K. Identification of preferred substrate sequences of microbial transglutaminase from Streptomyces mobaraensis using a phage-displayed peptide library. Arch. Biochem. Biophys. 2008, 477, 379–383. [Google Scholar] [CrossRef]
- Oteng-Pabi, S.K.; Keillor, J.W. Continuous enzyme-coupled assay for microbial transglutaminase activity. Anal. Biochem. 2013, 441, 169–173. [Google Scholar] [CrossRef]
- Lee, J.-H.; Song, C.; Kim, D.-H.; Park, I.-H.; Lee, S.-G.; Lee, Y.-S.; Kim, B.-G. Glutamine (Q)-peptide screening for transglutaminase reaction using mRNA display. Biotechnol. Bioeng. 2013, 110, 353–362. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Sawa, A.; Kawabata, R.; Nio, N.; Motoki, M. Substrate specificities of microbial transglutaminase for primary amines. J. Agric. Food Chem. 2000, 48, 6230–6233. [Google Scholar] [CrossRef]
- Nonaka, M.; Matsuura, Y.; Motoki, M. Incorporation of a lysine- and lysine dipeptides into as1-Caesin by Ca2+ -independent microbial transglutaminase. Biosci. Biotech. Biochem. 1996, 60, 131–133. [Google Scholar] [CrossRef]
- Ikura, K.; Sasaki, R.; Motoki, M. Use of transglutaminase in quality-improvement and processing of food proteins. Agric. Food. Chem. 1992, 2, 389–407. [Google Scholar]
- Lee, J.-H.; Song, E.; Lee, S.-G.; Kim, B.-G. High-throughput screening for transglutaminase activities using recombinant fluorescent proteins. Biotechnol. Bioeng. 2013, 110, 2865–2873. [Google Scholar] [CrossRef]
- Kulik, C.; Heine, E.; Weichold, O.; Möller, M. Synthetic substrates as amine donors and acceptors in microbial transglutaminase-catalysed reactions. J. Mol. Catal. B Enzym. 2009, 57, 237–241. [Google Scholar] [CrossRef]
- Gundersen, M.T.; Keillor, J.W.; Pelletier, J.N. Microbial transglutaminase displays broad acyl-acceptor substrate specificity. Appl. Microbiol. Biotechnol. 2013. [Google Scholar] [CrossRef] [Green Version]
- De Macédo, P.; Marrano, C.; Keillor, J.W. A direct continuous spectrophotometric assay for transglutaminase activity. Anal. Biochem. 2000, 285, 16–20. [Google Scholar] [CrossRef]
- Leblanc, A.; Gravel, C.; Labelle, J.; Keillor, J.W. Kinetic studies of guinea pig liver transglutaminase reveal a general-base-catalyzed deacylation mechanism. Biochemistry 2001, 40, 8335–8342. [Google Scholar] [CrossRef]
- Gillet, S.M.F.G.; Pelletier, J.N.; Keillor, J.W. A direct fluorometric assay for tissue transglutaminase. Anal. Biochem. 2005, 347, 221–226. [Google Scholar] [CrossRef]
- Gnaccarini, C.; Ben-Tahar, W.; Lubell, W.D.; Pelletier, J.N.; Keillor, J.W. Fluorometric assay for tissue transglutaminase-mediated transamidation activity. Bioorg. Med. Chem. 2009, 17, 6354–6359. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Fuchsbauer, H.; Blass, J.P.; Cooper, A.J.L. A sensitive fluorometric assay for tissue transglutaminase. Anal. Biochem. 2001, 206, 198–206. [Google Scholar]
- Wu, Y.; Tsai, Y. A rapid transglutaminase assay for high-throughput screening applications. J. Biomol. Screen. 2006, 11, 836–843. [Google Scholar] [CrossRef]
- Kenniston, J.A.; Conley, G.P.; Sexton, D.J.; Nixon, A.E. A homogeneous fluorescence anisotropy assay for measuring transglutaminase 2 activity. Anal. Biochem. 2013, 436, 13–15. [Google Scholar]
- Begg, G.E.; Holman, S.R.; Stokes, P.H.; Matthews, J.M.; Graham, R.M.; Iismaa, S.E. Mutation of a critical arginine in the GTP-binding site of transglutaminase 2 disinhibits intracellular cross-linking activity. J. Biol. Chem. 2006, 281, 12603–12609. [Google Scholar]
- Pinkas, D.M.; Strop, P.; Brunger, A.T.; Khosla, C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007, 5, e327. [Google Scholar] [CrossRef]
- Caron, N.S.; Munsie, L.N.; Keillor, J.W.; Truant, R. Using FLIM-FRET to measure conformational changes of transglutaminase type 2 in live cells. PLoS One 2012, 7, e44159. [Google Scholar]
- Clouthier, C.M.; Mironov, G.G.; Okhonin, V.; Berezovski, M.V.; Keillor, J.W. Real-time monitoring of protein conformational dynamics in solution using kinetic capillary electrophoresis. Angew. Chem. Int. Ed. Engl. 2012, 51, 12464–12468. [Google Scholar] [CrossRef]
- Mádi, A.; Kárpáti, L.; Kovács, A.; Muszbek, L.; Fésüs, L. High-throughput scintillation proximity assay for transglutaminase activity measurement. Anal. Biochem. 2005, 343, 256–262. [Google Scholar]
- Besheer, A.; Hertel, T.C.; Kressler, J.; Mäder, K.; Pietzsch, M. Enzymatically-catalyzed HESylation using microbial transglutaminase: Proof of feasibility. J. Pharm. Sci. 2009, 98, 4420–4428. [Google Scholar] [CrossRef]
- Abe, H.; Goto, M.; Kamiya, N. Protein lipidation catalyzed by microbial transglutaminase. Chemistry 2011, 17, 14004–14008. [Google Scholar]
- Mori, Y.; Wakabayashi, R.; Goto, M.; Kamiya, N. Protein supramolecular complex formation by site-specific avidin-biotin interactions. Org. Biomol. Chem. 2013, 11, 914–922. [Google Scholar]
- Touati, J.; Angelini, A.; Hinner, M.J.; Heinis, C. Enzymatic cyclisation of peptides with a transglutaminase. Chembiochem 2011, 12, 38–42. [Google Scholar]
- Strop, P.; Liu, S.-H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.-T.; Ho, W.-H.; Farias, S.; Casas, M.G.; Abdiche, Y.; Zhou, D.; Chandrasekaran, R.; Samain, C.; Loo, C.; Rossi, A.; Rickert, M.; Krimm, S.; Wong, T.; Chin, S.M.; Yu, J.; Dilley, J.; Chaparro-Riggers, J.; Filzen, G.F.; O’Donnell, C.J.; Wang, F.; Myers, J.S.; Pons, J.; Shelton, D.L.; Rajpal, A. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar] [CrossRef]
- Jeger, S.; Zimmermann, K.; Blanc, A.; Grünberg, J.; Honer, M.; Hunziker, P.; Struthers, H.; Schibli, R. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew. Chem. Int. Ed. Engl. 2010, 49, 9995–9997. [Google Scholar]
- Kamiya, N.; Abe, H. New fluorescent substrates of microbial transglutaminase and its application to peptide tag-directed covalent protein labeling. Bioconjugation Protoc. 2011, 751, 81–94. [Google Scholar]
- Kamiya, N.; Abe, H.; Goto, M.; Tsuji, Y.; Jikuya, H. Fluorescent substrates for covalent protein labeling catalyzed by microbial transglutaminase. Org. Biomol. Chem. 2009, 7, 3407–3412. [Google Scholar]
- Mori, Y.; Goto, M.; Kamiya, N. Transglutaminase-mediated internal protein labeling with a designed peptide loop. Biochem. Biophys. Res. Commun. 2011, 410, 829–833. [Google Scholar] [CrossRef]
- Gnaccarini, C.; Ben-Tahar, W.; Mulani, A.; Roy, I.; Lubell, W.D.; Pelletier, J.N.; Keillor, J.W. Site-specific protein propargylation using tissue transglutaminase. Org. Biomol. Chem. 2012, 10, 5258–5265. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hingorani, D. V; Randtke, E.A.; Pagel, M.D. A catalyCEST MRI contrast agent that detects the enzyme-catalyzed creation of a covalent bond. J. Am. Chem. Soc. 2013, 135, 6396–6398. [Google Scholar] [CrossRef]
- Kitaoka, M.; Tsuruda, Y.; Tanaka, Y.; Goto, M.; Mitsumori, M.; Hayashi, K.; Hiraishi, Y.; Miyawaki, K.; Noji, S.; Kamiya, N. Transglutaminase-mediated synthesis of a DNA-(enzyme)n probe for highly sensitive DNA detection. Chemistry 2011, 17, 5387–5392. [Google Scholar] [CrossRef]
- Takahara, M.; Hayashi, K.; Goto, M.; Kamiya, N. Tailing DNA aptamers with a functional protein by two-step enzymatic reaction. J. Biosci. Bioeng. 2013. [Google Scholar] [CrossRef]
- Kitaoka, M.; Mitsumori, M.; Hayashi, K.; Hiraishi, Y.; Yoshinaga, H.; Nakano, K.; Miyawaki, K.; Noji, S.; Goto, M.; Kamiya, N. Transglutaminase-mediated in situ hybridization (TransISH) system: a new methodology for simplified mRNA detection. Anal. Chem. 2012, 84, 5885–5891. [Google Scholar] [CrossRef]
- Watts, S.W.; Priestley, J.R.C.; Thompson, J.M. Serotonylation of vascular proteins important to contraction. PLoS One 2009, 4, e5682. [Google Scholar]
- Walther, D.J.; Stahlberg, S.; Vowinckel, J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 2011, 278, 4740–4755. [Google Scholar] [CrossRef]
- Lin, J.C.-Y.; Chou, C.-C.; Gao, S.; Wu, S.-C.; Khoo, K.-H.; Lin, C.-H. An in vivo tagging method reveals that Ras undergoes sustained activation upon transglutaminase-mediated protein serotonylation. Chembiochem 2013, 14, 813–817. [Google Scholar] [CrossRef]
- Chabot, N.; Moreau, S.; Mulani, A.; Moreau, P.; Keillor, J.W. Fluorescent probes of tissue transglutaminase reveal its association with arterial stiffening. Chem. Biol. 2010, 17, 1143–1150. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rachel, N.M.; Pelletier, J.N. Biotechnological Applications of Transglutaminases. Biomolecules 2013, 3, 870-888. https://doi.org/10.3390/biom3040870
Rachel NM, Pelletier JN. Biotechnological Applications of Transglutaminases. Biomolecules. 2013; 3(4):870-888. https://doi.org/10.3390/biom3040870
Chicago/Turabian StyleRachel, Natalie M., and Joelle N. Pelletier. 2013. "Biotechnological Applications of Transglutaminases" Biomolecules 3, no. 4: 870-888. https://doi.org/10.3390/biom3040870



