1. Introduction
Advancements in pervasive computation and communication technologies, coupled with micro- and nano-electronics have created opportunities for the seamless integration of electronics and flexible sensors. These flexible sensors made of textiles or possessing a clothing-like texture and flexibility result in functionalized textiles, commonly referred to as e-textiles or smart textiles. Tao [
1] describes smart textiles as a class of smart materials and structures that sense and react to environmental conditions or stimuli. Depending on the degree to which intelligence is imparted into these textiles, they may be passive, active or very smart. Passive textiles only acquires information about the environment [
2]; active textile reacts to its environment and adapts in different ways [
3]; and very smart textiles may be context-aware and adapt their responses based on the context [
4]. These three components may be confined to the presence of sensors, actuators and controlling units. For instance, a passive material requires sensors to detect signals. Constituents of an active material may require the coupling of an actuator or a controlling unit with a sensor. Similarly, for very smart textiles, a combination of sensors, actuators, controlling units and a context-aware material that functions as a processing unit may be essential. Context-aware intelligent materials are formulated by combining conventional textiles with novel materials, mechanics, processing techniques and the chemistry and biology of materials [
5].
In biomedical applications for long-term physiological signal monitoring, textiles are the preferred platform for sensors, because they are the most natural materials close to the skin. Traditionally, long-term ambulatory systems that perform biomedical monitoring use adhesives to hold the electrodes or sensors in place against the skin and use a conductive gel to make low impedance electrical contact with the skin. Advancements in analog front-end electronics with very high input impedance and a high common mode rejection ratio (CMRR) have enabled the use of dry electrodes without the need for gels [
6]. These dry electrodes when made on textiles can be seamlessly integrated into clothing to achieve an intuitive and comfortable alternative to currently available long-term monitoring systems. However, these dry electrodes have been known to have an initial settling time, wherein the contact impedance slowly drops to a value adequate to acquire signals with minimal noise from the environment [
7]. One approach to alleviate this problem is to use nanostructured sensors, which have significantly higher surface area, as compared to planar sensors, so that the desirable low contact impedance can be achieved sooner. In this paper, our focus is on the use of nanomaterials and nanostructures in textiles for wearable biomedical monitoring systems for cardiovascular health.
The review is organized as follows: First, we present the motivations for a textile integrated wearable system from a healthcare perspective for patients who have already been diagnosed with a cardiovascular disease (CVD) and for occult conditions that pose sudden and high risk to individuals under physical exertion. Second, we describe the research performed thus far on the use of nanostructured sensors and nanomaterials on textiles for cardiac monitoring. Finally, we present examples of end-to-end system implementations for multi-parameter monitoring through smart devices, namely the e-bra for women and the e-bro for men.
1.1. Cardiovascular Disease Patients
In the U.S., the cost of healthcare has increased by over 90%, from $1337 billion in 2000 to $2594 billion in 2010. It is projected to reach $4,487 billion in 2020 [
8]. Public spending on health and long-term care in Organization for Economic Co-operation and Development (OECD) member countries and BRIICS (Brazil, Russia, India, Indonesia, China, South Africa) is 6% of the gross domestic product (GDP) and is projected to increase up to 14% in the next 50 years [
9]. Chronic disease diagnosis and treatment are the primary causes for this increase. Patients suffering from chronic diseases need to repeatedly visit the hospital, which can be expensive. As a solution to this, remote point-of-care (POC) systems and remote patient monitoring (RPM) systems can be used. Remote patient monitoring for point-of-care facilitates the monitoring of a patient’s health condition at local or remote places without the need for hospital admission or visits. In the case of high risk patients, it can provide the patient with real-time feedback from a medical center.
1.2. Athletes: Football and Soccer
The sudden and fatal failure of an individual’s heart function is referred to as sudden cardiac death (SCD). It is well known that the risk of SCDs and acute myocardial infarctions (AMI) increase during and immediately after strenuous exercise [
10]. Nearly 58% of SCDs reported between 1980 and 2006 have been reported in basketball and football athletes. Recent studies have shown that the incidence of SCDs in young athletes in the U.S. at the high school and college level have been underestimated in previous studies [
11]. The most prevalent causes of SCD in young athletes are CVDs and sports-related injuries. Among CVD causes, the most prevalent are hypertrophic cardiomyopathy (HCM) (36% of cases) and coronary artery diseases (CAD) (17% of cases). Among sport injuries, commotio cordis and blunt trauma injuries together account for 25% of all SCDs recorded between 1985 and 2006 [
11]. The current strategy for the prevention of SCDs in young athletes is to prescreen them and diagnose any cardiovascular diseases that may put them at high risk for SCDs and promptly disqualify them from participation if diagnosed. The proven approach implemented in Italy has involved a mandatory prescreening with detailed history, physical examination and a 12-lead ECG with guidelines and criteria for the identification of cardiovascular abnormalities that may put the athlete at high risk for SCD [
12]. The American Heart Association (AHA), however, does not currently recommend the inclusion of 12-lead ECG as a part of the prescreening [
13,
14,
15] for several reasons: the high direct costs of the tests, a lack of dedicated trained athletics personnel to perform the prescreening in place of physicians, the sheer number of athletes to be screened and the reported low specificity, high false positives and false negatives of ECG interpretations [
16]. Although the positive diagnostic value of including a 12-lead ECG in prescreening has been identified by both the European Society of Cardiology (ESC) and the AHA [
17,
18], consensus panels for recommendations on cardiovascular screening of young athletes, the cost-effectiveness of including this test in the U.S. athletic prescreening protocol is still a subject of wide debate.
Despite the evidence suggesting the effectiveness and initial success of prescreening with ECG in Italy [
12], there are four limitations to the prescreening approach that need to be addressed:
First, there is still a wide debate on the differential diagnosis of HCM from the ECG changes brought on by training in many athletes with otherwise normal hearts (athlete’s heart) [
19,
20]. Reported differences in training-induced cardiac remodeling between athletes of African origin and others have made diagnoses based on ECG findings equivocal [
21]. Moreover, the remaining two prevalent causes for SCD (CADs and blunt trauma injuries) cannot be diagnosed during prescreening, as CADs do not manifest as ECG abnormalities and blunt trauma injuries are non-pathological and can occur in any athlete with an otherwise healthy heart.
Second, recommendations [
22] suggest that for the differentiation of HCM from athlete’s heart, Brugada-like ECG abnormalities, arrhythmogenic right ventricular cardiomyopathy or dysplasia and features, like prolonged PR intervals, short PR intervals, early repolarization and inverted or biphasic T waves, can be further evaluated using an exercise test to improve the specificity. However, this is to be done in addition to the preliminary ECG screening at an added cost.
Third, from the perspective of secondary prevention,
i.e., through the adoption of strict guidelines on sudden cardiac arrest (SCA) resuscitation, it is imperative that an SCA is promptly recognized, cardiopulmonary resuscitation (CPR) is started immediately and a defibrillating shock is applied as soon as possible [
23]. The target resuscitation time recommended by the AHA is between 3 and 5 min, from the time the athlete’s collapse was witnessed to the application of the defibrillating shock. It has been shown that survival chances may drop by 7%–10% for every minute that defibrillation is delayed. In the absence of a real-time ECG, the emergency responder or rescuer has to first identify an SCA with accurate pulse or respiration assessments, while the athlete may be gasping or having myoclonic jerks or seizure-like activity, which may be inconsistent with an SCA.
Fourth, the various mechanisms for SCD have been studied extensively at the cellular process and ionic channels level [
24]. This work needs to be augmented with real-time studies on the mechanism of SCD using non-invasive techniques, like ECG, which are lacking. The ECG is rarely or never available during a sudden cardiac arrest episode. Therefore, a system for the real-time monitoring of cardiac electrophysiology during exertion, which puts the athletes at higher risk of SCDs, is an important step in the prevention and treatment of sudden cardiac arrest in athletes.
1.3. Military Recruits under Training
Among U.S. military recruits between the age of 18 and 25 years, the incidence rate of non-traumatic SCDs were found to be 11.1:100,000 in a 25-year period [
25]. Recently, a cohort study of active military personnel who died on duty showed that 92% of the sudden death occurrences during military training were during organized physical training. According to the same study, 31.6% of the sudden deaths occurred during running, which was part of the organized physical training. The study further concluded that among persons ≥35 years of age, more emphasis should be placed on evaluating the risk of atherosclerotic coronary disease [
26].
Based on the three scenarios described above, we can formulate a system requirement as follows:
An ergonomic, intuitive and wearable, fully-integrated platform to acquire ECG and blood pressure in real time.
Wireless communication to transfer the acquired biomedical signal in real time to a data logging device or a computational device that does real-time analysis.
2. Sensors on Textiles
Smart textile (fabric) can be made from materials ranging from traditional cotton, polyester and nylon, to advanced Kevlar with integrated functionalities. However, in the scope of the present review, fabrics with electrical conductivity are of interest. There are two kinds of smart textile (fabric) products that have been developed and studied for health monitoring fabric with textile-based sensor electronics [
27,
28,
29,
30,
31] and fabric that envelopes traditional sensor electronics [
32,
33]. Pioneering research work, done by Jayaraman and co-workers [
27,
28], showed that weaving can be used to incorporate electrically-conductive yarn into fabric to obtain a textile that can be used as a “Wearable Motherboard”. It can connect multiple sensors on the body, such as wet gel ECG electrodes, to the signal acquisition electronics. Later research has shown that conductive yarns can be instrumental in the fabrication of textile-based sensors made of fabric [
29,
30] or metallic meshes [
31] coated with silver or conductive metal cores woven into the fabric [
34].
There are two broad approaches to the fabrication of garments with ECG sensor electrodes in research:
Finished garments through functionalization or integration of finished garments with sensor elements. This approach involves the integration of finished electrodes into finished garments by simply stitching the electrodes at the appropriate locations on the garment or using deposition techniques to transfer the functional materials at the appropriate locations.
Unfinished garments—the introduction of smart materials during the garment fabrication process. This approach entails the use of textile fabrication techniques to form woven or non-woven fabrics with the inclusion of functional materials.
2.1. Incorporation in Finished Garments
Several flexible and rigid materials have been fabricated and evaluated for use as electrodes. Among resistive electrodes are flexible polydimethylsiloxane (PDMS) [
35], CNT array electrodes named ENOBIO [
36], carbon nanotube (CNT)/PDMS nanocomposites [
37], flexible polymeric dry electrodes [
38] and vertically-aligned metallic nanowires on flexible substrates [
39].
Among capacitive electrodes are Ti/TiN electrodes [
40], IrO-coated electrodes [
41] and MEMs spiked electrodes [
42]. A comprehensive review of these contact and non-contact dry electrodes has been presented in [
43]. Smart textile implementations can be achieved by stitching these electrodes onto finished garments.
2.2. Textiles as Electrodes: Unfinished Garments
The smallest units of the textile are fibers or filaments. Innumerable combinations of these units can result in many textile materials with varying length, cross-section areas and shapes and surface roughness. The intelligent functionality, conductivity in this case, can be introduced into textiles at different levels. At the fiber level, a coating can be applied or conductive threads can be added to make a composite textile. Fibers of different types can be arranged at random or in a strictly organized way in yarns or fabrics to form even 3D structures. These structures can be metallized or functionalized to fabricate a conductive textile electrode and other functional surfaces with micro- or nano-rods, or micro- or nano-coil arrays. Accordingly, there are two strategies followed to make fabrics conductive: inclusion of thin conductive filaments in the yarns used to make the fabric or coating of the finished fabric with conductive materials by various coating techniques.
The former approach has been studied using stainless steel [
44]. Mechanical, as well as conductive property enhancement has been documented with the absorption of single-walled carbon nanotubes (SWCNT) in cotton [
45].
The latter approach uses deposition and coating techniques, like sputtering, screen printing, electro-spinning, carbonizing and evaporation deposition. Conductive coatings on fabric result in higher conductivities, but are less durable, especially through wash cycles [
34].
Nanotextiles are formed by the fabrication of nanostructures on textile fabrics or forming nanoscale filamentous structures on fabric, rather than incorporating nanomaterials into fabrics. InOh
et al. [
46] have fabricated electrospun silver-plated polyvinylidene fluoride (PVDF) nanofibers for use as long-term dry electrodes. Vertically aligned nanostructures using a fabric flocking technique have been fabricated and tested by Rai
et al. [
47].
2.3. Nanocomposite Inks for Conductive Traces and Connections
Implementations of wearable systems on textile require a cost-effective, as well as scalable way to form interconnects between the various components. One approach to achieving this goal is to have functional inks that can be printed onto a textile to essentially form printed circuit boards on textile. In the case of ECG smart textiles, this involves the formulation of conductive inks or printable capacitive structures on fabric. Polymer thick film (PTF) technologies have been used to form conductive traces and transmission lines on a non-woven fabric in [
48]. Alternatively, inks with conductive fillers and polymer binders have been used in the formulation of several inks that are stretchable and resistant to wear and tear. Stretchable conductive inks on textiles have been demonstrated through conductive traces using silver flakes in polyurethane-based binder by Araki
et al. [
49]. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) films have been successfully deposited on rubber latex substrates using ink-jet printing by Romaguera
et al. [
50].
Multi-wall carbon nanotubes (MWCNTs) and polyaniline nanoparticle (PANP) core shell-based nanocomposite conductive inks were synthesized and successfully screen printed on woven cotton by Rai
et al. [
51]. Conductive traces drawn from inks with silver flakes in an acrylic binder have been used to make connections between ECG electrodes and flexible printed circuit boards (PCBs) [
52].
In addition to conductive materials for resistive-type ECG electrodes, a flexible capacitive-type ECG electrode structure consisting of conductive CNT-acrylic nanocomposite inks sandwiched between two layers of acrylic inks has been fabricated and tested for ECG acquisition by Kumar
et al. [
53].
3. Examples of Systems Implementation
Wearable systems for monitoring physiological signals have been reported in previous studies. Some of them are in the form of wearable accessories, such as wrist watch for cuffless blood pressure monitoring [
54]. Systems can be off-the-shelf electronics with dry electrodes fabricated on FR4 boards [
55] that can be embedded/encapsulated in clothing, such as a cap or shirt. The systems either display only or transmit to a remote location through Bluetooth-enabled cellular phone/Zigbee-enabled PC and wireless data transmission or the Internet for remote patient monitoring (RPM) [
56,
57]. The systems can be microelectronics fabricated on flexible substrates, which are easy to incorporate into the textile [
58,
59,
60]. These electronics can accomplish multiple biometric monitoring, such as sweat rate, temperature, ECG, blood oxygen level,
etc., along with wireless transmission. In combination with global positioning technology, they can facilitate medical intervention and augment ubiquitous healthcare [
59].
Sensors that are encapsulated, enmeshed or textile-based are predominantly electrical, optical or piezoelectric monitor physiological signals close to the body. Dry electrodes are the most popular type of sensors for biopotential measurement in wearable systems. These electrodes have varying designs, which include fabric made of woven conductive thread, an array of metalized needles, a conductive surface and an Ag/AgCl back electrode (conductive gel free) [
61] and capacitively-coupled dry electrodes [
62]. They have been shown to acquire biopotential signals. In addition to that, they have been proposed for cardiac, as well as neural stimulation. The textile-based dry electrodes have also been demonstrated as bioimpedance spectroscopy devices for plethysmography monitoring [
63]. Optical sensors are mainly off the shelf microelectronics or optical fiber-based sensors. The piezoelectric sensors are off-the-shelf microelectronics, flexible piezoelectric membranes or functionalized piezoelectric yarn.
Wireless sensor systems generate large amounts of data that require data management and post processing. Research in database architecture has shown that database organization for the derivation of medical parameters for diagnostics and archiving of diagnostic parameters solves the problem of data volume [
64]. In addition to that, data processing through cloud computing and remote access to stored data improves the computing performance [
65].
In the following sections, implementations of wearable systems with textile-based nanosensors and state-of-the-art wireless communication systems are described.
3.1. E-Bro
The textile platform in this application is an inner vest that can incorporate nano-biosensors, such as gold nanowire electrodes [
66] or nanostructured textile electrodes [
67] and composite piezoelectric films [
68,
69]. It can also incorporate an infrared emitter-detection system for plethysmography and temperature sensors. The e-bro system is an implementation of a multichannel wearable wireless textile-based nano-biosensor that monitors ECG and blood pressure.
3.1.1. System Description
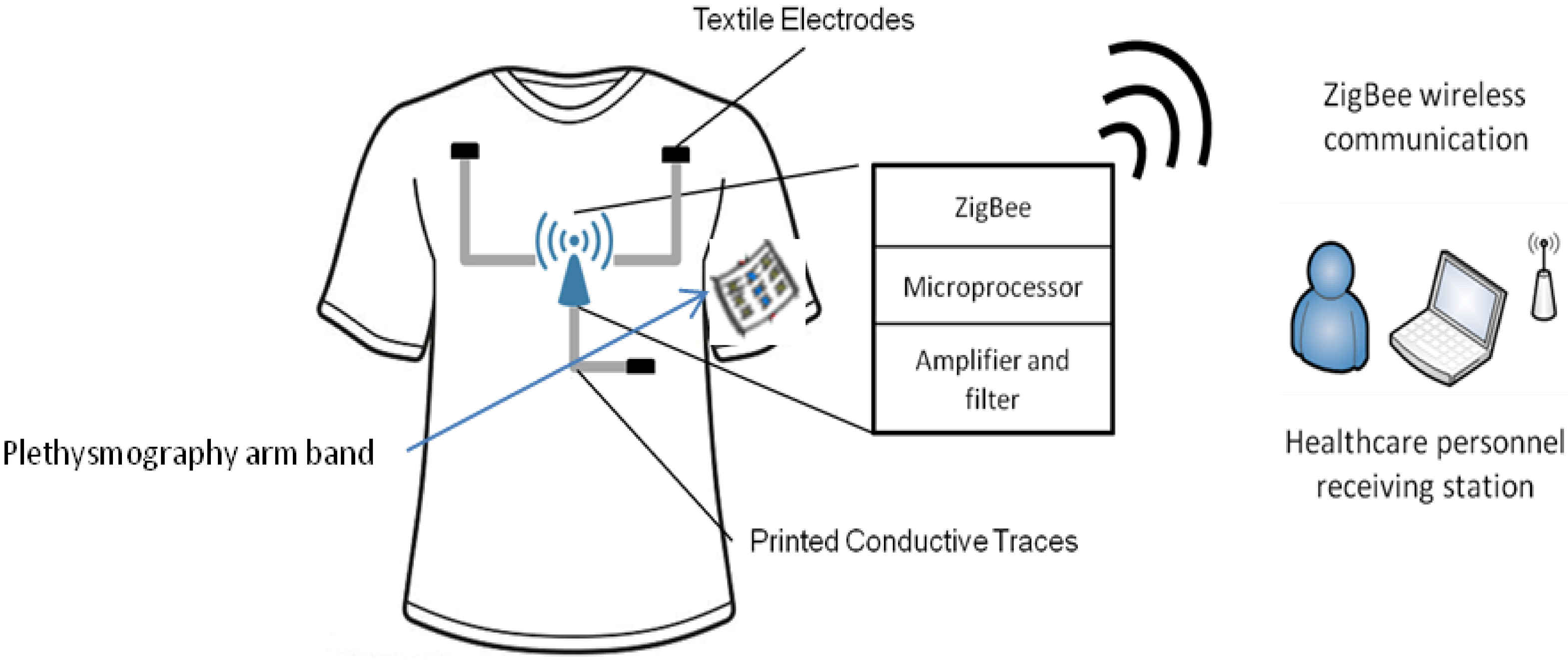
Figure 1 shows the overall system, which consists of four components: firstly, a compression inner vest, referred to as the e-bro, with the textile electrode sensors and the printed connection traces that connect the electrodes to a sensor electronics module (SEM); secondly, a photoplethysmography arm band that has near-IR LEDs and photodiodes, which are connected to the SEM through conductive traces printed from the left arm; thirdly, the SEM that consists of an amplifier, filter circuits, a microcontroller and a ZigBee wireless radio; lastly, a software program running on a PC that receives and plots incoming data from the person wearing it.
Figure 1.
Schematic showing overall system for the acquisition of ECG through the e-bro.
Figure 1.
Schematic showing overall system for the acquisition of ECG through the e-bro.
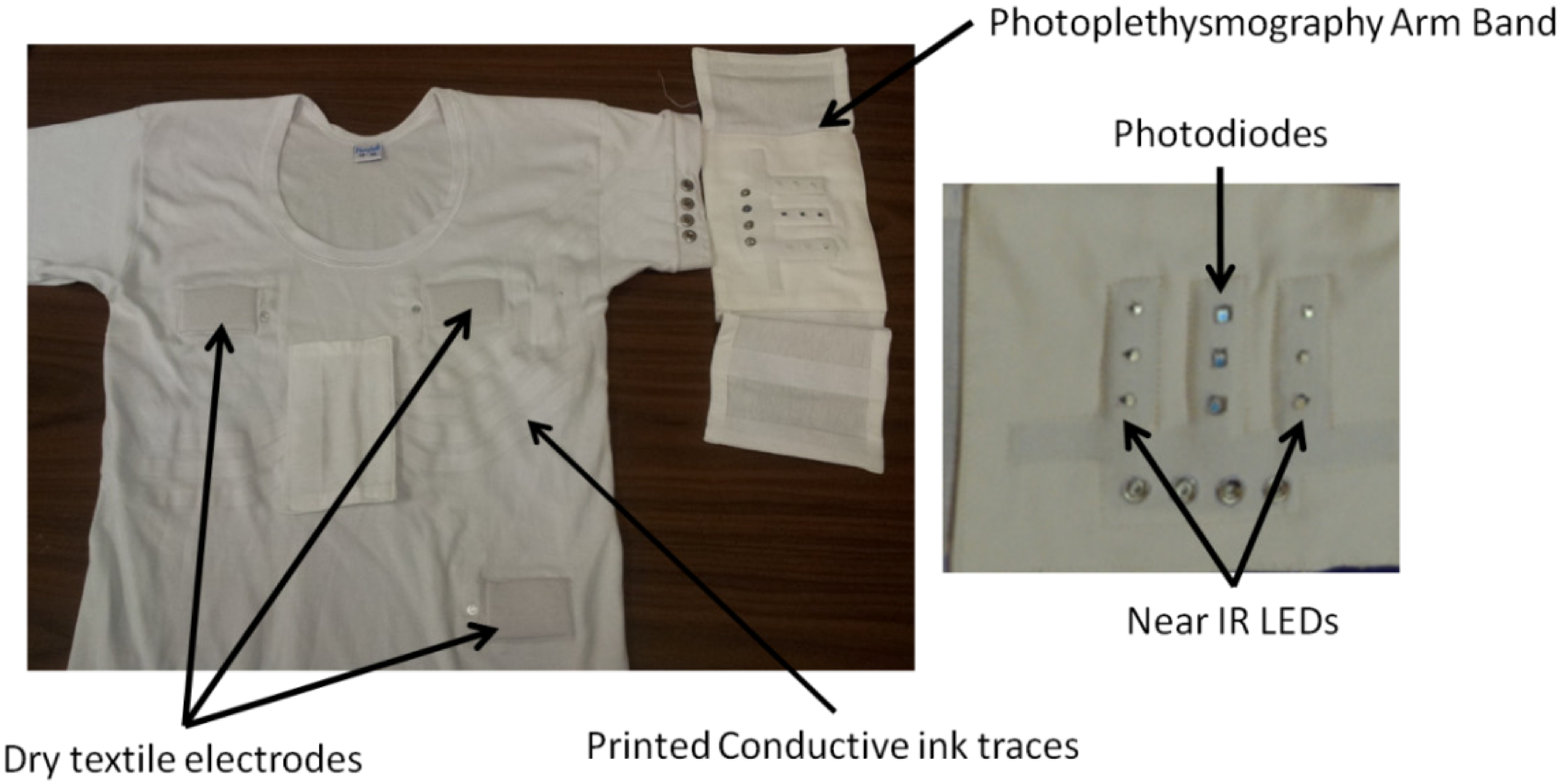
The photoplethysmography arm band consists of two arrays of near-IR photodiodes and a central array of three photodiodes. This assembly was described in detail in [
52]. The electrodes and the arm band are connected to the SEM through conductive traces, an ink formulation of silver nanoparticle fillers in an elastic acrylic-based binder, printed on the vest, and snap buttons. The conductive traces were made using conductive inks, and screen printing was used, which is textile manufacturing compatible. The arm band is removable and simple snaps on to the vest through four snap buttons (
Figure 2). The removable SEM and arm band makes the e-bro washable.
Figure 2.
E-bro and the photoplethysmography arm band. Reproduced with permission from [
47]. Copyright 2014, The Electrochemical Society.
Figure 2.
E-bro and the photoplethysmography arm band. Reproduced with permission from [
47]. Copyright 2014, The Electrochemical Society.
3.1.2. Sensor Electronics Module (SEM)
The amplifier, which is a part of the SEM, consists of four channels: three channels for the bipolar limb leads, Lead I, Lead II and Lead III, and the fourth channel amplifies the potential difference across the photodiode, which detects the reflected IR waves from the brachial artery. The amplifiers used in the SEM had a band pass of 0.2 Hz to 70 Hz and a mid-band gain of 50 dB for the three ECG channels. The gain was increased to 55 dB for the photoplethysmography sensors for a band of 0.2 Hz to 15 Hz. The amplified signals from the amplifier are digitized using the onboard microcontroller for transmission;
Figure 3.
Figure 3.
(
a) Schematic of the sensor electronics module (SEM); (
b) actual SEM used in e-bro system. Reproduced with permission from [
47]. Copyright 2014, The Electrochemical Society.
Figure 3.
(
a) Schematic of the sensor electronics module (SEM); (
b) actual SEM used in e-bro system. Reproduced with permission from [
47]. Copyright 2014, The Electrochemical Society.
The choice of the ZigBee radio module was motivated by two desired functions. Firstly, it has to support data rates higher than 9,600 bps, because four channels of digitized ECG and BP signal have to be transmitted in real time. Secondly, it provides communication ranges as high as possible for applications in sports, military expedition and high risk work environments, such as firefighters.
3.2. E-Bra
The systems incorporated in the inner vest for men can also be integrated in an inner garment for women, such as a brassier. The various sensors listed in the previous section can be incorporated in the e-bra and the signals from the sensors brought to the eNanoflex [
70] module through printed conductive traces or conductive threads.
Figure 4 shows a picture of the e-bra, the eNanoflex module used for data acquisition and wireless transmission and the simple signal display interface that plots the data received from the eNanoflex module.
Figure 4.
(a) Real-time data plotted on the laptop; (b) the eNanoflex module; (c) the e-bra worn by the test subject; (d) multi-channel signal smartphone display; (e) heart rate and blood pressure (systolic and diastolic) smartphone display.
Figure 4.
(a) Real-time data plotted on the laptop; (b) the eNanoflex module; (c) the e-bra worn by the test subject; (d) multi-channel signal smartphone display; (e) heart rate and blood pressure (systolic and diastolic) smartphone display.
3.2.1. Multichannel Data Acquired
The data acquired by the e-bro can be transmitted wirelessly to a PC. The data received by the PC was then filtered using an adaptive filter algorithm to minimize the effect of motion on the ECG signal baseline [
71]. The data acquisition and adaptive filter were developed using MATLAB (Mathworks, Natick, MA, USA). However, the same can be achieved on a JAVA platform and can be deployed on a smartphone.
Figure 4a,d shows the original three ECG signals, Lead I, Lead II and Lead III. It also plots the pulse waveform, the heart rate and the estimated systolic and diastolic blood pressure.
Figure 4b shows the eNanoflex module used for data acquisition and wireless transmission. The derived pulse transit time (PTT) values are then used to estimate the systolic and diastolic blood pressure values (
Figure 4e) based on the calibration equations previously obtained in [
52]. Other sensor systems can be incorporated to develop wearable applications to monitor respiration, temperature and blood oxygen level.
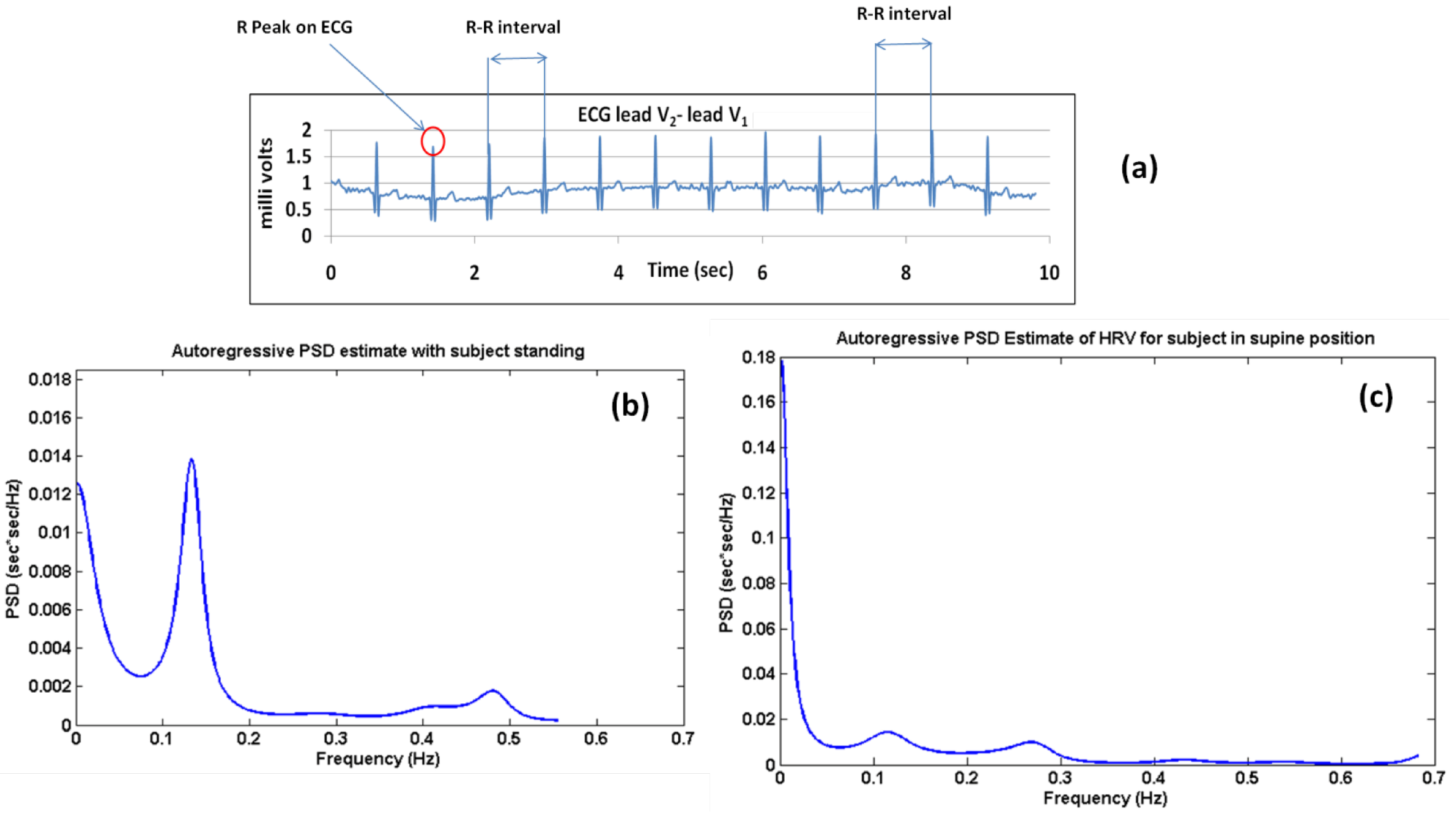
Post processing of ECG can also calculate heart rate variability (HRV), which is a prognostic and diagnostic tool. HRV is described as the sequence formed by concatenating the difference in heart rate between consecutive beats (
Figure 5a). It is calculated as the inverse of the difference in the intervals between consecutive R-peaks. The R-peak detection algorithm used for the calculation of the RR interval (RRI) was as given in [
72]. The autoregressive (AR) power spectrum estimation technique was used to obtain the power spectrum density (PSD) plot of the RRI sequence. The characteristic LF and HF peaks were observed [
73].
Figure 5b shows the AR PSD computed from the RRI series for the standing-up case.
Figure 5c shows the same for supine ECG. AR PSDs in both figures show a classic shift in the power distribution between LF and HF components with respect to total power.
Figure 5.
.(a) R-R interval calculations from ECG; (b) plot of the autoregressive (AR) power spectrum density (PSD) computed from the RR interval (RRI) series for the standing case; (c) plot of the AR PSD computed from the RRI series for the supine position.
Figure 5.
.(a) R-R interval calculations from ECG; (b) plot of the autoregressive (AR) power spectrum density (PSD) computed from the RR interval (RRI) series for the standing case; (c) plot of the AR PSD computed from the RRI series for the supine position.
Thus, these implementations of the e-bro and e-bra systems can be used for the tracking of chronic conditions related to autonomous nervous regulation of cardiac activity. Continuous multiple lead ECG monitoring can be used for the detection of T-wave inversion, which is indicative of a change in ventricular repolarization. Automated post processing of ECG by algorithm for the detection of T wave inversion can serve as an alarm system that will trigger a subroutine to initiate the ECG signal relay through a remote server to a doctor’s office for diagnosis.
4. Conclusions
The need for wearable solutions for continuous cardiovascular monitoring from a clinical perspective was discussed in detail in this paper. Although research efforts have been underway over the past two decades in the field of dry electrodes for biopotential electrodes, recent advances in the use of nanomaterials and nanostructures in textiles have shown significant improvements in the performance of dry electrodes. In this review, we have identified the most recent advancements in the areas of nanotechnology in non-invasive cardiac biopotential monitoring systems that are wearable and textile based.
Furthermore, we have briefly discussed the wireless communication architectures, which also play a key role in the implementation of continuous wireless cardiovascular monitoring. Over the past few years, several research efforts have been underway to determine the communication architectures that could adequately support endeavors, like continuous remote patient monitoring and real-time analytics of biomedical signals, in a massively scalable fashion through cloud computing and the Internet of Things. Questions in this area are in areas such as data security, computational costs, sustainability in terms of energy and infrastructure costs. In this regard, we have briefly discussed the work done by our group, as well as alternative approaches to implementations.
Nonetheless, research on the communications technology perspective as well as the materials and sensors perspective have made significant strides in this direction over the past decade. The ultimate objective has been to design a truly personalized healthcare platform that can intelligently cater to each unique individual’s healthcare needs. Recent trends in research are leading to this realization by tapping the benefits of nanotechnology, like the large surface area of dry electrodes with a small footprint.
Acknowledgments
This research was conducted with the endowment, for smart textiles and healthcare, from the Global Institute for Nanotechnology in Engineering and Medicine Inc. (700 Research Center Blvd., Fayetteville, AR 72701, USA). The authors would like to acknowledge Raj Mittra for suggesting that we communicate our work in this special issue for wearable electronics.
Author Contributions
The research objectives were framed by Vijay Varadan and Robert E. Harbaugh. The introductory section and motivation for work were written by Prashanth Shyamkumar and Pratyush Rai, and reviewed by Vijay Varadan and Robert E. Harbaugh. The sections on sensors on textiles were written by Prashanth Shyamkumar and Pratyush Rai. Sections on systems implementation were written by Sechang Oh, Pratyush Rai and Mouli Ramasamy. Vijay Varadan and Robert E. Harbaugh provided guidance on the overall organization and preparation of the manuscript. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, X. Smart Fibers, Fabrics and Clothing; Woodhead Publishing: Cambridge, UK, 2001. [Google Scholar]
- Zhang, X.; Tao, X. Smart textiles: Passive smart. Textil Asia 2001, 45–49. [Google Scholar]
- Zhang, X.; Tao, X. Smart textile: Active smart. Textil Asia 2001, 49–52. [Google Scholar]
- Zhang, X.; Tao, X. Smart textiles: Very smart. Textile Asia 2001, 35–37. [Google Scholar]
- Van Langenhove, L. Smart Textiles for Medicine and Healthcare, 1st ed.; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Van Helleputte, N.; Konijnenburg, M.; Hyejung, K.; Pettine, J.; Dong-Woo, J.; Breeschoten, A.; Morgado, A.; Torfs, T.; de Groot, H.; van Hoof, C.; et al. A multi-parameter signal-acquisition SoC for connected personal health applications. In Proceedings of the 2014 IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC), San Francisco, CA, USA, 9–13 February 2014; pp. 314–315.
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271. [Google Scholar] [CrossRef]
- California HealthCare Foundation. Snapshot Health Care Costs 101. Available online: http://www.chcf.org (accessed on 21 November 2013).
- De la Maisonneuve, C.; Martins, J.O. Public Spending on Health and Long-Term Care: A New Set of Projections; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Thompson, P.D.; Franklin, B.A.; Balady, G.J.; Blair, S.N.; Corrado, D.; Estes, N.A., III; Fulton, J.E.; Gordon, N.F.; Haskell, W.L.; Link, M.S.; et al. Exercise and acute cardiovascular events placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007, 115, 2358–2368. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 2006, 296, 1593–1601. [Google Scholar] [CrossRef]
- Myerburg, R.J.; Vetter, V.L. Electrocardiograms should be included in preparticipation screening of athletes. Circulation 2007, 116, 2616–2626. [Google Scholar] [CrossRef]
- Chaitman, B.R. An electrocardiogram should not be included in routine preparticipation screening of young athletes. Circulation 2007, 116, 2610–2614. [Google Scholar] [CrossRef]
- Faber, L.; van Buuren, F. Athlete screening for occult cardiac disease: No risk, no fun? J. Am. Coll. Cardiol. 2008, 51, 1040–1041. [Google Scholar] [CrossRef]
- Maron, B.J. National electrocardiography screening for competitive athletes: Feasible in the United States? Ann. Int. Med. 2010, 152, 324–326. [Google Scholar]
- Corrado, D.; Pelliccia, A.; Bjornstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; Panhuyzen-Goedkoop, N.; Deligiannis, A.; Solberg, E.; Dugmore, D.; et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: Proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2005, 26, 516–524. [Google Scholar]
- Maron, B.J.; Thompson, P.D.; Ackerman, M.J.; Balady, G.; Berger, S.; Cohen, D.; Dimeff, R.; Douglas, P.S.; Glover, D.W.; Hutter, A.M., Jr.; et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: A scientific statement from the American Heart Association Council on Nutrition, PhysicalActivity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation 2007, 115, 1643–1655. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Culasso, F.; di Paolo, F.M.; Spataro, A.; Biffi, A.; Caselli, G.; Piovano, P. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 2000, 102, 278–284. [Google Scholar] [CrossRef]
- Pelliccia, A.; di Paolo, F.M.; Quattrini, F.M.; Basso, C.; Culasso, F.; Popoli, G.; de Luca, R.; Spataro, A.; Biffi, A.; Thiene, G.; et al. Outcomes in athletes with marked ECG repolarization abnormalities. N. Engl. J. Med. 2008, 358, 152–161. [Google Scholar] [CrossRef]
- Pelliccia, A. Differences in cardiac remodeling associated with raceimplications for pre-participation screening and the unfavorable situation of black athletes. J. Am. Coll. Cardiol. 2008, 51, 2263–2265. [Google Scholar] [CrossRef]
- Uberoi, A.; Stein, R.; Perez, M.V.; Freeman, J.; Wheeler, M.; Dewey, F.; Peidro, R.; Hadley, D.; Drezner, J.; Sharma, S.; et al. Interpretation of electrocardiogram of young athletes. Circulation 2011, 124, 746–757. [Google Scholar] [CrossRef]
- Drezner, J.A.; Courson, R.W.; Roberts, W.O.; Mosesso, V.N., Jr.; Link, M.S.; Maron, B.J. Inter-association task force recommendations on emergency preparedness and management of sudden cardiac arrest in high school and college athletic programs: A consensus statement. J. Athl. Train. 2007, 42, 143–158. [Google Scholar]
- Rubart, M.; Zipes, D.P. Mechanisms of sudden cardiac death. J. Clin. Invest. 2005, 115, 2305–2515. [Google Scholar] [CrossRef]
- Eckart, R.E.; Scoville, S.L.; Campbell, C.L.; Shry, E.A.; Stajduhar, K.C.; Potter, R.N.; Pearse, L.A.; Virmani, R. Sudden death in young adults: A 25-year review of autopsies in military recruits. Ann. Int. Med. 2004, 141, 829–834. [Google Scholar]
- Eckart, R.E.; Shry, E.A.; Burke, A.P.; McNear, J.A.; Appel, D.A.; Castillo-Rojas, L.M.; Avedissian, L.; Pearse, L.A.; Potter, R.N.; Tremaine, L.; et al. Sudden death in young adults—An autopsy-based series of a population undergoing active surveillance. J. Am. Coll. Cardiol. 2011, 58, 1254–1261. [Google Scholar] [CrossRef]
- Park, S.; Gopalsamy, C.; Rajamanickam, R.; Jayaraman, S. The wearable motherboard™: An information infrastructure or sensate liner for medical applications. Stud. Health Technol. Inform. 1999, 62, 252–258. [Google Scholar]
- Park, S.; Jayaraman, S. Wearable biomedical systems: Research to reality. In Proceedings of the IEEE International Conference on Portable Information Devices PORTABLE07, Orlando, FL, USA, 25–29 May 2007; pp. 1–7.
- Coosemans, J.; Hermans, B.; Peurs, R. Intergrating wireless ECG in textiles. Sens. Actuators A 2006, 130–131, 48–53. [Google Scholar] [CrossRef]
- Lee, Y.D.; Chung, W.Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuators B 2009, 140, 390–395. [Google Scholar] [CrossRef]
- Alzaidi, A.; Zhang, L.; Bajwa, H. Smart Textiles Based Wireless ECG System. In Proceedings of the 2012 IEEE Long Island Systems, Applications and Technology Conference (LISAT), Farmingdale, NY, USA, 4 May 2012; pp. 1–5.
- Smart T-shirt by GOW Trainer. Available online: http://www.gowtrainer.com (accessed on 21 November 2013).
- Wearable Wellness System from Smartex s.r.l. Pisa Italy. Available online: http://www.smartex.it (accessed on 22 November 2013).
- Rattfalt, L.; Chedid, M.; Hult, P.; Linden, M.; Ask, P. Electrical properties of textile electrodes. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2007. EMBS 2007, Lyon, France, 22–26 August 2007; pp. 5735–5738.
- Fernandes, M.S.; Lee, K.S.; Ram, R.J.; Correia, J.H.; Mendes, P.M. Flexible PDMS-based dry electrodes for electro-optic acquisition of ECG signals in wearable devices. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3503–3506.
- Ruffini, G.; Dunne, S.; Farres, E.; Cester, I.; Watts, P.C.P.; Ravi, S.; Silva, P.; Grau, C.; Fuentemilla, L.; Marco-Pallares, J.; et al. ENOBIO dry electrophysiology electrode; first human trial plus wireless electrode system. In Proceedings of 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2007, EMBS 2007, Lyon, France, 22–26 August 2007; pp. 6689–6693.
- Jung, H.; Moon, J.; Baek, D.; Lee, J.H.; Choi, Y.Y.; Hong, J.S.; Lee, S.H. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef]
- Baek, J.; An, J.-H.; Choi, J.-M.; Park, K.-S.; Lee, S.-H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators A 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Varadan, V.K.; Oh, S.; Kwon, H.; Hankins, P. Wireless point-of-care diagnosis for sleep disorder with dry nanowire electrodes. J. Nanotechnol. Eng. Med. 2010, 1, 031012. [Google Scholar] [CrossRef]
- Fiedler, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Zentner, L.; Zanow, F.; Haueisen, J. Novel Ti/TiN dry electrodes and Ag/AgCl: A direct comparison in multichannel EEG. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2012; pp. 1011–1014.
- Dias, N.S.; Carmo, J.P.; Ferreira da Silva, A.; Mendes, P.M.; Correia, J.H. New dry electrodes based on iridium oxide (IrO) for non-invasive biopotential recordings and stimulation. Sens. Actuators A 2010, 164, 28–34. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Ko, L.-W.; Lin, C.-T.; Hong, C.-T.; Jung, T.-P.; Liang, S.-F.; Jeng, J.-F. Using novel MEMS EEG sensors in detecting drowsiness application. In Proceedings of the 2006 IEEE Biomedical Circuits and Systems Conference (BioCAS 2006), London, UK, 29 November–1 December 2006; pp. 33–36.
- Chi, Y.M.; Tzyy-Ping, J.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef]
- Mestrovic, M.A.; Helmer, R.J.N.; Kyratzis, L.; Kumar, D. Preliminary study of dry knitted fabric electrodes for physiological monitoring. In Proceedings of the 3rd International Conference on Intelligent SensorsSensor Networks and Information, 2007, ISSNIP 2007, Melbourne, Australia, 3–6 December 2007; pp. 601–606.
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- InOh, T.; Yoon, S.; Kim, T.E.; Wi, H.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Nanofiber web textile dry electrodes for long-term biopotential recording. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 204–211. [Google Scholar] [CrossRef]
- Rai, P.; Oh, S.; Shyamkumar, P.; Ramasamy, M.; Harbaugh, R.E.; Varadan, V.K. Nano-bio-textile sensors with mobile wireless platform for wearable health monitoring of neurological and cardiovascular disorders. J. Electrochem. Soc. 2014, 161, B3116–B3150. [Google Scholar] [CrossRef]
- Karaguzel, B.; Merritt, C.R.; Kang, T.; Wilson, J.M.; Nagle, H.T.; Grant, E.; Pourdeyhimi, B. Utility of nonwovens in the production of integrated electrical circuits via printing conductive inks. J. Textile Inst. 2008, 99, 37–45. [Google Scholar] [CrossRef]
- Araki, T.; Nogi, M.; Suganuma, K.; Kogure, M.; Kirihara, O. Printable and stretchable conductive wirings comprising silver flakes and elastomers. IEEE Electron Device Lett. 2011, 32, 1424–1426. [Google Scholar] [CrossRef]
- Romaguera, V.S.; Madec, M.B.; Yeates, S.G. Inkjet printing of conductive polymers for smart textiles and flexible electronics. Mater. Res. Soc. Symp. Proc. 2009, 1192, 26–31. [Google Scholar]
- Rai, P.; Lee, J.; Mathur, G.N.; Varadan, V.K. Carbon nanotubes polymer nanoparticle inks for healthcare textile. Proc. SPIE 2012, 8548. [Google Scholar] [CrossRef]
- Rai, P.; Kumar, P.S.; Oh, S.; Kwon, H.; Mathur, G.N.; Varadan, V.K.; Agarwal, M.P. Smart healthcare textile sensor system for unhindered-pervasive health monitoring. Proc. SPIE 2012, 8344. [Google Scholar] [CrossRef]
- Kumar, P.S.; Rai, P.; Oh, S.; Kwon, H.; Varadan, V.K. Nanocomposite electrodes for smartphone enabled healthcare garments: E-bra and smart vest. Proc. SPIE 2012, 8548. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Poon, C.C.Y.; Chan, C.-H.; Tsang, M.W.W.; Wu, K.F. A health-shirt using e-textile materials for the continuous and cuffless monitoring of arterial blood pressure. In Proceedings of 3rd IEEE/EMBS International Summer School on Medical Devices and Biosensors, 2006, Cambridge, MA, USA, 4–6 September 2006; pp. 86–89.
- Ghoshdastider, U.; Lange, C.; Viga, R.; Grabmaier, A. A modular and wireless exg signal acquisition system with a dense array of dry electrodes. In Proceedings of 2012 IEEE Sensors, Taipei, Taiwan, 28–31 October 2012; pp. 1–4.
- Bifulco, P.; Cesarelli, M.; Fratini, A.; Ruffo, M.; Pasquariello, G.; Gargiulo, G. A wearable device for recording of biopotentials and body movements. In Proceedings of 2011 IEEE International Workshop on Medical Measurements and Applications Proceedings (MeMeA), Bari, Italy, 30–31 May 2011; pp. 469–472.
- Lopez, G.; Custodio, V.; Moreno, J.I. LOBIN: E-Textile and Wireless-Sensor-network-Based Platform for Healthcare Monitoring in Future Hospital Environment. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1446–1458. [Google Scholar] [CrossRef]
- Coyle, S.; Lau, K.-T.; Moyna, N.; O’Gorman, D.; Diamond, D.; Di Francesco, F.; Costanzo, D.; Salvo, P.; Trivella, M.G.; de Rossi, D.E.; et al. BIOTEX—Biosensing Textiles for Personalised Healthcare Management. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.; Lee, C.C.; Chan, W.M.; Sheung-On, C.; Kwok, P. Development of a wearable system integrated with novel biomedical sensors for ubiquitous healthcare. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 5802–5805.
- Axisa, F.; Scmitt, P.M.; Gehin, C.; Delhomme, G.; McAdams, E.; Dittmar, A. Flexible Technologies and Smart Clothing for Citizen Medicine, Home Healthcare, and Disease Prevention. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 325–336. [Google Scholar] [CrossRef]
- Steltenkamp, S.; Becher, K.; Doerge, T.; Ruff, R.; Hoffmann, K.-P. Electrode structures for acquisition and neural stimulation controlling the cardiovascular system. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009, EMBC 2009, Minneapolis, MN, USA, 3–6 September 2009; pp. 5478–5481.
- Chamadiya, B.; Mankodiya, K.; Wagner, M.; Nasreddine, R.B.; Hofmann, U.G. Non-contact, non-obtrusive electrocardiography in clinical environements. In Proceedings of the the 2011 5th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth), Dublin, Ireland, 23–26 May 2011; pp. 101–106.
- Beckmann, L.; Jacob, M.; Antink, C.H.; Cordes, A.; Pikkemaat, R.; Jungbecker, N.; Gries, T.; Leonhardt, S. Portable bioimpedance spectroscopy device and textile electrodes for mobile monitoring applications. Int. Conf. Electr. Bioimpedance 2010, 224, 1–4. [Google Scholar]
- Lagunar, C.M.; Barca, C.C.; Padrón, A.M.Q.; Planes, X.; Wattenberg, F.S.; López, C.A.; Martín-Fernández, M.; Hernández, N.M.; Oliveras, E.C.; Herranz, J.C. Ubiquitous Tele-monitoring Kit (UTK): Measuring physiological signals anywhere at anytime. In Ambient Assisted Living and Home Care; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7657, pp. 183–191. [Google Scholar]
- Doukas, C.; Maglogiannis, I. Managing wearable sensor data through cloud computing. In Proceedings of the 2011 IEEE Third International Conference on Cloud Computing Technology and Science (CloudCom), Athens, Greece, 29 November–1 December 2011; pp. 440–445.
- Yoon, H.; Deshpande, D.C.; Ramachandran, V.; Varadan, V.K. Aligned nanowires growth using lithography-assisted bonding of a polycarbonate template for neural probe electrodes. Nanotechnology 2008, 19, 025304. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometer diameter fibers of polymer, produced by electrospining. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Laukhina, E.; Pfattner, R.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Laukhin, V. Flexible film-based sensors structured with a high piezoresistive organic molecular conductor as an active component. In Proceedings of the 2010 First International Conference on Sensor Device Technologies and Applications (SENSORDEVICES), Venice, Italy, 18–25 July 2010; pp. 4–9.
- Castellanos, J.; Navas-Gonzalez, R.; Ochoteco, E.; Vidal-Verdu, F. Evaluation of a low cost piezorresistive material for high resolution tactile sensors. In Proceedings of the 2008 2nd European Conference & Exhibition on Integration Issues of Miniaturized Systems - MOMS, MOEMS, ICS and Electronic Components (SSI), Barcelona, Spain, 9–10 April 2008; pp. 1–3.
- Varadan, V.K.; Kumar, P.S.; Oh, S.; Kwon, H.; Rai, P.; Banerjee, N.; Harbaugh, R.E. E-nanoflex sensor systems: Smartphone-based roaming health monitor. J. Nanotechnol. Eng. Med. 2011, 2, 011016. [Google Scholar] [CrossRef]
- Kwon, H.; Oh, S.; Varadan, V.K. Motion artifact removal algorithm by ICA for e-bra: A women ECG measurement system. Proc. SPIE 2013, 8691. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Task force of the european society of cardiology the north american society of pacing electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).