Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Dry Spinning of Gelatin Fiber
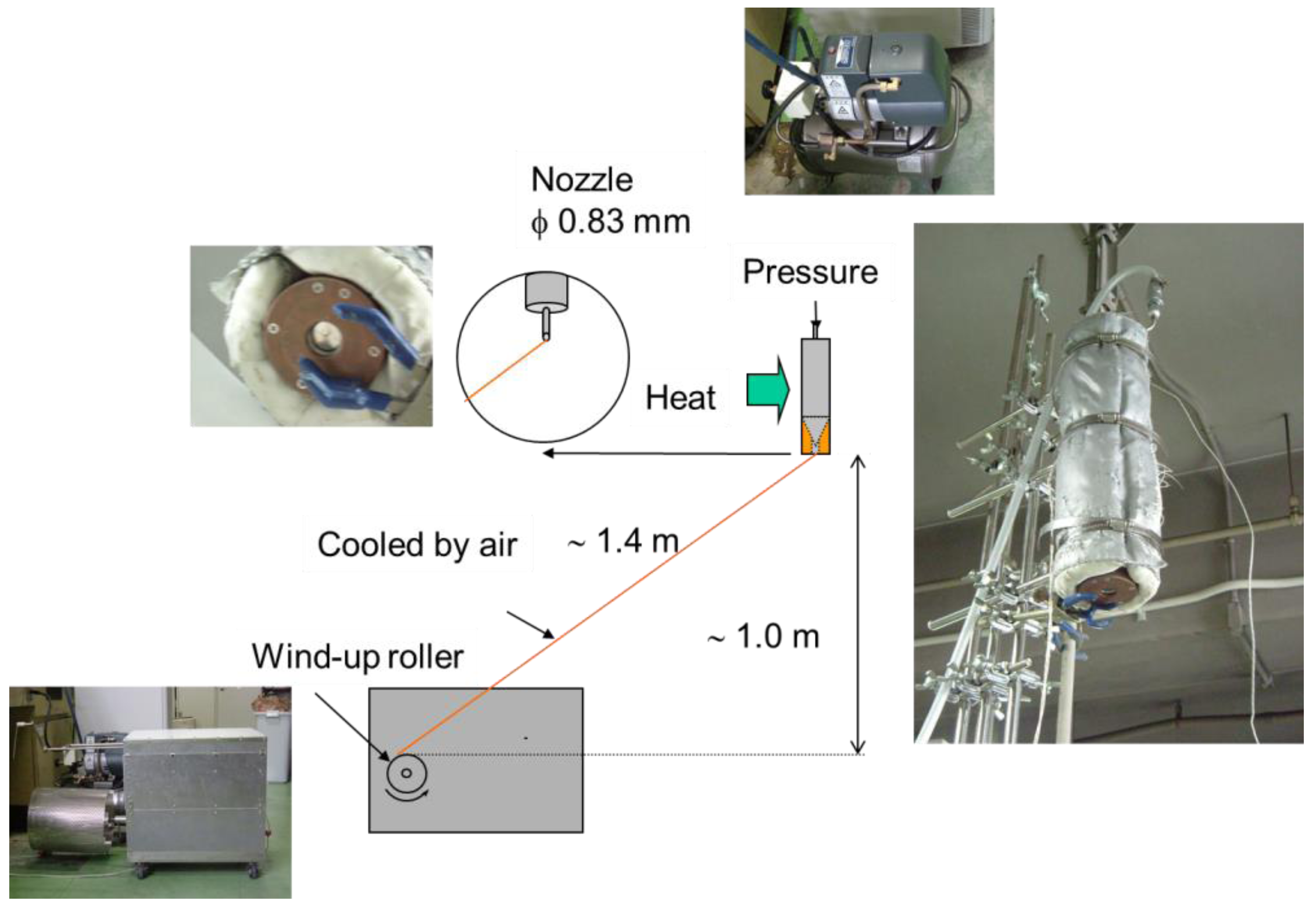
2.3. Preparation of Crosslinked Gelatin Fibers
2.3.1. Crosslinking with Sugars
2.3.2. Crosslinking with Denacol
2.3.3. Crosslinking by GTA Treatment
2.4. Water Resistance
2.5. Measurements
3. Results and Discussion
3.1. Preparation of Gelatin Fibers
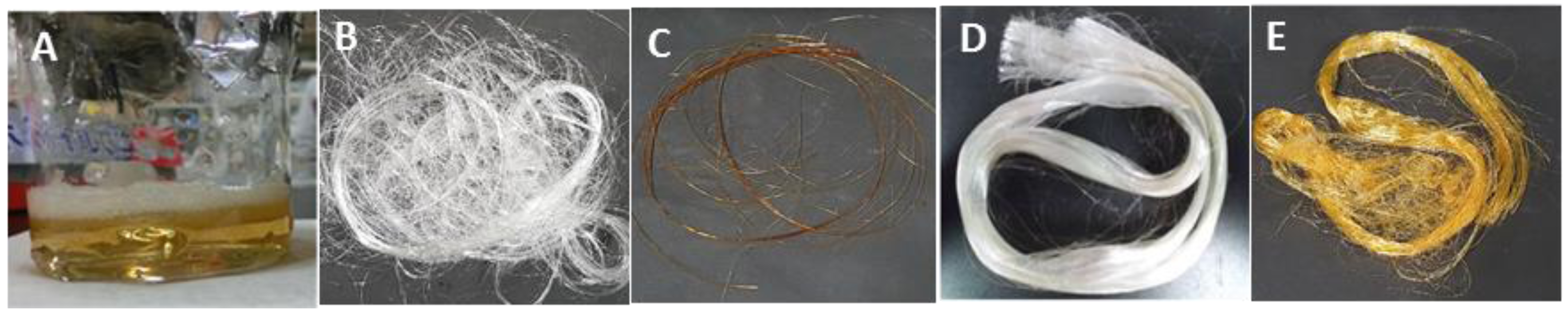

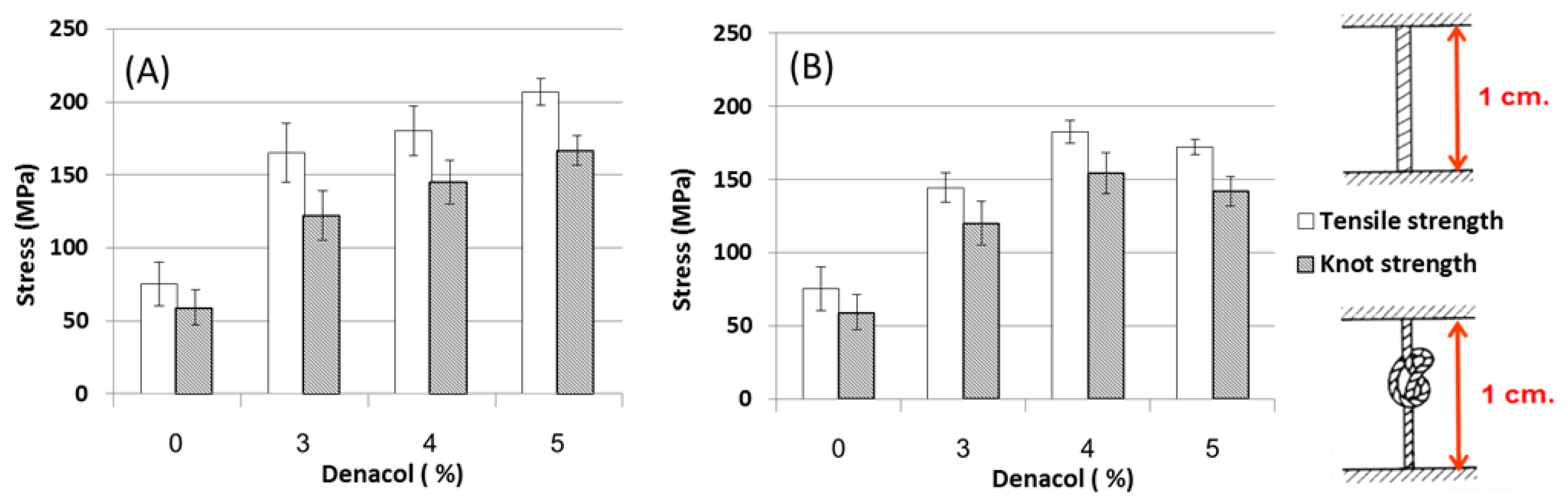
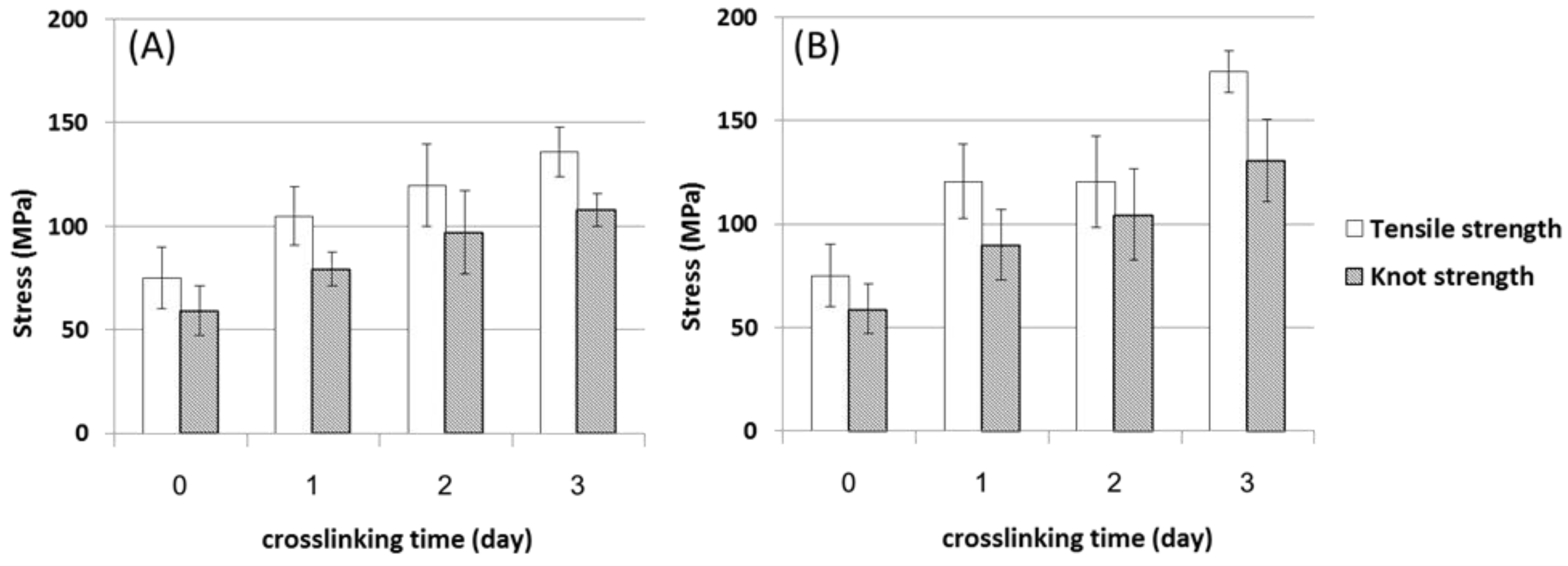
3.2. Effects of Crosslinkers

| Denacol (%) | Crosslinking Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
| 0 | × | × | ○ | ○ | ○ | ○ | ○ |
| 3 | × | △ | ○ | ○ | △ | △ | × |
| 4 | × | △ | ○ | ○ | △ | △ | × |
| 5 | × | △ | ○ | ○ | △ | × | × |
3.3. FT-IR Spectroscopy
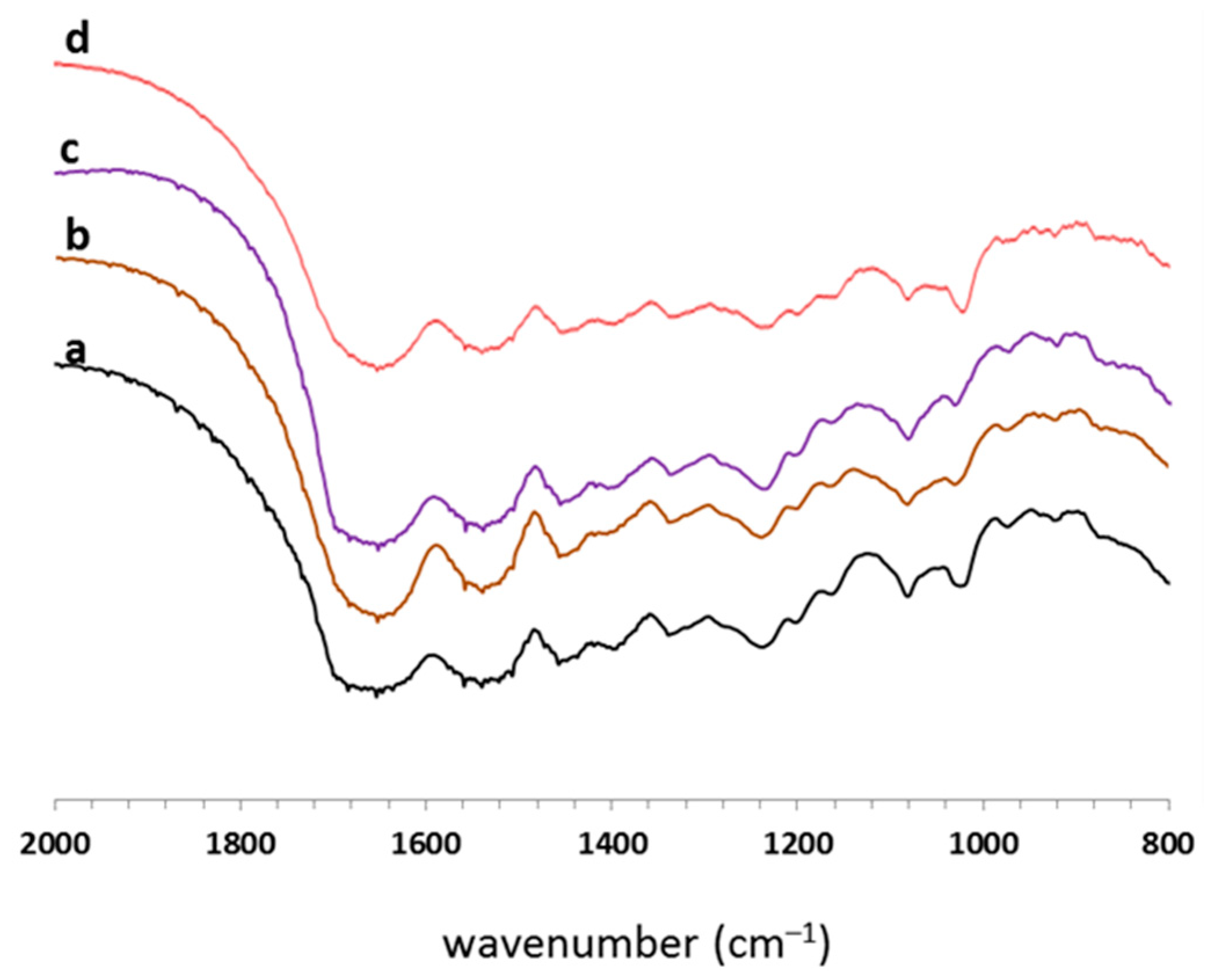
3.4. Water Resistance
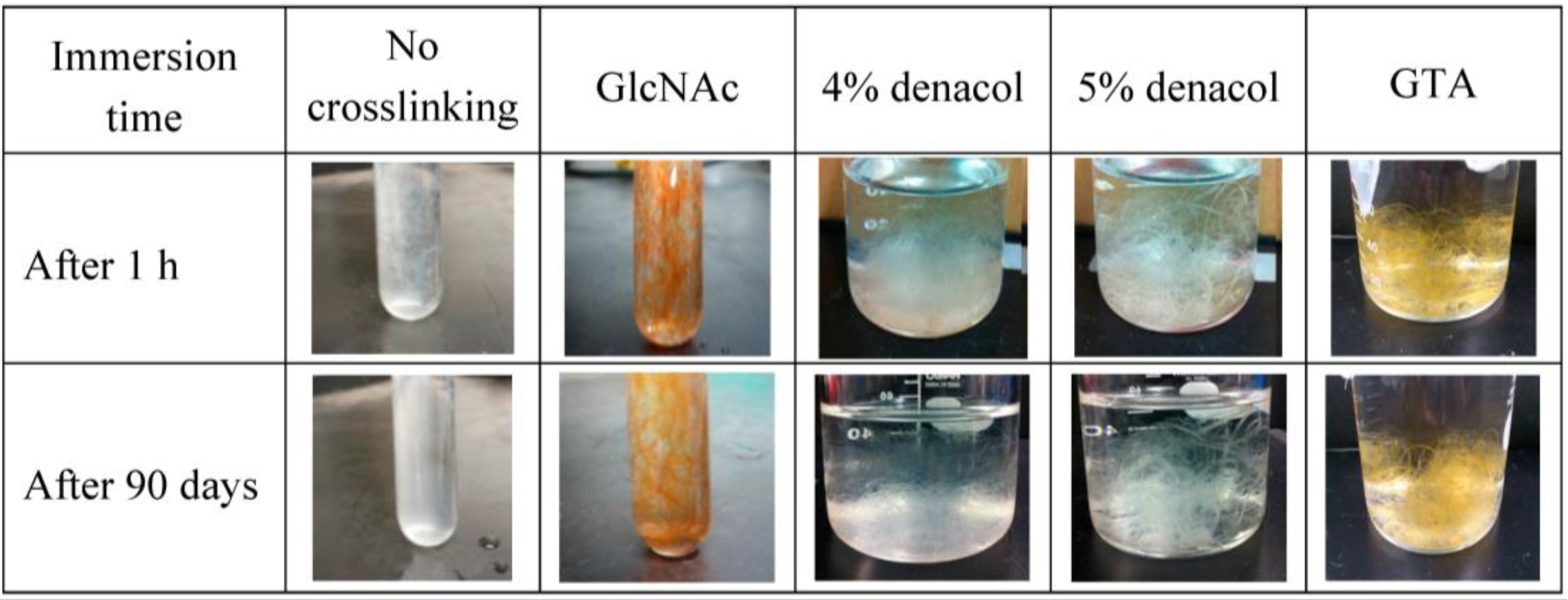
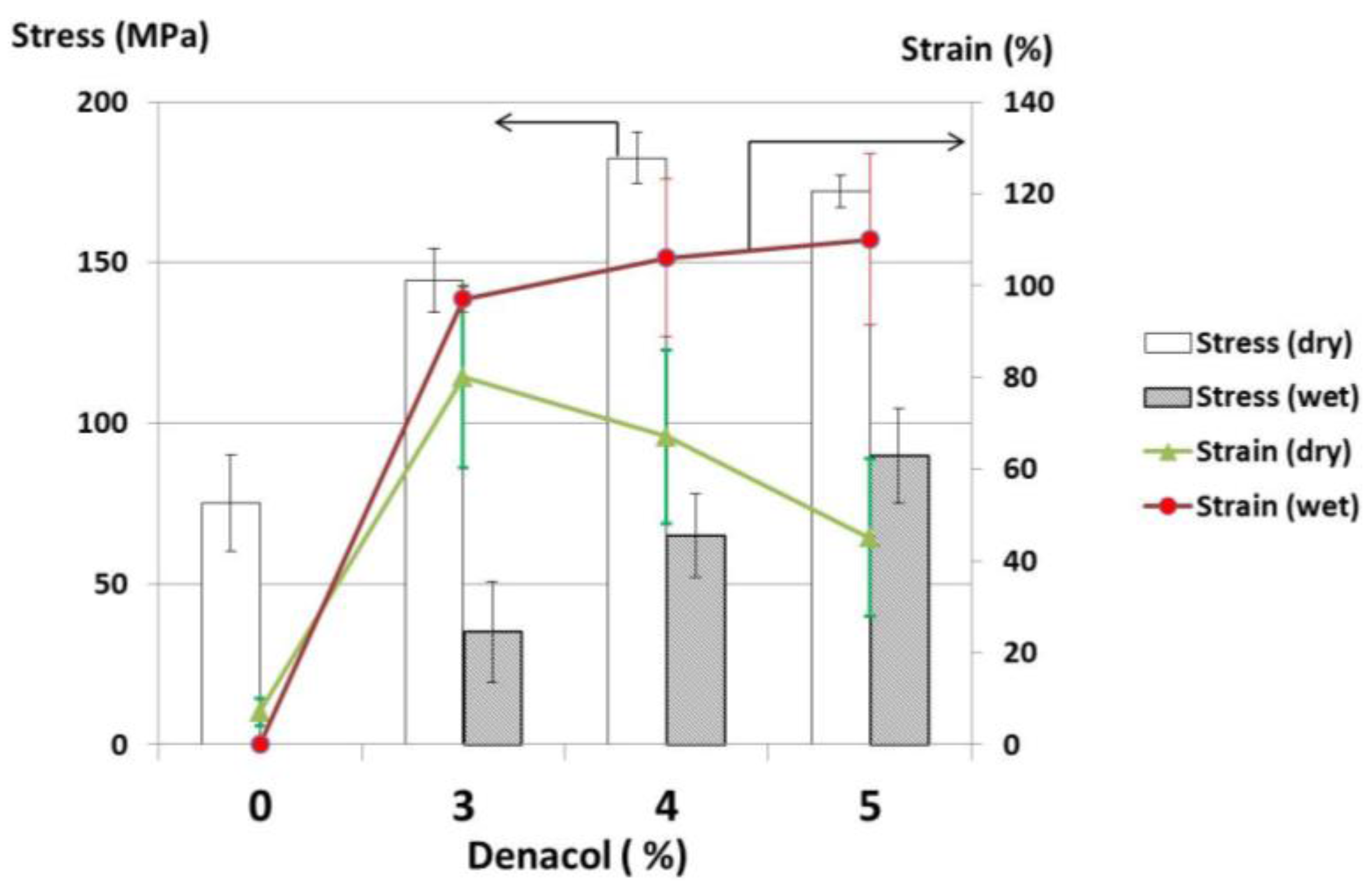
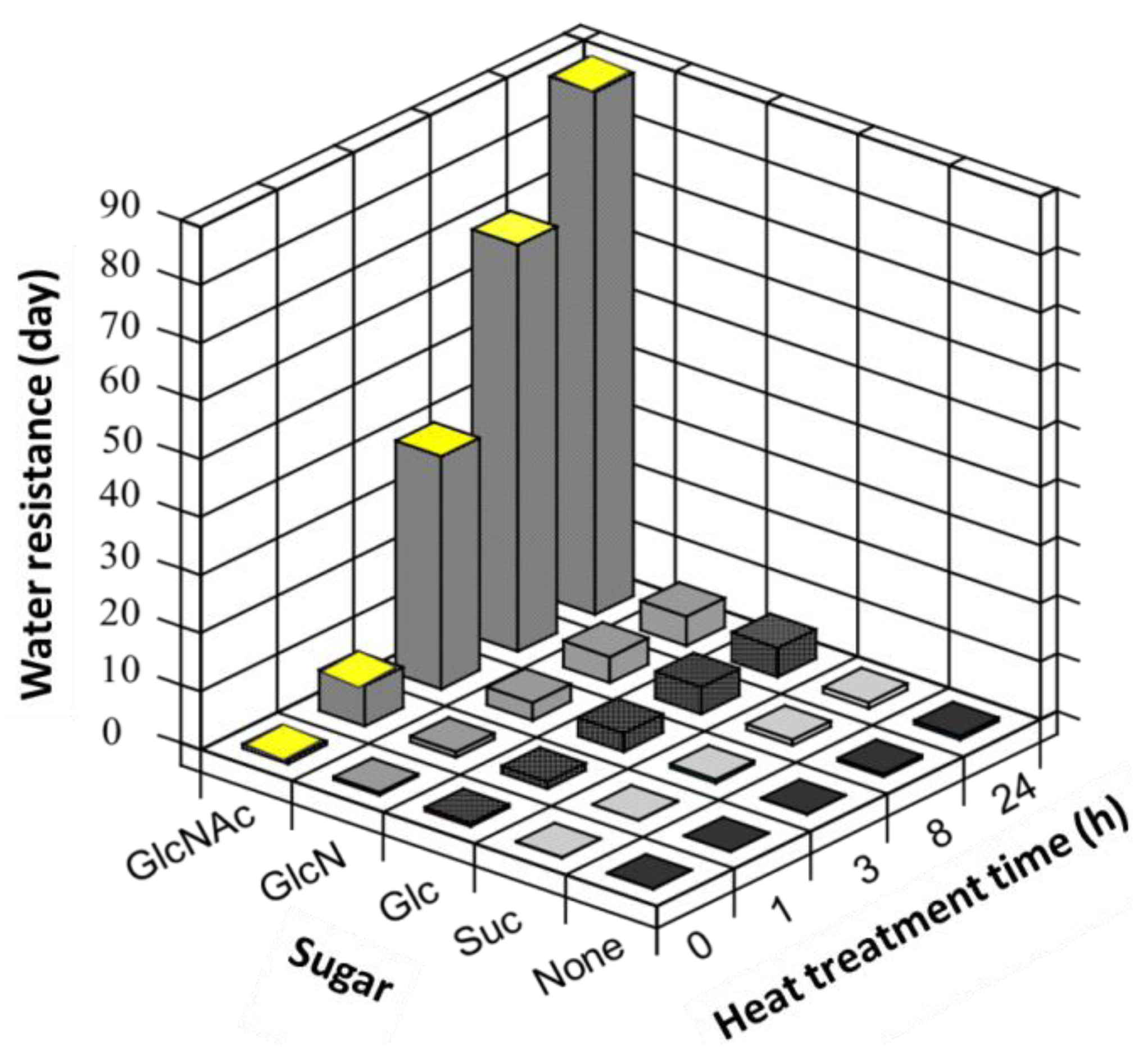
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. CRC Crit. Rev. Food Sci. Nutr. 2010, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Tokura, S.; Tamura, H.; Itoh, N. Gelatin Fiber and Process for Producing the Same. Patent WO2005,054,553, 14 June 2005. [Google Scholar]
- Fukae, R.; Midorikawa, T. Preparation of gelatin fiber by gel spinning and its mechanical properties. J. Appl. Polym. Sci. 2008, 110, 4011–4015. [Google Scholar] [CrossRef]
- Midorikawa, T.; Lawal, O.S.; Sasaki, Y.; Fukae, R. Structure and physical properties of gelatin fibers prepared by gel-spinning in ethylene glycol. J. Appl. Polym. Sci. 2012, 125, E332–E338. [Google Scholar] [CrossRef]
- Stoessel, P.R.; Raso, R.A.; Kaufmann, T.; Grass, R.N.; Stark, W.J. Fibers mechanically similar to sheep wool obtained by wet spinning of gelatin and optional plasticizers. Macromol. Mater. Eng. 2015, 300, 234–241. [Google Scholar] [CrossRef]
- Stoessel, P.R.; Krebs, U.; Hufenus, R.; Halbeisen, M.; Zeltner, M.; Grass, R.N.; Stark, W.J. Porous, water-resistant multifilament yarn spun from gelatin. Biomacromolecules 2015, 16, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Nastruzzi, C.; Davis, S.S. Sugar cross-linked gelatin for controlled release: Microspheres and disks. Biomaterials 1998, 19, 1641–1649. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Burczak, K.; Shiraki, K.; Ikada, Y. Crosslinking and biodegradation of native and denatured collagen. In Polymers of Biological and Biomedical Significance; Shalaby, S.W., Ikada, Y., Langer, R., Williams, J., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 275–286. [Google Scholar]
- Nagahama, H.; Kashiki, T.; Nwe, N.; Jayakumar, R.; Furuike, T.; Tamura, H. Preparation of biodegradable chitin/gelatin membranes with GlcNAc for tissue engineering applications. Carbohydr. Polym. 2008, 73, 456–463. [Google Scholar] [CrossRef]
- Lederer, M.O.; Gerum, F.; Severin, T. Cross-linking of proteins by Maillard processes-model reactions of d-glucosecor methylglyoxal with butylamine and guanidine derivatives. Bioorg. Med. Chem. 1998, 6, 993–1002. [Google Scholar] [CrossRef]
- Nakajima, K.; Sato, M.; Hattori, M.; Yoshida, T.; Yoshimura, K.; Takahashi, K. Soft textural and emulsifiable gelatin formed by conjugating with fatty-acylated saccharide. Biosci. Biotechnol. Biochem. 2008, 72, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Cui, C.; Ren, J.; Yang, B.; Zhao, M. Effect of xylose on the molecular and particle size distribution of peanut hydrolysate in Maillard reaction system. J. Sci. Food Agric. 2011, 91, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Masutani, E.M.; Kinoshita, C.K.; Tanaka, T.T.; Ellison, A.K.D.; Yoza, B.A. Increasing thermal stability of gelatin by UV-induced cross-linking with glucose. Int. J. Biomater. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Shen, S.H.; Lin, D.; Hata, C.; Thyagarajan, K.; Noishiki, Y.; Quijano, R.C. Fixation of bioprosthetic tissues with monofunctional and polyepoxy compounds. J. Biomed. Mater. Res. 1994, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Tseng, H.J.; Wu, M.S. Comparative in vitro evaluation of two different preparations of small diameter polyurethane vascular graft. Artif. Organs 2000, 24, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.S.M.; Sousa, F.; Gübitz, G.; Paulo, A.C. Chemical modifications on proteins using glutaraldehyde. Food Technol. Biotech. 2004, 42, 51–56. [Google Scholar]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [PubMed]
- Olde Damink, L.H.H.; Dijkstra, P.J.; van Luyn, M.J.A.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Akin, H.; Hasirci, N. Preparation and characterization of crosslinked gelatin microspheres. J. Appl. Polym. Sci. 1995, 58, 95–100. [Google Scholar] [CrossRef]
- Harland, R.S.; Peppas, N.A. Solute diffusion in swollen membranes. VII Diffusion in semicrystalline network. Colloid Polym. Sci. 1989, 267, 218–225. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tobolsky, A.V. Cross-linking of gelatine by dehydration. Nature 1967, 215, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Ruijgrok, J.M.; de Wijn, J.R.; Boon, M.E. Optimising glutaraldehyde crosslinking of collagen: Effects of time, temperature and concentrations as measured by shrinkage temperature. J. Mater. Sci. Mater. Med. 1994, 5, 80–87. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaochai, T.; Imai, Y.; Furuike, T.; Tamura, H. Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning. Fibers 2016, 4, 2. https://doi.org/10.3390/fib4010002
Chaochai T, Imai Y, Furuike T, Tamura H. Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning. Fibers. 2016; 4(1):2. https://doi.org/10.3390/fib4010002
Chicago/Turabian StyleChaochai, Thitirat, Yusuke Imai, Tetsuya Furuike, and Hiroshi Tamura. 2016. "Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning" Fibers 4, no. 1: 2. https://doi.org/10.3390/fib4010002
APA StyleChaochai, T., Imai, Y., Furuike, T., & Tamura, H. (2016). Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning. Fibers, 4(1), 2. https://doi.org/10.3390/fib4010002





