Effects of Germanium Tetrabromide Addition to Zinc Tetraphenyl Porphyrin / Fullerene Bulk Heterojunction Solar Cells
Abstract
:1. Introduction
2. Experimental Section
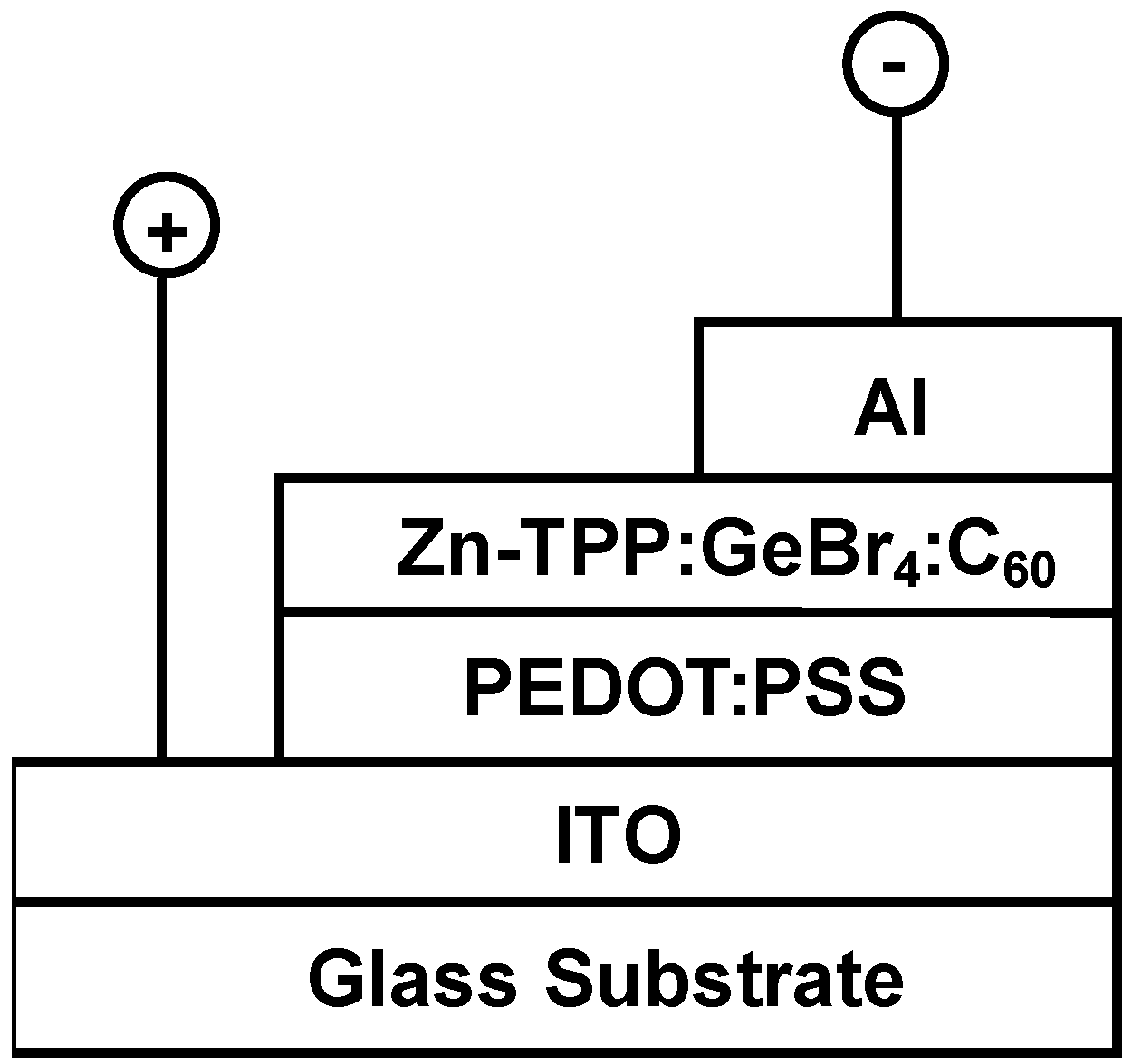
3. Results and Discussion
| Zn-TPP:C60 | η (%) | FF | Voc (V) | Jsc (mA cm−2) |
|---|---|---|---|---|
| 3:7 | 2.2 × 10−5 | 0.23 | 0.058 | 0.0017 |
| 2:8 | 9.5 × 10−4 | 0.20 | 0.25 | 0.019 |
| 1:9 | 1.3 × 10−2 | 0.29 | 0.26 | 0.17 |
| Additive-free | ||||
| 1:9 | 6.4 × 10−3 | 0.20 | 0.23 | 0.14 |

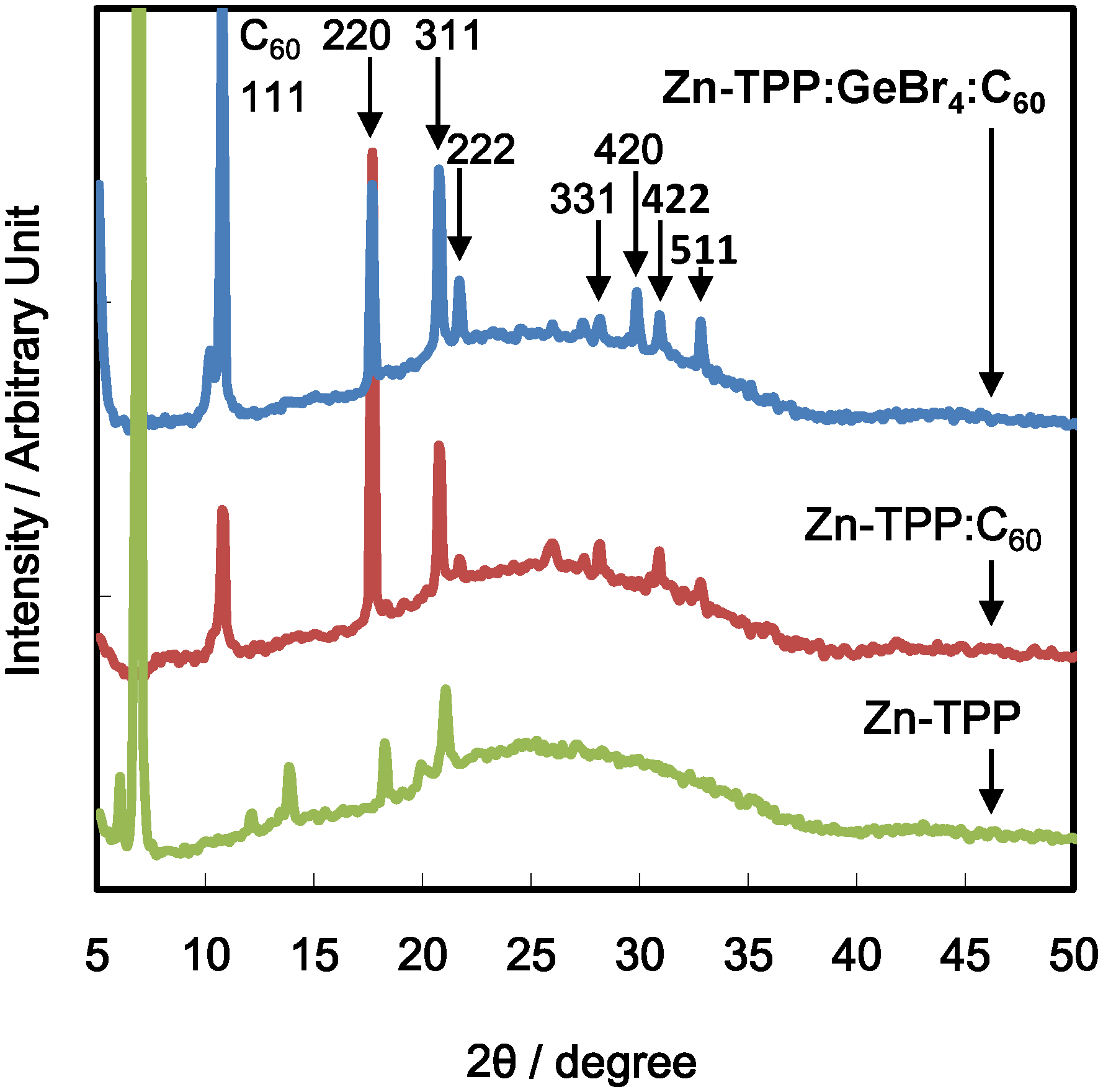


4. Conclusions
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Steven, C.E.; Warren, E.P. Theoretical Fermi-Surface Properties and Superconducting Parameters for K3C60. Science 1991, 254, 842–845. [Google Scholar]
- Allemand, P.-M.; Khemani, K.C.; Koch, A.; Wudl, F.; Holczer, K.; Donovan, S.; Gruner, G.; Thompson, J.D. Organic Molecular Soft Ferromagnetism in a Fullerene C60. Science 1991, 253, 301–302. [Google Scholar]
- Mihailovic, D.; Arcon, D.; Venturini, P.; Blinc, R.; Omerzu, A.; Cevc, P. Orientational and Magnetic Ordering of Buckyballs in TDAE-C60. Science 1995, 268, 400–402. [Google Scholar]
- Lappas, A.; Prassides, K.; Vavekis, K.; Arcon, D.; Blinc, R.; Cevc, P.; Amato, A.; Feyerherm, R.; Gygax, F.N.; Schenck, A. Spontaneous Magnetic Ordering in the Fullerene Charge-Transfer Salt (TDAE)C60. Science 1995, 267, 1799–1802. [Google Scholar]
- Khlyabich, P.P.; Burkhart, B.; Rudenko, A.E.; Barry, C. Optimization and Simplification of Polymer-Fullerene Solar Cells through Polymer and Active Layer Design. Polymer 2013, 54, 5267–5298. [Google Scholar] [CrossRef]
- Marrocchi, A.; Lanari, D.; Facchettibc, A.; Vaccaro, L. Poly(3-hexylthiophene): Synthetic Methodologies and Properties in Bulk Heterojunction Solar Cells. Energy Environ. Sci. 2012, 5, 8457–8474. [Google Scholar] [CrossRef]
- Roncali, J. Single Material Solar Cells: The Next Frontier for Organic Photovoltaics? Adv. Energy Mater. 2011, 1, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef]
- Liao, H.C.; Tsao, C.S.; Lin, T.H.; Jao, M.H.; Chuang, C.M.; Chang, S.Y.; Huang, Y.C.; Shao, Y.T.; Chen, C.Y.; Su, C.J.; et al. Nanoparticle-Tuned Self-Organization of a Bulk Heterojunction Hybrid Solar Cell with Enhanced Performance. ACS Nano 2012, 6, 1657–1666. [Google Scholar] [CrossRef]
- Chen, W.; Xu, T.; He, F.; Wang, W.; Wang, C.; Strzalka, J.; Liu, Y.; Wen, J.; Miller, D.J.; Chen, J.; et al. Hierarchical Nanomorphologies Promote Exciton Dissociation in Polymer/Fullerene Bulk Heterojunction Solar Cells. Nano Lett. 2011, 11, 3707–3713. [Google Scholar] [CrossRef]
- Brousse, B.; Ratier, B.; Moliton, A. Vapor deposited solar cells based on heterojunction or interpenetrating networks of zinc phthalocyanine and C60. Thin Solid Films 2004, 451–452, 81–85. [Google Scholar] [CrossRef]
- Donzello, M.P.; Ercolani, C.; Kadish, K.M.; Ricciardi, G.; Rosa, A.; Stuzhin, P.A. Tetrakis (thiadiazole)porphyrazines. 5. Electrochemical and DFT/TDDFT studies of the free-base macrocycle and its MgII, ZnII, and CuII complexes. Inorg. Chem. 2007, 46, 4145–4157. [Google Scholar] [CrossRef]
- David, C.; Hans, G.; Mathieu, O.; Jef, P.; Paul, H. Stacked organic solar cells based on pentacene and C60. Solar Energy Mater. Solar Cells 2007, 91, 399–404. [Google Scholar] [CrossRef]
- Mauro, M.; Matthias, W.; Alberta, B.; Nikos, K.; Sean, S.; Markus, S.; Zhengguo, Z.; David, W.; Russell, G.; Christoph, B. Bipolar Charge Transport in PCPDTBT-PCBM Bulk-Heterojunctions for Photovoltaic Applications. Adv. Funct. Mater. 2008, 18, 1757–1766. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, H.R.; Chen, J.; Shinokubo, H.; Osuka, A.; Kim, D. Photoelectrochemical Properties of Doubly β-Functionalized Porphyrin Sensitizers for Dye-Sensitized Nanocrystalline-TiO2 Solar Cells. J. Phys. Chem. C 2008, 112, 16691–16699. [Google Scholar] [CrossRef]
- Ruimin, M.; Ping, G.; Linlin, Y.; Lianshun, G.; Xianxi, Z.; Mohammad, K.N.; Michael, G. Theoretical Screening of −NH2-, −OH-, −CH3-, −F-, and −SH-Substituted Porphyrins As Sensitizer Candidates for Dye-Sensitized Solar Cells. J. Phys. Chem. A 2010, 114, 1973–1979. [Google Scholar] [CrossRef]
- Dastoor, P.C.; McNeill, C.R.; Frohne, H.; Foster, C.J.; Dean, B.; Fell, C.J.; Belcher, W.J.; Campbell, W.M.; Officer, D.L.; Blake, I.M.; et al. Understanding and Improving Solid-State Polymer/C60-Fullerene Bulk-Heterojunction Solar Cells Using Ternary Porphyrin Blends. J. Phys. Chem. C 2007, 111, 15415–15426. [Google Scholar] [CrossRef]
- Chen, F.C.; Tseng, H.C.; Ko, C.J. Solvent mixtures for improving device efficiency of polymer photovoltaic devices. Appl. Phys. Lett. 2008, 92, 103316. [Google Scholar] [CrossRef]
- Lou, S.J.; Szarko, J.M.; Xu, T.; Yu, L.; Marks, T.J.; Chen, L.X. Effects of Additives on the Morphology of Solution Phase Aggregates Formed by Active Layer Components of High-Efficiency Organic Solar Cells. J. Am. Chem. Soc. 2011, 133, 20661–20663. [Google Scholar]
- Freitas, J.N.D.; Nogueira, A.F. Incorporation of Inorganic Nanoparticles into Bulk Heterojunction Organic Solar Cells. Nanoenergy 2013. [Google Scholar] [CrossRef]
- Lua, A.J.; Beaupréb, S.; Leclercb, M.; Taoa, Y. Control of the active layer nanomorphology by using co-additives towards high-performance bulk heterojunction solar cells. Org. Electron. 2012, 13, 1736–1741. [Google Scholar] [CrossRef]
- Patrick, B.; Prashant, V.K. Quantum Dot Solar Cells. Electrophoretic Deposition of CdSe-C60 Composite Films and Capture of Photogenerated Electrons with nC60 Cluster Shell. J. Am. Chem. Soc. 2008, 130, 8890–8891. [Google Scholar] [CrossRef]
- Nozik, A.J. Quantum dot solar cells. Physica E 2002, 14, 115–120. [Google Scholar] [CrossRef]
- Istvan, R.; Vaidyanathan, S.; Masaru, K.; Prashant, V.K. Quantum Dot Solar Cells. Harvesting Light Energy with CdSe Nanocrystals Molecularly Linked to Mesoscopic TiO2 Films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef]
- Richard, D.S.; Vladimir, M.A.; Victor, C.K. High-efficiency carrier multiplication through direct photogeneration of multi-excitons via virtual single-exciton states. Nat. Phys. 2005, 1, 189–194. [Google Scholar] [CrossRef]
- Randy, J.E.; Matthew, C.B.; Justin, C.J.; Pingrong, Y.; Olga, I.M.; Arthur, J.N.; Andrew, S.; Alexander, L.E. Highly efficient multiple exciton generation in colloidal PbSe and PbS quantum dots. Nano Lett. 2005, 5, 865–871. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A.; López, N.; Antolín, E.; Cánovas, E.; Stanley, C.; Farmer, C.; Caballero, L.J.; Cuadra, L.; Balenzategui, J.L. Experimental analysis of the quasi-Fermi level split in quantum dot intermediate-band solar cells. Appl. Phys. Lett. 2005, 87, 083505:1–083505:3. [Google Scholar]
- Oku, T.; Noma, T.; Suzuki, A.; Kikuchi, K.; Kikuchi, S. Fabrication and characterization of fullerene/porphyrin bulk heterojunction solar cells. J. Phys. Chem. Solid. 2010, 71, 551–555. [Google Scholar] [CrossRef]
- Oku, T.; Kumada, K.; Suzuki, A.; Kikuchi, K. Effects of germanium addition to copper phthalocyanine/fullerene-based solar cells. Cent. Eur. J. Eng. 2012, 2, 248–252. [Google Scholar] [CrossRef]
- Nagata, A.; Oku, T.; Kikuchi, K.; Suzuki, A.; Yamasaki, Y.; Osawa, E. Fabrication, nanostructures and electronic properties of nanodiamond-based solar cells. Prog. Nat. Sci. 2010, 20, 38–42. [Google Scholar] [CrossRef]
- Vijayarangamuthu, K.; Rath, S.; Kabiraj, D.; Avasthi, D.K.; Kulriya, P.K.; Singh, V.N.; Mehta, B.R. Ge nanocrystals embedded in a GeOx matrix formed by thermally annealing of Ge oxide films. J. Vac. Sci. Technol. A 2009, 27, 731–733. [Google Scholar] [CrossRef]
- Ardyanian, M.; Rinnert, H.; Vergnat, M. Structure and photoluminescence properties of evaporated GeOx/SiO2 multilayers. J. App. Phys. 2006, 100, 113106:1–113106:4. [Google Scholar]
- Frank, E.; Sherif, Z.E.A. Nanoscale electrodeposition of germanium on Au (111) from an ionic liquid: An in situ STM study of phase formation Part I. Ge from GeBr4. Phys. Chem. Chem. Phys. 2002, 4, 1640–1648. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suzuki, A.; Nishimura, K.; Oku, T. Effects of Germanium Tetrabromide Addition to Zinc Tetraphenyl Porphyrin / Fullerene Bulk Heterojunction Solar Cells. Electronics 2014, 3, 112-121. https://doi.org/10.3390/electronics3010112
Suzuki A, Nishimura K, Oku T. Effects of Germanium Tetrabromide Addition to Zinc Tetraphenyl Porphyrin / Fullerene Bulk Heterojunction Solar Cells. Electronics. 2014; 3(1):112-121. https://doi.org/10.3390/electronics3010112
Chicago/Turabian StyleSuzuki, Atsushi, Kenta Nishimura, and Takeo Oku. 2014. "Effects of Germanium Tetrabromide Addition to Zinc Tetraphenyl Porphyrin / Fullerene Bulk Heterojunction Solar Cells" Electronics 3, no. 1: 112-121. https://doi.org/10.3390/electronics3010112
APA StyleSuzuki, A., Nishimura, K., & Oku, T. (2014). Effects of Germanium Tetrabromide Addition to Zinc Tetraphenyl Porphyrin / Fullerene Bulk Heterojunction Solar Cells. Electronics, 3(1), 112-121. https://doi.org/10.3390/electronics3010112





