Synthesis and Characterization of Novel PVA/SiO2-TiO2 Hybrid Fibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PVA/SiO2-TiO2 Hybrid Sols
2.3. Spinnability of the Hybrid Sols and Preparation of the Hybrid Fibers
2.4. Characterizations
3. Results and Discussion
3.1. Spinnability of the Hybrid Sols
| Order | n(TEOS) (mol) | n(H2O) (mol) | n(HCl) (mol) | n(SiO2):n (TiO2) | w(PVA in the hybrid fibers) (%) |
|---|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.05 | 10 | 17 |
| 2 | 0.5 | 1 | 0.05 | 10 | 20 |
| 3 | 0.5 | 1 | 0.05 | 10 | 25 |
| 4 | 0.5 | 1 | 0.05 | 10 | 33 |
| 5 | 0.5 | 1 | 0.05 | 20 | 17 |
| 6 | 0.5 | 1 | 0.05 | 5 | 17 |
| 7 | 0.5 | 1 | 0.05 | 2.5 | 17 |
| 8 | 0.5 | 1 | 0.05 | 1.25 | 17 |
| 9 | 0 | 0 | 0 | 0 | 100 |
| Sample order | Spinnability | Spinning time (min) | Spinning length (m) |
|---|---|---|---|
| 1 | yes | 50 | 1.4 |
| 2 | yes | 148 | 1.5 |
| 3 | yes | 193 | 1.7 |
| 4 | yes | 205 | 2.5 |
| 5 | yes | 292 | 3.0 |
| 6 | yes | 35 | 1.0 |
| 7 | yes | 14 | 0.5 |
| 8 | no | 0 | 0 |
| 9 | yes | – | 2.1 |
3.2. Structure and Morphology of the Hybrid Fibers
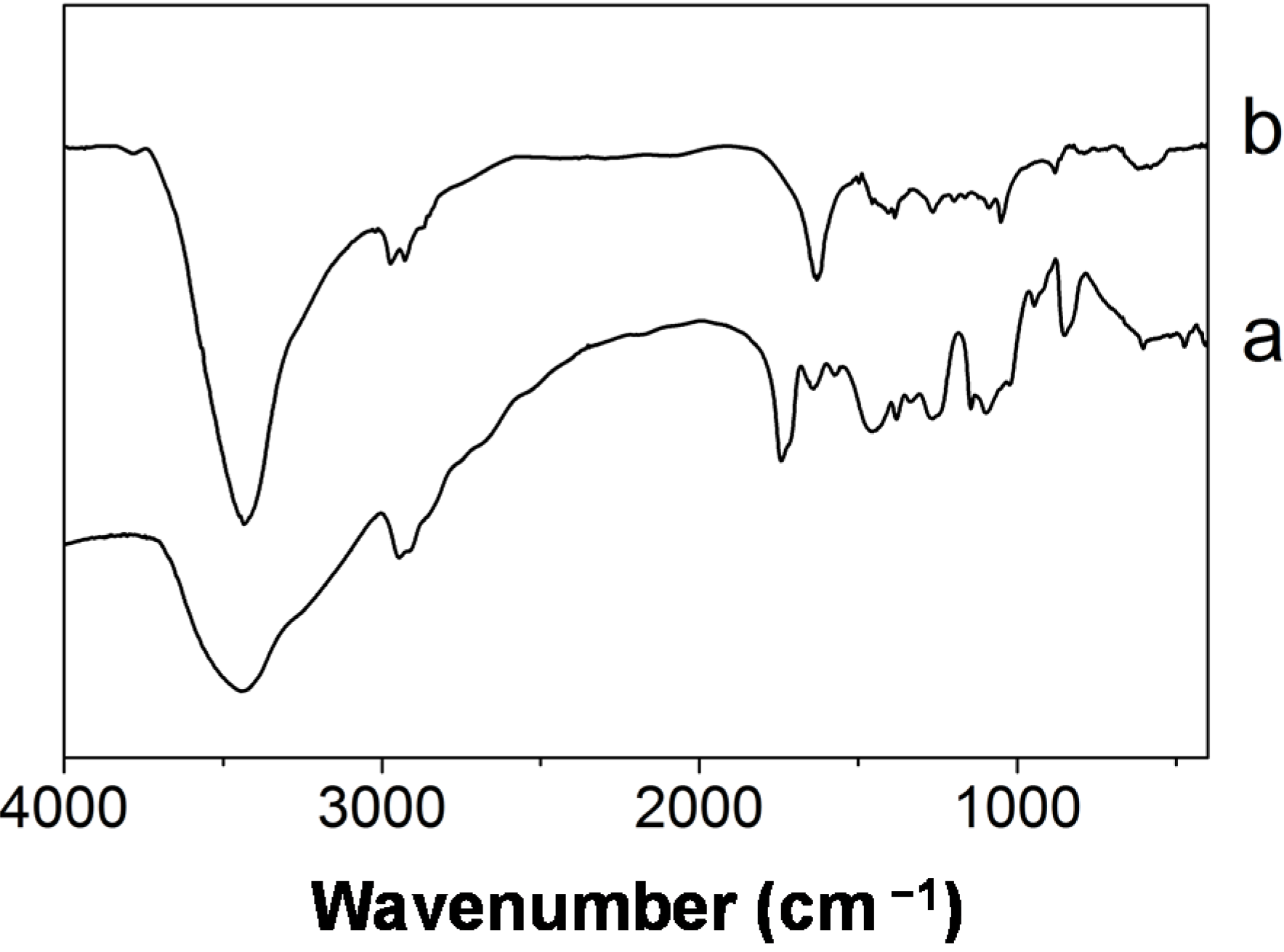
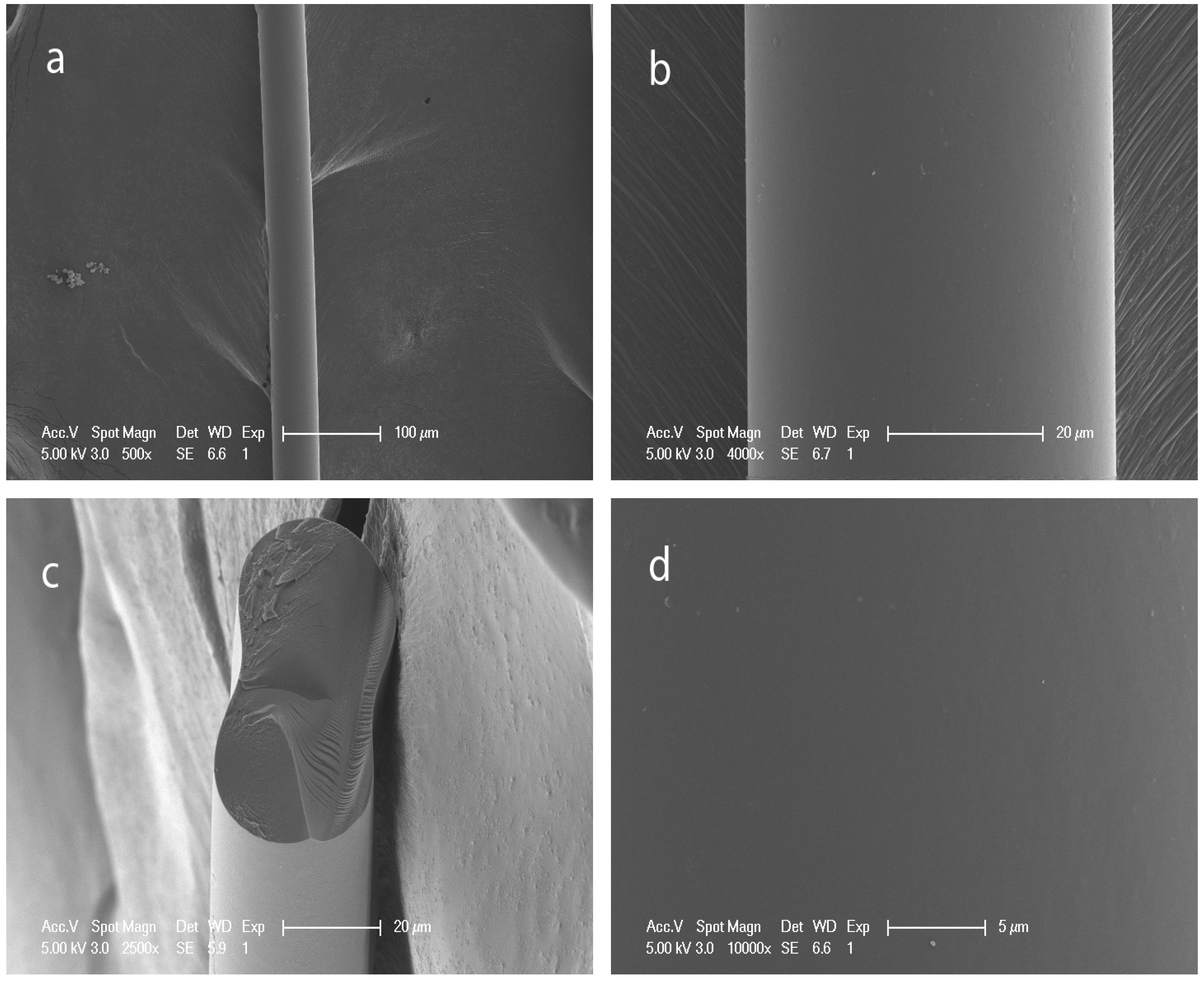

3.3. UV-shielding Properties of the Hybrid Fibers

3.4. Thermal Properties of Hybrid Fibers
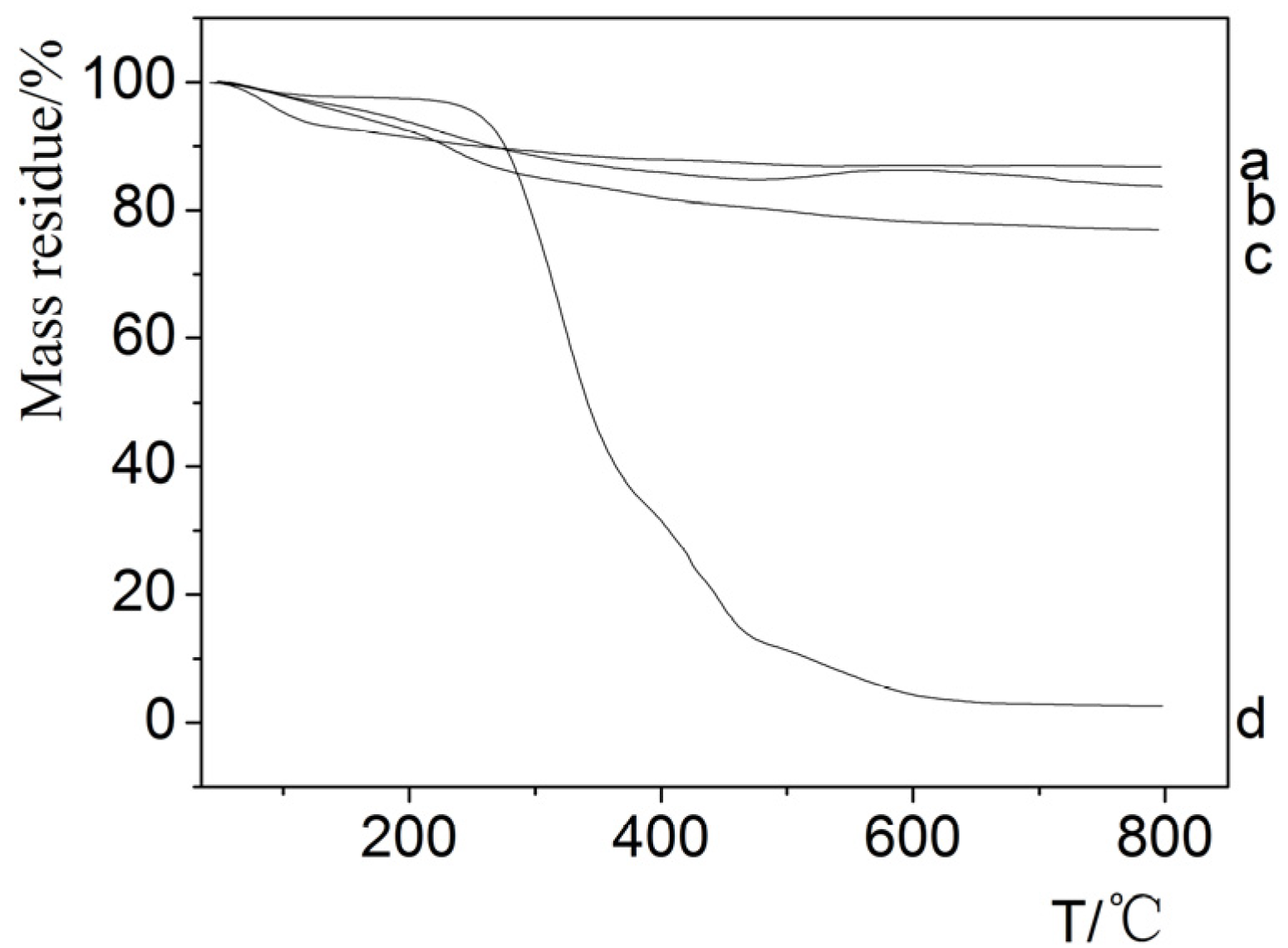
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Zhou, X.J.; Chen, Y. Titanium dioxide fibers prepared by sol-gel process and centrifugal spinning. J. Sol-Gel Sci. Technol. 2014, 71, 102–108. [Google Scholar] [CrossRef]
- Hosgor, Z.; Kayaman-Apohan, N.; Karatas, S. Nonisocyanate polyurethane/silica hybrid coatings via a sol-gel route. Adv. Polym. Technol. 2012, 31, 390–400. [Google Scholar] [CrossRef]
- You, Y.; Zhang, S.Y.; Wan, L. Preparation of continuous TiO2 fibers by sol-gel method and its photocatalytic degradation on formaldehyde. Appl. Surf. Sci. 2012, 258, 3469–3474. [Google Scholar] [CrossRef]
- Yu, L.Y.; Xu, Z.L.; Shen, H.M.; Yang, H. Preparation and characterization of PVDF-SiO2 composite hollow fiber UF membrane by sol-gel method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.J.; Li, X.L. Preparation of PVDF/Al2O3 hybrid membrane via the sol-gel process and characterization of the hybrid membrane. Desalin. Water Treat. 2013, 51, 3685–3690. [Google Scholar] [CrossRef]
- Hasegawa, I.; Fukuda, Y.; Kajiwara, M. Inorganic–organic hybrid route to synthesis of ZrC and Si–Zr–C fibres. Ceram. Int. 1999, 25, 523–527. [Google Scholar] [CrossRef]
- Leroy, C.M.; Achard, M.F.; Babot, O.; Steunou, N.; Massé, P.; Binet, L.; Brun, N.; Livage, J.; Backov, R. Designing nanotexturated vanadium oxide based microscopic fibers: Applications as alcohol sensors. Chem. Mater. 2007, 19, 3988–3999. [Google Scholar] [CrossRef]
- Serier, H.; Achard, M.-F.; Steunou, N.; Maquet, J.; Livage, J.; Leroy, C.; Babot, O.; Backov, R. Designing width and texture of vanadium oxide macroscopic fibers toward controlling their mechanical and alcohol-sensing properties. Adv. Funct. Mater. 2006, 16, 1745–1753. [Google Scholar] [CrossRef]
- Kinadjian, N.; le Bechec, M.; Prouzet, E.; Henrist, C.; Lacombe, S.; Backov, R. Varying TiO2 macroscopic fiber morphologies toward tuning their photocatalytic properties. ACS Appl. Mater. Inter. 2014, 6, 11211–11218. [Google Scholar] [CrossRef]
- Kinadjian, N.; le Bechec, M.; Pigot, T.; Dufour, F.; Bentaleb, A.; Prouzet, E.; Lacombe, S.; Backov, R. Photocatalytic TiO2 macroscopic fibers obtained through integrative chemistry. Eur. J. Inorg. Chem. 2012, 2012, 5350–5359. [Google Scholar] [CrossRef]
- Cao, J.Y.; Shi, T.J.; Wang, H.L. Study on spinnable PMMA/SiO2 hybrid solution. Polym. Mater. Sci. Eng. 2007, 23, 204–207. [Google Scholar]
- Li, D.; Mathew, B.; Mao, C. Biotemplated synthesis of hollow double-layered core/shell titania/silica nanotubes under ambient conditions. Small 2012, 8, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Siwinska-Stefanska, K.; Ciesielczyk, F.; Kolodziejczak-Radzimska, A. TiO2-SiO2 inorganic barrier composites: from synthesis to application. Pigment. Resin Technol. 2012, 41, 139–148. [Google Scholar] [CrossRef]
- Kim, E.Y.; Whang, C.M.; Lee, W.I. Photocatalytic property of SiO2/TiO2 nanoparticles prepared by sol-hydrothermal process. J. Electroceram. 2006, 17, 899–902. [Google Scholar] [CrossRef]
- Xavier Orignac, H.C.; Vasconcelos, H.C.; Du, X.M.; Almeida, R.M. Influence of solvent concentration on the microstructure of SiO2-TiO2 sol-gel films. J. Sol-Gel Sci. Technol. 1997, 8, 243–248. [Google Scholar]
- Tang, X.Y.; Yu, Y.X.; Yang, D.X. SiO2/TiO2 fibers from titanium-modified polycarbosilane. J. Membr. Sci. 2010, 45, 2670–2674. [Google Scholar]
- Gimenez, V.; Mantecon, A.; Cadiz, V. Modification of poly(vinyl alcohol) with acid chlorides and crosslinking with difunctional hardeners. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 925–934. [Google Scholar] [CrossRef]
- Krumova, M.; Lopez, D.; Benavente, R.; Perena, J.M. Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol). Polymer 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Kinadjian, N.; Achard, M.-F.; Julián-López, B.; Maugey, M.; Poulin, P.; Prouzet, E.; Backov, R. ZnO/PVA macroscopic fibers bearing anistropic photonic properties. Adv. Funct. Mater. 2012, 22, 3994–4003. [Google Scholar] [CrossRef]
- Tamaki, R.; Chujo, Y. Synthesis of poly(vinyl alcohol)/silica gel polymer hybrids by in-situ hydrolysis method. Appl. Organomet. Chem. 1998, 12, 755–762. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, W.; Zhang, L.Y.; Yao, X. New method for making porous SiO2 thin films. Thin Solid Films 1999, 353, 124–128. [Google Scholar] [CrossRef]
- Xu, Y.D.; Zhou, W.C.; Zhang, L.T.; Cheng, L.F. Spinnability and crystallizability of silica glass fiber by the sol–gel method. J. Mater. Process Technol. 2000, 101, 44–46. [Google Scholar] [CrossRef]
- Song, Q.S.; Shi, T.J.; Wang, H.L.; Hang, G.P. Preparation and characterization of continuous fibres of PVA/SiO2 hybrid by sol-gel method. Chin. Polym. Mater. Sci. Eng. 2007, 23, 216–219. [Google Scholar]
- Sheng, D.Y. The Application of IR in Polymer Research; Science Press: Beijing, China, 1988; pp. 83–88. (in Chinese) [Google Scholar]
- Koji, N.; Tomonori, Y.; Kenji, I.; Fumio, S. Properties and structure of poly (vinyl alcohol)/silica composites. J. Appl. Polym. Sci. 1999, 74, 133–138. [Google Scholar] [CrossRef]
- Zhang, L.D.; Mou, J.M. Nano-Materials Science; Liaoning Science and Technology Press: Shenyang, Liaoning, China, 1994; pp. 38–44. (in Chinese) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Shi, T.; Song, Q. Synthesis and Characterization of Novel PVA/SiO2-TiO2 Hybrid Fibers. Fibers 2014, 2, 275-284. https://doi.org/10.3390/fib2040275
Ma H, Shi T, Song Q. Synthesis and Characterization of Novel PVA/SiO2-TiO2 Hybrid Fibers. Fibers. 2014; 2(4):275-284. https://doi.org/10.3390/fib2040275
Chicago/Turabian StyleMa, Haihong, Tiejun Shi, and Qiusheng Song. 2014. "Synthesis and Characterization of Novel PVA/SiO2-TiO2 Hybrid Fibers" Fibers 2, no. 4: 275-284. https://doi.org/10.3390/fib2040275




