Nanocarriers for Delivery of Antioxidants on the Skin
Abstract
:1. Introduction
2. Antioxidants
3. Nanocarriers

3.1. Nanocarriers for Resveratrol Administration

3.2. Nanocarriers for Vitamin C
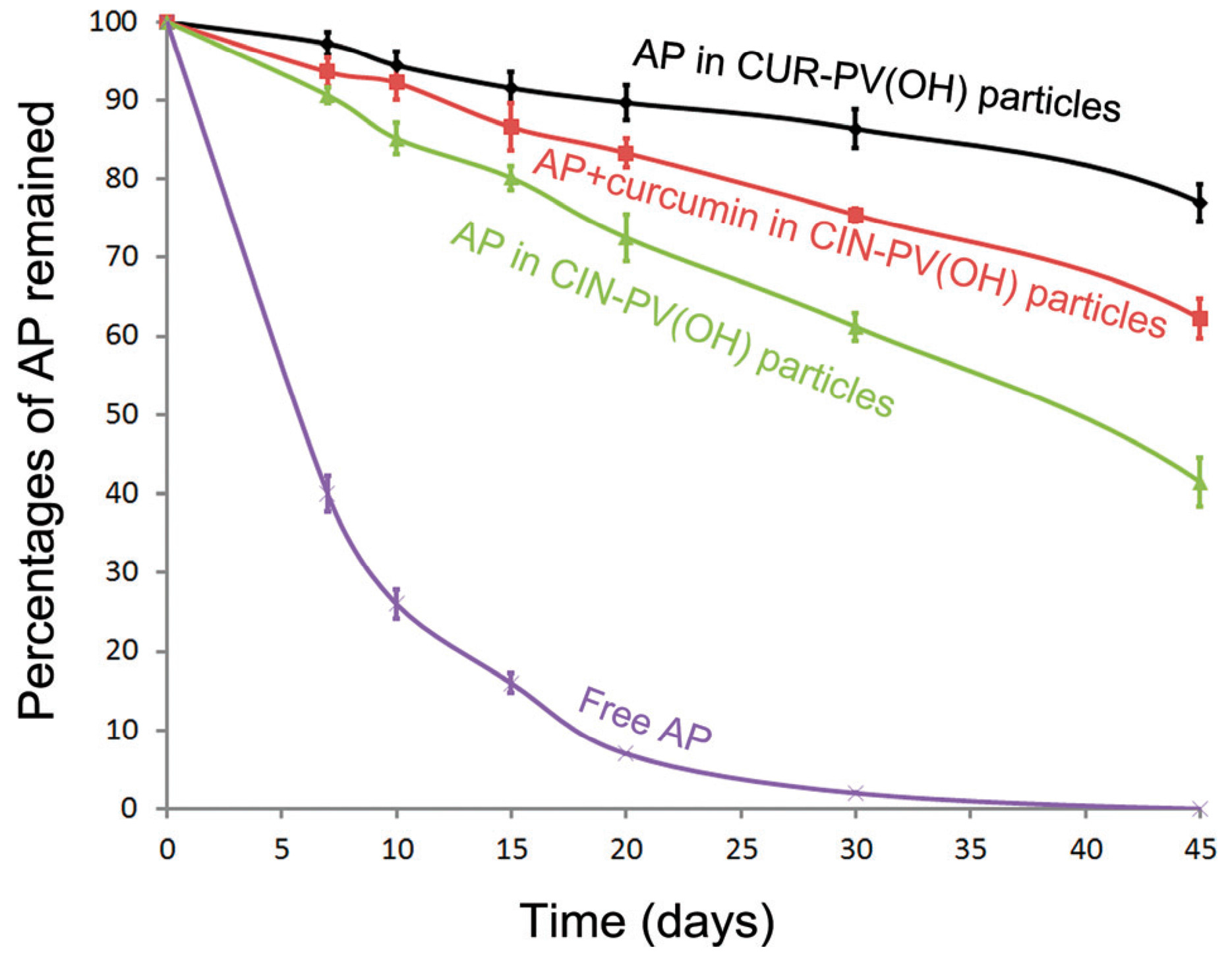
3.3. Nanocarriers for Quercetin Delivery
3.4. Nanocarriers for Coenzyme Q10 Delivery
3.5. Nanocarriers for Other Antioxidants
| Antioxidant | Nanoformulation | Methods | Advantages | Reference |
|---|---|---|---|---|
| Resveratrol | Solid lipid nanoparticles | In vitro photo-degradation | Protection from photo-degradation | Carloti et al. 2012 [25] |
| In vitro porcine skin | Enhance uptake in skin | |||
| Resveratrol | Fosfolipid vesicels | DPPH radical scavenging activity assay | Increase efficiency and stability of carriers | Caddeo et al. 2013 [27] |
| Resveratrol + curcumin | Lipid-core nanocapsules | In vitro static Franz diffusion cell human skin | Increase delivery of resveratrol into skin | Friedrich et al. 2015 [28] |
| Resveratrol + curcumin | Niosomes | In vitro static Franz diffusion cell rabbit skin DPPH radical scavenging activity assay | Increase delivery of resveratrol into skin Increase antioxidant activity | Tavano et al. 2014 [29] |
| Q10 | Ultra small nano-structured lipid carrier (NLC) | In vitro static Franz diffusion cell porcine skin | Increase delivery of Q10 into skin | Schwarz et al. 2013 [42] |
| Q10 | Ultra-small lipid nanoparticles (usNLC) | In vitro human keratinocyte cell line HaCaT | Strongest reduction of the radical formation and non-toxic | Lohan et al. 2015 [42] |
| Quercetin | Solid lipid nanosystems | In vitro static Franz diffusion cell human skin | Higher amounts of quercetin within the skin | Bose et al. 2013 [38] |
| Quercetin | Colloidal silica emulsion | In vivo human penetration assay | Enhance penetration into stratum corneum in human | Scalia et al. 2012 [35] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dreher, F.; Maibach, H.I. Protective effects of topical antioxidants in humans. Curr. Probl. Dermatol. 2001, 29, 157–164. [Google Scholar] [PubMed]
- Yoshihisa, Y.; Honda, A.; Zhao, Q.L.; Makino, T.; Abe, R.; Matsui, K.; Shimizu, H.; Miyamoto, Y.; Kondo, T.; Shimizu, T. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 2010, 19, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitro evaluation. Int. J. Pharm. 2012, 434, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.J.; Banga, A.K. Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int. J. Cosmet. Sci. 2014, 36, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The missing link—Light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Phys. 2009, 22, 31–44. [Google Scholar]
- Darvin, M.E.; Haag, S.F.; Meinke, M.C.; Sterry, W.; Lademann, J. Determination of the influence of IR radiation on the antioxidative network of the human skin. J. Biophotonics 2011, 4, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Syring, F.; Schanzer, S.; Haag, S.F.; Graf, R.; Loch, M.; Gersonde, I.; Groth, N.; Pflücker, F.; Lademann, J. Radical protection by differently composed creams in the UV/VIS and IR spectral ranges. Photochem. Photobiol. 2013, 89, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Haag, S.F.; Kleemann, A.; Lademann, J.; Meinke, M.C. Radical protection in the visible and infrared by a hyperforin-rich cream—In vivo versus ex vivo methods. Exp. Dermatol. 2013, 22, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Melot, M.; Pudney, P.D.; Williamson, A.M.; Caspers, P.J.; van der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Control. Release 2009, 138, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R. Skin antioxidants: Their role in aging and in oxidative stress—New approaches for their evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Hung, C.F.; Lin, Y.K.; Huang, Z.R.; Fang, J.Y. Delivery of resveratrol, a red wine polyphenol polyphenol, from solutions and hydrogels via the skin. Biol. Pharm. Bull. 2008, 31, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hung, C.F.; Chiu, H.C.; Wang, J.J.; Chan, T.F. Efficacy and irritancy of enhancers on the in vitro and in vivo percutaneous absorption of curcumin. J. Pharm. Pharmacol. 2003, 55, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Suwannateep, N.; Wanichwecharungruang, S.; Haag, S.F.; Devahastin, S.; Groth, N.; Fluhr, J.W.; Lademann, J.; Meinke, M.C. Encapsulated curcumin results in prolonged curcumin activity in vitro and radical scavenging activity ex vivo on skin after UVB-irradiation. Eur. J. Pharm. Biopharm. 2012, 82, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Marti-Mestres, G.; Mestres, J.P.; Bres, J.; Martin, S.; Ramos, J.; Vian, L. The in vitro percutaneous penetration of three antioxidant compounds. Int. J. Pharm. 2007, 331, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jurkovic, P.; Šentjurc, M.; Gašperlin, M.; Kristl, J.; Pecar, S. Skin protection against ultraviolet induced free radicals with ascorbylpalmitate in microemulsions. Eur. J. Pharm. Biopharm. 2003, 56, 59–66. [Google Scholar] [CrossRef]
- Abramovits, W.; Granowski, P.; Arrazola, P. Applications of nanomedicine in dermatology: Use of nanoparticles in varioustherapies and imaging. J. Cosmet. Dermatol. 2010, 9, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Schafer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Castro, G.A.; Coelho, A.L.; Oliveira, C.A.; Mahecha, G.A.; Orefice, R.L.; Ferreira, L.A. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int. J. Pharm. 2009, 381, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S. Resveratrol in solid lipid nanoparticles. J. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Pando, D.; Caddeo, C.; Manconib, M.; Fadda, A.M.; Pazos, C. Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J. Pharm. Pharmacol. 2013, 65, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Manconi, M.; Faddda, M.A.; Lai, F.; Lanpis, S.; Diez-Sale, O.; Sinico, C. Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B. 2013, 11, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H.L.; Beck, R.C.; Windbergs, M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B Biointerfaces 2014, 114, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Kristl, J.; Volk, B.; Gasperlin, M.; Sentjurc, M.; Jurkovic, P. Effect of colloidal carriers on ascorbylpalmitate stability. Eur. J. Pharm. Sci. 2003, 19, 181–189. [Google Scholar] [CrossRef]
- Janesirisakule, S.; Sinthusake, T.; Wanichwecharungruang, S. Nanocarrier with self-antioxidative property for stabilizing and delivering ascorbylpalmitate into skin. J. Pharm. Sci. 2013, 102, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Tanaka, Y.; Tanaka, M.; Endo, K.; Yoshii, A. Enhanced skin delivery of quercetin by microemulsion. J. Pharm. Pharmacol. 2009, 61, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative evaluation of the effect of permeation enhances, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Skin Pharmacol. Physiol. 2013, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K. New insights into skin structure: Scatching the surface. Adv. Drug Deliv. Rev. 2002, 54, S3–S17. [Google Scholar] [CrossRef]
- Guo, C.; Yang, C.; Li, Q.; Qi, T.; Xi, Y.; Liu, W.; Zhai, G. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak-Kohn, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 441, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Muta-Takada, K.; Terada, T.; Yamanishi, H.; Ashida, Y.; Inomata, S.; Nishiyama, T.; Amano, S. Coenzyme Q10 protects against oxidative stress-induced cell death and enhances the synthesis of basement membrane components in dermal and epidermal cells. Biofactors 2009, 35, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Bonina, F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Baisaeng, N.; Hoppel, M.; Löw, M.; Keck, C.M.; Valenta, C. Ultra-small NLC for improved dermal delivery of coenzyme Q10. Int. J. Pharm. 2013, 447, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.B.; Bauersachs, S.; Ahlberg, S.; Baisaeng, N.; Keck, C.M.; Müller, R.H.; Witte, E.; Wolk, K.; Hackbarth, S.; Röder, B.; et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur. J. Pharm. Biopharm. 2015, 89, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Brugè, F.; Damiani, E.; Puglia, C.; Offerta, A.; Armeni, T.; Littarru, G.P.; Tiano, L. Nanostructured lipid carriers loaded with CoQ10: Effect on human dermal fibroblast under normal and UVA-mediated oxidative conditions. Int. J. Pharm. 2013, 455, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mahamongkol, H.; Bellantone, R.A.; Stagni, G.; Plakogiannis, F.M. Permeation study of five formulations of alpha-tocopherol acetate through human cadaver skin. J. Cosmet. Sci. 2005, 56, 91–103. [Google Scholar] [PubMed]
- Ben-Shabat, S.; Kazdan, Y.; Beit-Yannai, E.; Sintov, A.C. Use of alpha-tocopherol esters for topical vitamin E treatment: Evaluation of their skin permeation and metabolism. J. Pharm. Pharmacol. 2013, 65, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.T.; Reynolds, C.A.; Gatz, M. Age-related differences and change in positive and negative affect over 23 years. J. Personal. Soc. Psychol. 2001, 80, 136–151. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qui, M.; Jiang, X.; Lai, R.; Chen, H. A novel small odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamics study on amyloid-b25-35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Sherif, S.; Bendas, E.R.; Badawy, S. The clinical efficacy of cosmeceutical application of liquid crystalline nanostructured dispersions of alpha lipolic acid as anti-wrinkle. Eur. J. Pharm. Biopharm. 2014, 86, 252–259. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinardell, M.P.; Mitjans, M. Nanocarriers for Delivery of Antioxidants on the Skin. Cosmetics 2015, 2, 342-354. https://doi.org/10.3390/cosmetics2040342
Vinardell MP, Mitjans M. Nanocarriers for Delivery of Antioxidants on the Skin. Cosmetics. 2015; 2(4):342-354. https://doi.org/10.3390/cosmetics2040342
Chicago/Turabian StyleVinardell, María Pilar, and Montserrat Mitjans. 2015. "Nanocarriers for Delivery of Antioxidants on the Skin" Cosmetics 2, no. 4: 342-354. https://doi.org/10.3390/cosmetics2040342







