Effectiveness of a Novel Compound HAIR & SCALP COMPLEX on Hair Follicle Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cord Blood Stem Cell (CBSC)-Derived Exosomes
2.2. Plant-Derived Nanovesicles (PDNVs)
2.3. Isolation and Characterization of Colostrum-Derived Exosomes
2.4. Colostrum-Derived Exosome Imaging with Scanning Electron Microscopy
2.5. GF20 Preparation and Detection of Bioactive Factors via ELISA
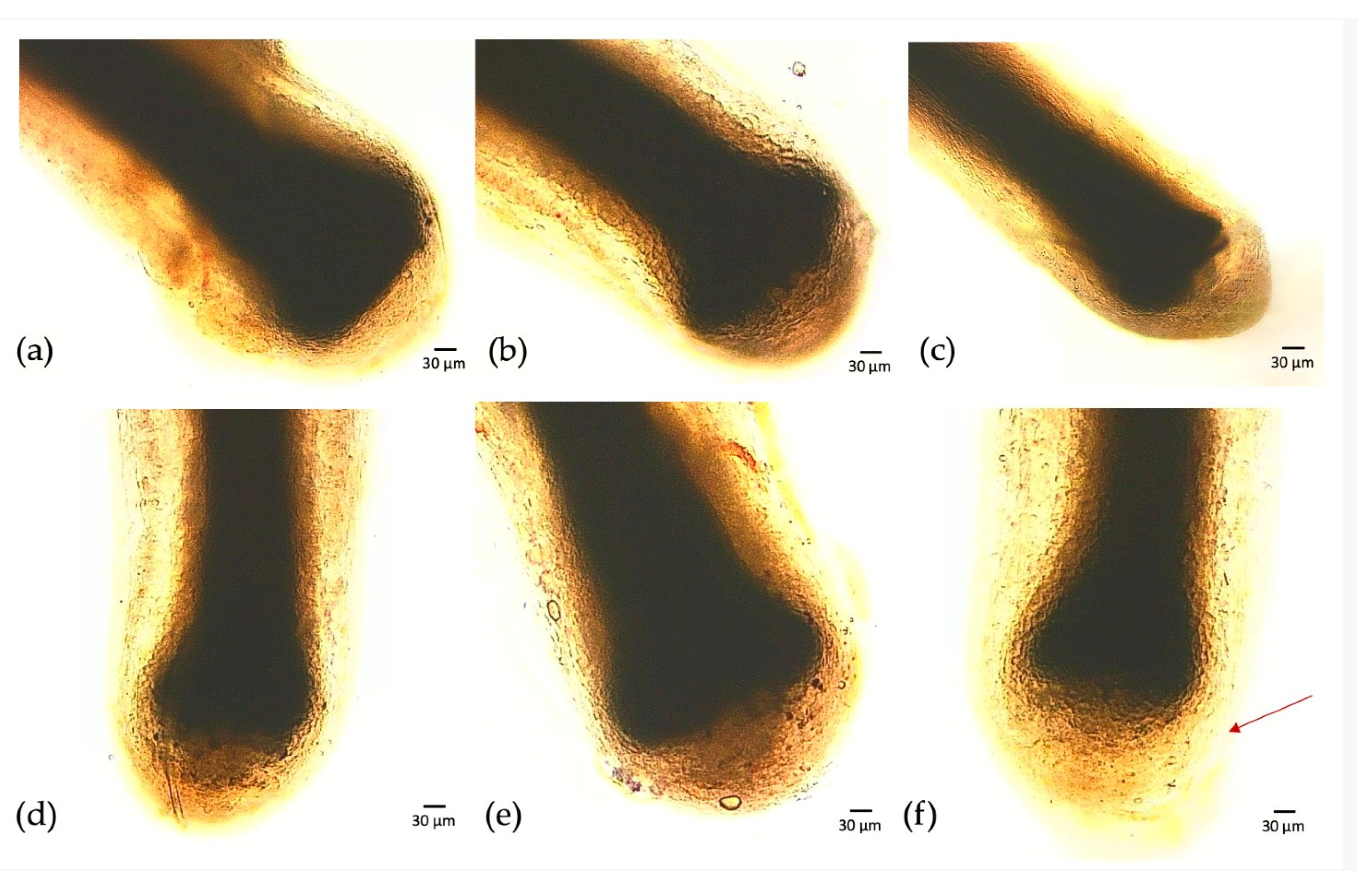
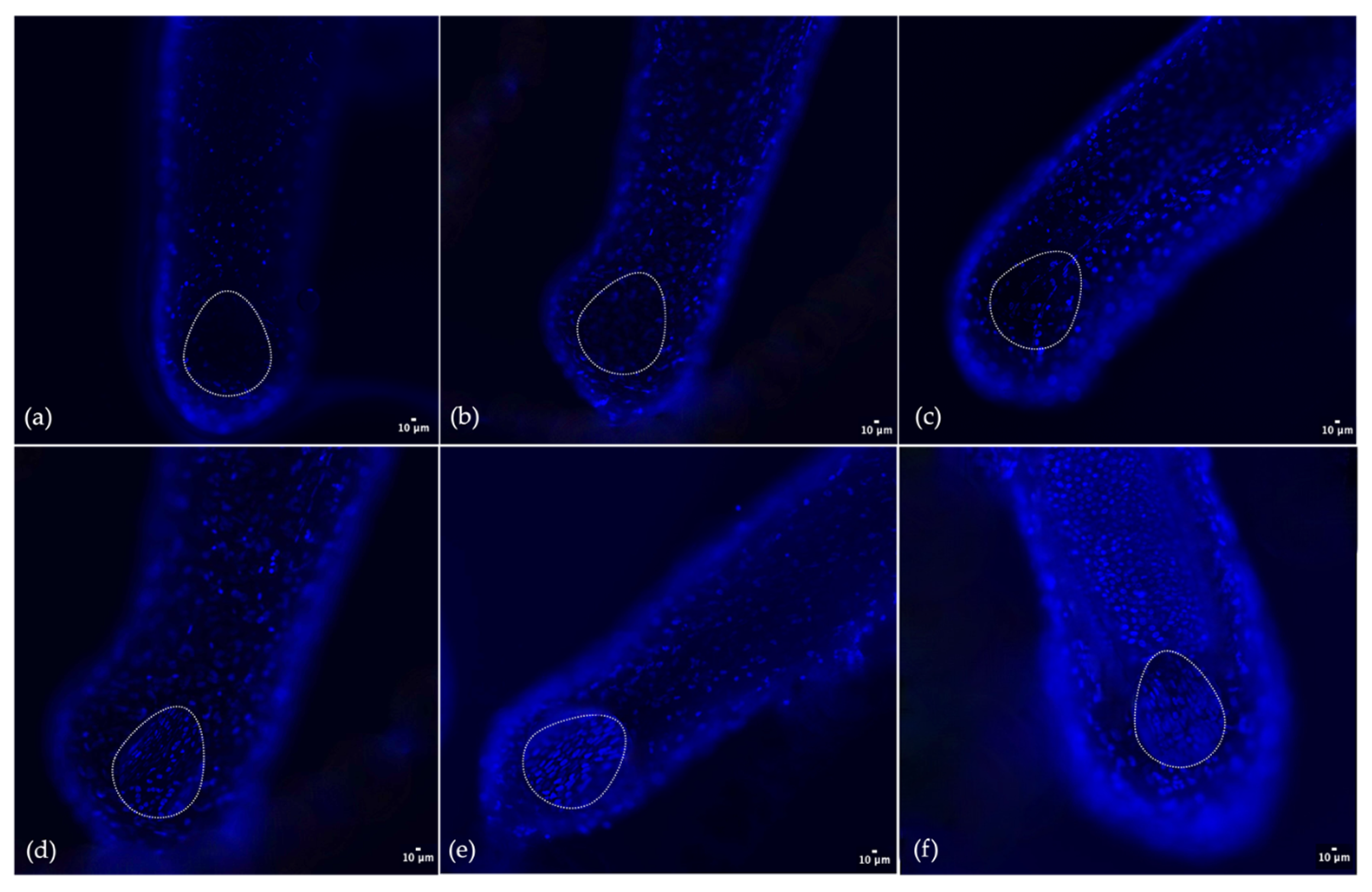
2.6. In Vitro Culture of Human Hair Follicle
2.7. Identification and Analysis of Hair Follicle Derma Papilla Cells
2.8. Statistical Analysis
3. Results
3.1. Characterization of CBSC-Derived Exosomes
3.2. Characterization of Plant-Derived Nanovesicles
3.3. Characterization of Colostrum-Derived Exosomes
3.4. Bioactive Components of Colostrum
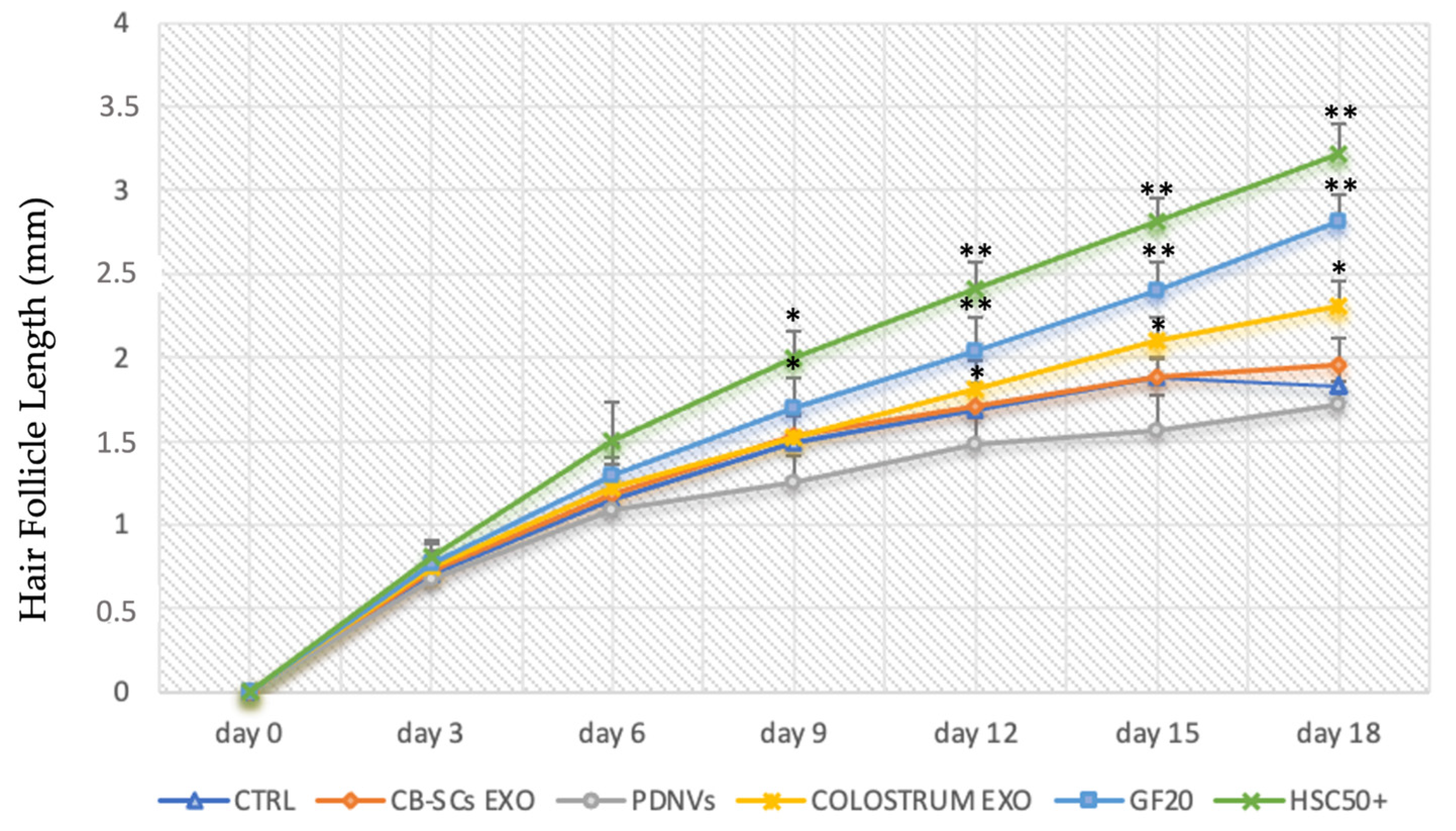
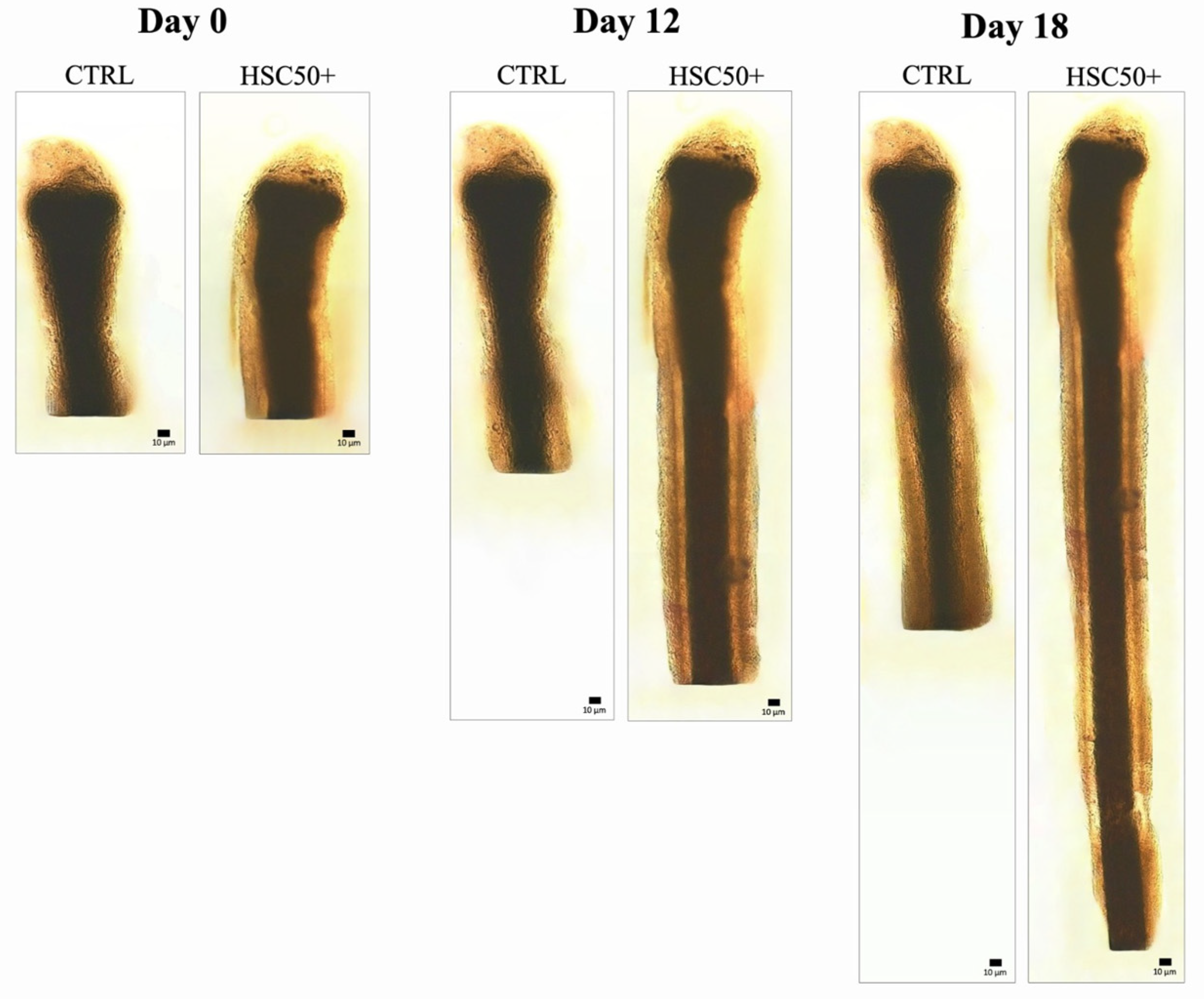
3.5. In Vitro Culture of Human Hair Follicles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thadanipon, K.; Suchonwanit, P. Measuring Patient Quality of Life Following Treatment for Alopecia. Patient Prefer. Adherence 2021, 15, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Dhami, L. Psychology of Hair Loss Patients and Importance of Counseling. Indian J. Plast. Surg. 2021, 54, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Saknite, I.; Valdebran, M.; Balu, M.; Lentsch, G.; Williams, J.N.; Koenig, K.; Tromberg, B.J.; Atanaskova Mesinkovska, N. Feature characterization of scarring and non-scarring types of alopecia by multiphoton microscopy. Lasers Surg. Med. 2019, 51, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.I. Overview of Alopecia: A Dermatopathologist’s Perspective. Mo. Med. 2015, 112, 308–312. [Google Scholar]
- Piraccini, B.M.; Alessandrini, A. Androgenetic alopecia. G. Ital. Dermatol. Venereol. 2014, 149, 15–24. [Google Scholar] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Hibino, T.; Nishiyama, T. Role of TGF-beta2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Koh, Y.G.; Lee, W.G.; Seok, J.; Park, K.Y. The use of epidermal growth factor in dermatological practice. Int. Wound J. 2023, 20, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Pi, L.Q.; Hwang, S.T.; Lee, W.S. Effect of IGF-I on Hair Growth Is Related to the Anti-Apoptotic Effect of IGF-I and Up-Regulation of PDGF-A and PDGF-B. Ann. Dermatol. 2012, 24, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Suh, W.; Sung, J.H. Hair Growth Regulation by Fibroblast Growth Factor 12 (FGF12). Int. J. Mol. Sci. 2022, 23, 9467. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Skin. Appendage Disord. 2018, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- El-Refai, A.M.; Elhabak, D.M.; Khashaba, R.A. More is Not Always Better in Hair Growth Factors. Epidermal Growth Factor: Hair Growth Factor Involved in Alopecia Areata Pathogenesis. Int. J. Trichology 2020, 12, 182–187. [Google Scholar]
- Yoon, S.Y.; Kim, K.T.; Jo, S.J.; Cho, A.R.; Jeon, S.I.; Choi, H.D.; Kim, K.H.; Park, G.S.; Pack, J.K.; Kwon, O.S.; et al. Induction of hair growth by insulin-like growth factor-1 in 1,763 MHz radiofrequency-irradiated hair follicle cells. PLoS ONE 2011, 6, e28474. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, W.; Wang, S.; Zhang, T.; Si, H.; Yang, F.; Li, G. Epidermal Growth Factor Promotes Proliferation and Migration of Follicular Outer Root Sheath Cells via Wnt/β-Catenin Signaling. Cell Physiol. Biochem. 2016, 39, 360–370. [Google Scholar] [CrossRef]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Chen, Q.; Yang, N.; Bao, Z.; Hu, S.; Chen, Y.; Wu, X. A Treatment Combination of IGF and EGF Promotes Hair Growth in the Angora Rabbit. Genes 2020, 12, 24. [Google Scholar] [CrossRef]
- Xie, M.; Wu, D.; Li, G.; Yang, J.; Zhang, Y.S. Exosomes targeted towards applications in regenerative medicine. Nano Sel. 2021, 2, 880–908. [Google Scholar] [CrossRef]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Gong, H.; Luo, S.; Cui, Y. The Role of Exosomes and Their Applications in Cancer. Int. J. Mol. Sci. 2021, 22, 12204. [Google Scholar] [CrossRef]
- Xie, F.; Huang, Y.; Zhan, Y.; Bao, L. Exosomes as drug delivery system in gastrointestinal cancer. Front. Oncol. 2023, 12, 1101823. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S. Extracellular Vesicles in Neurodegenerative Diseases: A Double-Edged Sword. Tissue Eng. Regen. Med. 2017, 14, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J. Potential for Therapeutic-Loaded Exosomes to Ameliorate the Pathogenic Effects of α-Synuclein in Parkinson’s Disease. Biomedicines 2023, 11, 1187. [Google Scholar] [CrossRef]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke, D.J., 2nd. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef]
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef]
- Shkryl, Y.; Tsydeneshieva, Z.; Degtyarenko, A.; Yugay, Y.; Balabanova, L.; Rusapetova, T.; Bulgakov, V. Plant Exosomal Vesicles: Perspective Information Nanocarriers in Biomedicine. Appl. Sci. 2022, 12, 8262. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Kim, M.; Jang, H.; Kim, W.; Kim, D.; Park, J.H. Therapeutic Applications of Plant-Derived Extracellular Vesicles as Antioxidants for Oxidative Stress-Related Diseases. Antioxidants 2023, 12, 1286. [Google Scholar] [CrossRef]
- Di Giulio, S.; Carata, E.; Mariano, S.; Panzarini, E. Plant Extracellular Vesicles: Investigating Their Utilization as Beneficial Nutrients in Diet. Appl. Sci. 2023, 13, 6656. [Google Scholar] [CrossRef]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomedicine 2020, 29, 102271. [Google Scholar] [CrossRef]
- Naeem, P.; Baumgartner, A.; Ghaderi, N.; Sefat, F.; Alhawamdeh, M.; Heidari, S.; Shahzad, F.; Swaminathan, K.; Akhbari, P.; Isreb, M.; et al. Anticarcinogenic impact of extracellular vesicles (exosomes) from cord blood stem cells in malignant melanoma: A potential biological treatment. J. Cell Mol. Med. 2023, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.S.; Rodrigues, S.C.; Gomes, C.F.; Duarte, F.V.; Romao, M.; Leal, E.C.; Freire, P.C.; Neves, R.; Simões-Correia, J. Development of an optimized and scalable method for isolation of umbilical cord blood-derived small extracellular vesicles for future clinical use. Stem Cells Transl. Med. 2021, 10, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Rapaport, J.A. Systematic review of exosome treatment in hair restoration: Preliminary evidence, safety, and future directions. J. Cosmet. Dermatol. 2023, 22, 2424–2433. [Google Scholar] [CrossRef]
- Kim, H.; Jang, Y.; Kim, E.H.; Jang, H.; Cho, H.; Han, G.; Song, H.K.; Kim, S.H.; Yang, Y. Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase. Front. Cell Dev. Biol. 2022, 10, 815205. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhang, B.; He, B. The Role of Cancer Stem Cell-Derived Exosomes in Cancer Progression. Stem Cells Int. 2022, 4, 9133658. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Musumeci, P.; Baeri, P.; Messina, E.; Boninelli, S.; Compagnini, G.; Calcagno, L. Rotational dynamics of gold nanoparticle chains in water solution. J. Nanopart Res. 2012, 14, 1308. [Google Scholar] [CrossRef]
- Zimbone, M.; Baeri, P.; Calcagno, L.; Musumeci, P.; Contino, A.; Barcellona, M.L.; Bonaventura, G. Dynamic light scattering on bioconjugated laser generated gold nanoparticles. PLoS ONE 2014, 9, e89048. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.E.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J. Dairy. Sci. 2013, 96, 1745–1754. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef]
- Wheeler, T.T.; Hodgkinson, A.J.; Prosser, C.G.; Davis, S.R. Immune components of colostrum and milk—A historical perspective. J. Mammary Gland. Biol. Neoplasia 2007, 12, 237–247. [Google Scholar] [CrossRef]
- El-Agamy, E.I. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoulm, H.F.J.; Warnerm, J.O.; van Neervenm, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Xiang, L.J.; Shi, H.X.; Zhang, J.; Jiang, L.P.; Cai, P.T.; Lin, Z.L.; Lin, B.B.; Huang, Y.; Zhang, H.L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed. Res. Int. 2015, 2015, 730139. [Google Scholar]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Zheng, X.; Zhu, H.; Qi, Q.; Liu, S.; Zhang, H.; Che, J. Latest Trend of Milk Derived Exosomes: Cargos, Functions, and Applications. Front. Nutr. 2021, 8, 747294. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treat ment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Hoffmann, R.; Eicheler, W.; Huth, A.; Wenzel, E.; Happle, R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch. Dermatol. Res. 1996, 288, 153–156. [Google Scholar] [CrossRef]

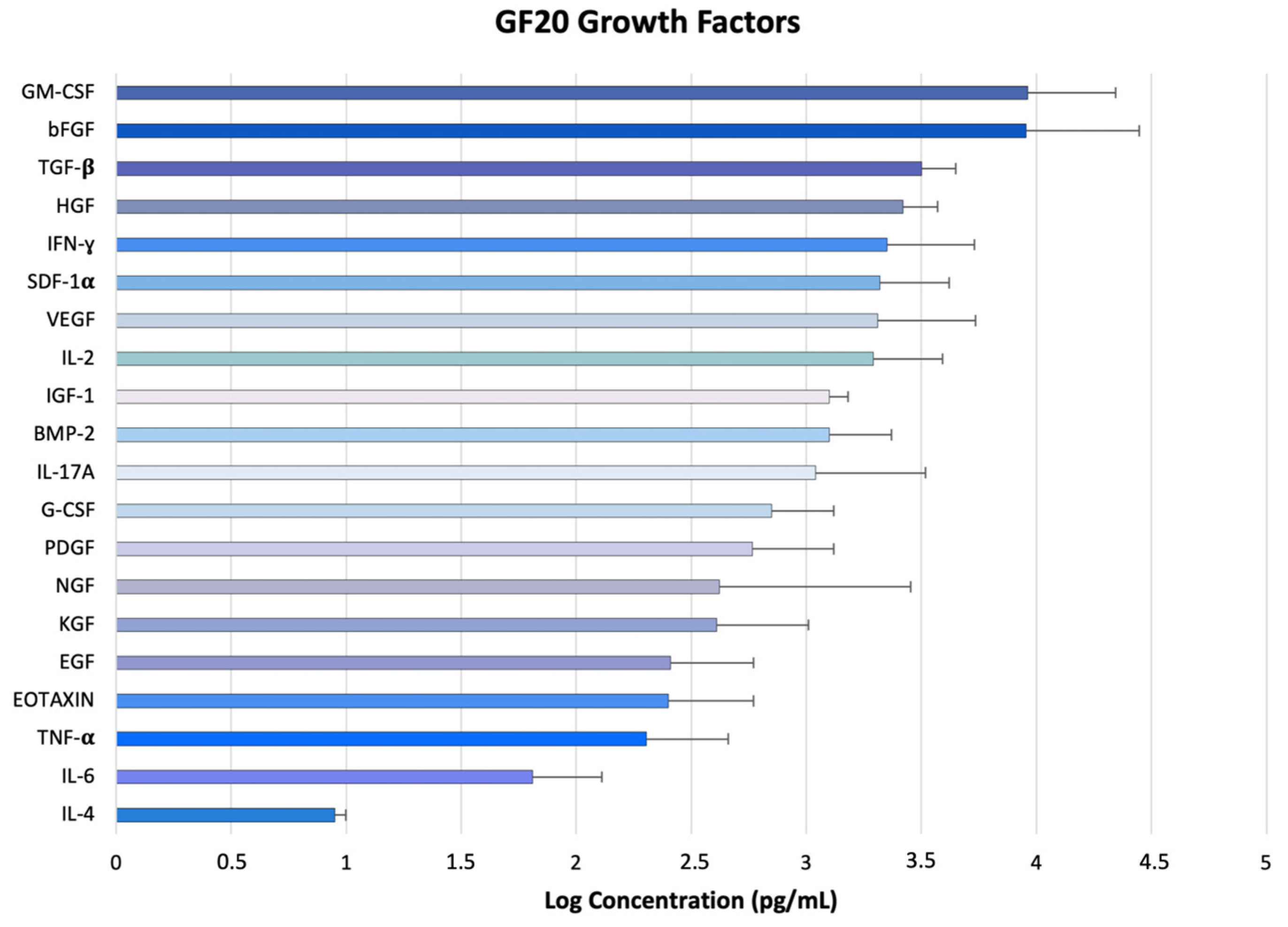

| Growth Factors and Cytokines Detected via ELISA |
|---|
| Transforming Growth Factors β (TGF-β) |
| Insulin-Like Growth Factor 1 (IGF-1) |
| Basic Fibroblast Growth Factor (bFGF) |
| Vascular Endothelial Growth Factor (VEGF) |
| Epidermal Growth Factor (EGF) |
| Platelet-Derived Growth Factor (PDGF) |
| Keratinocyte Growth Factor (KGF) |
| Hepatocyte Growth Factor (HGF) |
| Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) |
| Granulocyte-Colony Stimulating Factor (G-CSF) |
| EOTAXIN-CCL11 |
| Tumor Necrosis Factor α (TNF-α) |
| Nerve Growth Factor (NGF) |
| Gamma Interferon (INF-γ) |
| Bone Morphogenetic Protein 2 (BMP-2) |
| Stromal-Cell-Derived Factor 1α (SDF1-α) |
| Interleukin-2 (IL-2) |
| Interleukin-4 (IL-4) |
| Interleukin-6 (IL-6) |
| Interleukin-17A (IL-17A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferruggia, G.; Contino, M.; Zimbone, M.; Brundo, M.V. Effectiveness of a Novel Compound HAIR & SCALP COMPLEX on Hair Follicle Regeneration. Cosmetics 2024, 11, 10. https://doi.org/10.3390/cosmetics11010010
Ferruggia G, Contino M, Zimbone M, Brundo MV. Effectiveness of a Novel Compound HAIR & SCALP COMPLEX on Hair Follicle Regeneration. Cosmetics. 2024; 11(1):10. https://doi.org/10.3390/cosmetics11010010
Chicago/Turabian StyleFerruggia, Greta, Martina Contino, Massimo Zimbone, and Maria Violetta Brundo. 2024. "Effectiveness of a Novel Compound HAIR & SCALP COMPLEX on Hair Follicle Regeneration" Cosmetics 11, no. 1: 10. https://doi.org/10.3390/cosmetics11010010





