Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives
Abstract
:1. Introduction
- the polymerization of functional (semi)metal alkoxides, organosilanes, and macromonomers;
- the encapsulation of organic components in sol-gel derived organo-silicas or hybrid metal oxides; and
- the use of such different strategies as templated or self-assembly, nano-building block approaches, microporous metal organic frameworks, integrative synthesis, or coupled processes and so on.
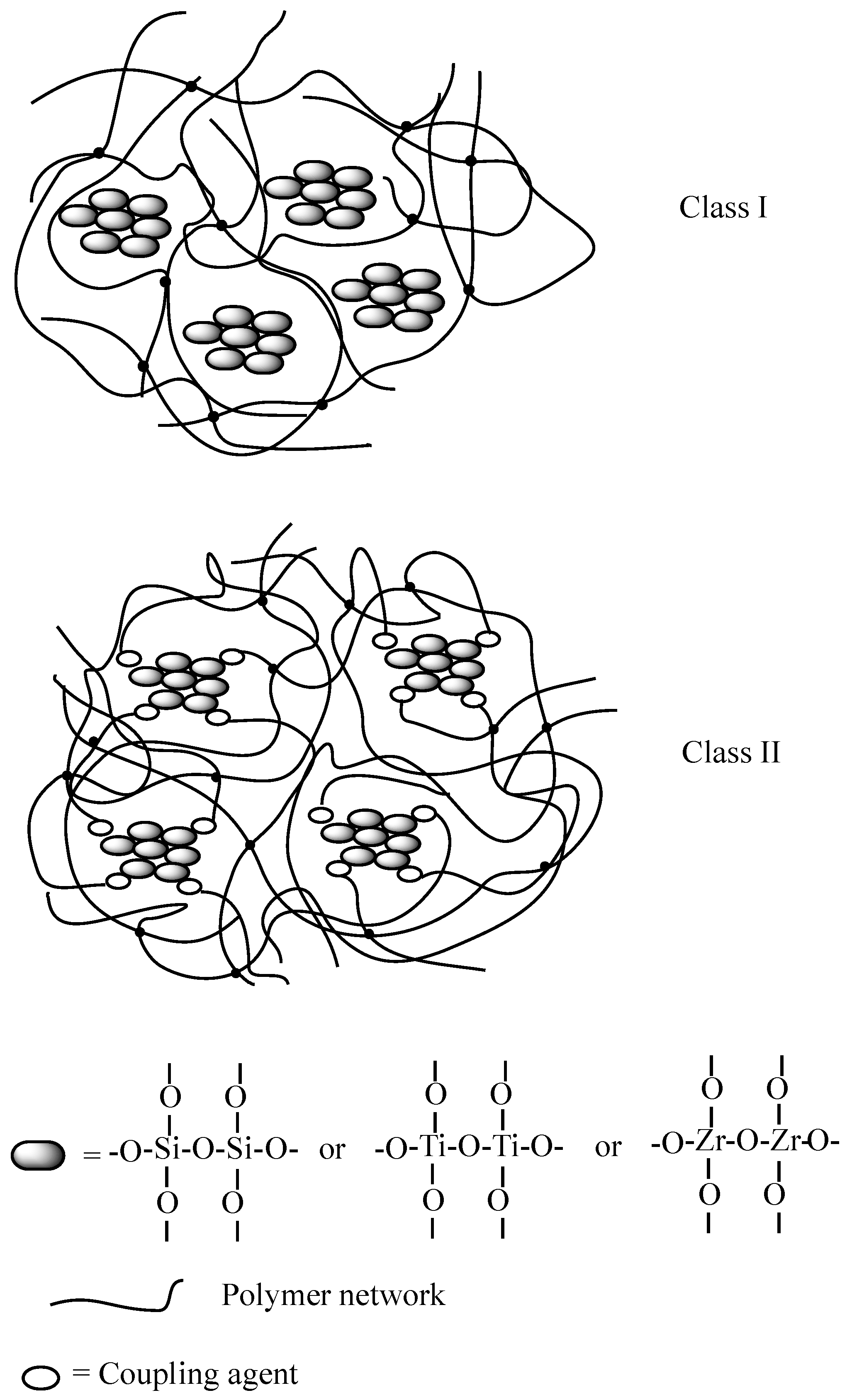
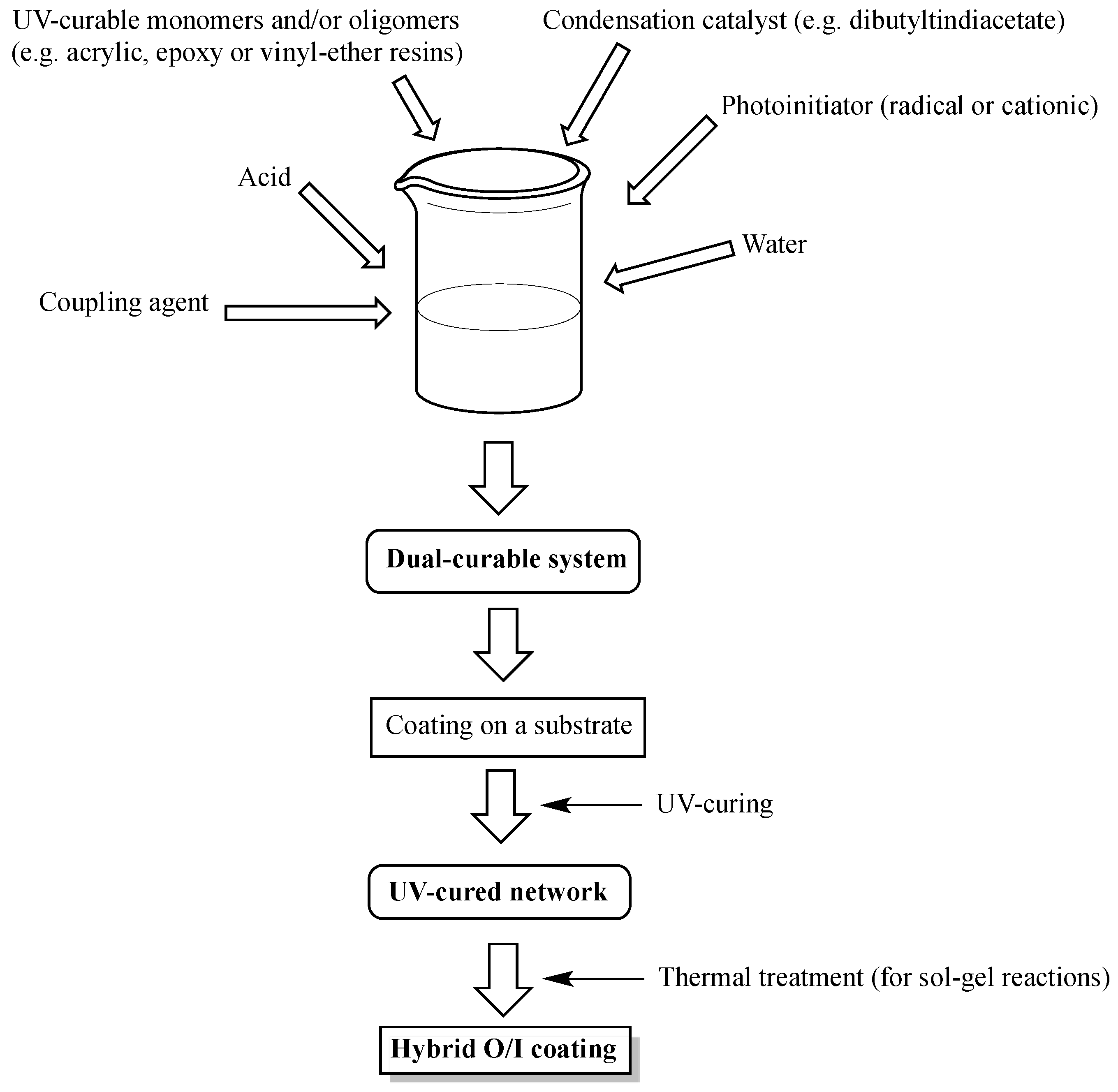
2. Design of Dual-Cured Hybrid O/I Systems
- Structure of the monomers/oligomers employed in the formulation of the UV-curable systems: indeed, the flexibility and mechanical properties of the final obtained hybrid coating will be affected by the crosslinking density of the polymer network, regardless of the presence of the inorganic domains formed after the sol-gel reactions, which could further limit the mobility of the polymer segments.
- Coupling agent: allows covalently linking the organic and inorganic phases, hence favoring the creation of strong interfaces in between the organic and inorganic parts.
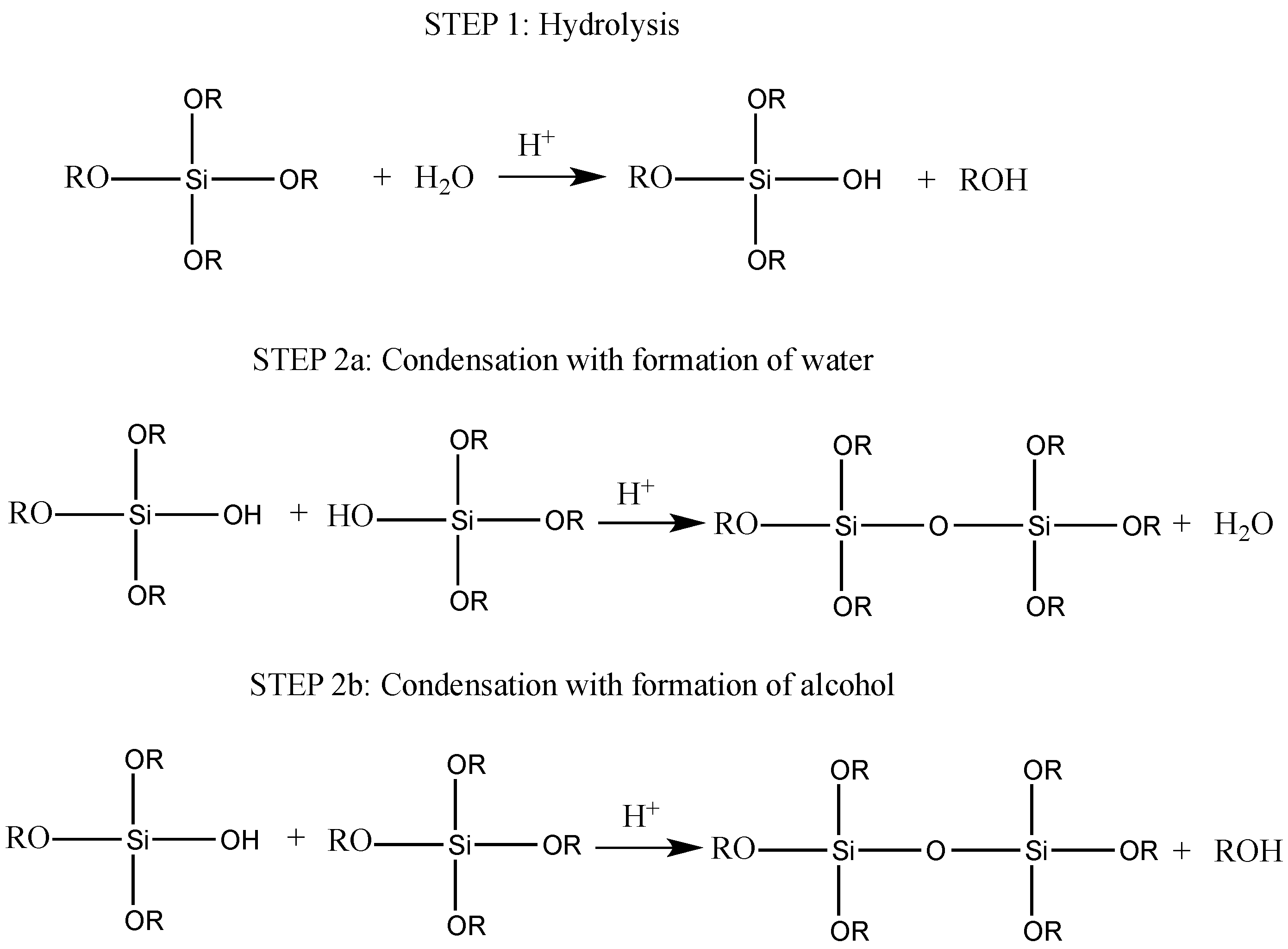
- Concentration and type of (semi)metal alkoxides: they affect the rates of the both hydrolysis and condensation reactions, hence giving rise to the formation of reactive species at different rates.
- pH: the microstructure of the oxidic phases obtained by sol-gel process depends on the hydrolysis and condensation reactions that are generally controlled by the solution pH. More specifically, under acid-catalyzed conditions, the hydrolysis kinetics is favored instead of the condensation, which usually starts when hydrolysis is completed. Conversely, in alkali-catalyzed reactions, condensation is faster than hydrolysis, resulting in the formation of highly condensed species that may agglomerate into fine particles [25].
- Water/precursor molar ratio: the water amount in the UV-cured system that has to undergo the sol-gel process significantly influences the hydrolysis and condensation kinetics of this latter. In particular, at fixed inorganic precursor concentration, an increase in water content leads to a corresponding increase in hydrolysis and condensation rate.
- Presence of sol-gel catalysts: this allows speeding up the sol-gel reactions, hence promoting the formation of hybrid O/I coatings with stable properties and, thus, suitable for practical uses.
3. Recent Examples of Dual-Cured Hybrid O/I Systems
4. Conclusions
Conflicts of Interest
References
- Drisko, G.L.; Sanchez, C. Hybridization in materials science—Evolution, current state, and future aspirations. Eur. J. Inorg. Chem. 2012, 2012, 5097–5105. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J. Chem. 1994, 18, 1007–1047. [Google Scholar]
- Yano, S.; Iwata, K.; Kurita, K. Physical properties and structure of organic-inorganic hybrid materials produced by sol-gel process. Mater. Sci. Eng. C 1998, 6, 75–90. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Gomez-Ribero, P.; Sanchez, C. Functional Hybrid Materials; Wiley-VCH: Wienheim, Germany, 2004. [Google Scholar]
- Mascia, L. Developments in Organic-Inorganic Polymeric Hybrids: Ceramers. Trends Polym. Sci. 1995, 3, 61–66. [Google Scholar]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Nicole, L.; Laberty-Robert, C.; Rozes, L.; Sanchez, C. Hybrid materials science: A promised land for the integrative design of multifunctional materials. Nanoscale 2014, 6, 6267–6292. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Hybrid Materials; Wiley-VCH: Wienheim, Germany, 2003. [Google Scholar]
- Letailleur, A.A.; Ribot, F.; Boissière, C.; Teisseire, J.; Barthel, E.; Desmazieres, B.; Chemin, N.; Sanchez, C. Sol-gel derived hybrid thin films: The chemistry behind processing. Chem. Mater. 2011, 23, 5082–5089. [Google Scholar] [CrossRef]
- Hajji, P.; David, L.; Gerard, J.F.; Pascault, J.P.; Vigier, G. Synthesis, structure, and morphology of polymer–silica hybrid nanocomposites based on hydroxyethyl methacrylate. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3172–3179. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Xu, J.; Baran, G.; Qiu, K.Y. Mechanical properties of interface-free polyacrylate-silica hybrid sol-gel materials for potential dental applications. Polym. Adv. Technol. 2001, 12, 361–368. [Google Scholar] [CrossRef]
- Wu, K.H.; Chang, T.C.; Wang, Y.T.; Chiu, Y.S. Organic-inorganic hybrid materials. I. Characterization and degradation of poly(imide-silica) hybrids. J. Polym. Sci. Polym. Chem. 1999, 37, 2275–2284. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Yin, J.; Qi, Z. Preparation and properties of organosoluble polyimide/silica hybrid materials by sol-gel process. J. Appl. Polym. Sci. 1999, 73, 2977–2984. [Google Scholar]
- Innocenzi, P.; Brusatin, G.; Babonneau, F. Competitive polymerization between organic and inorganic networks in hybrid materials. Chem. Mater. 2000, 12, 3726–3732. [Google Scholar] [CrossRef]
- Malucelli, G. Preparation and characterization of nanocomposite coatings obtained through UV-curing processes. In Nanocomposites: Synthesis, Characterization and Applications; Wang, X., Ed.; NOVA Science Publishers, Inc.: New York, NY, USA, 2011; pp. 139–167. [Google Scholar]
- Cho, J.; Ju, H.; Hong, J. Photocuring kinetics of UV-initiated free-radical photopolymerizations with and without silica nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 658–670. [Google Scholar] [CrossRef]
- Malucelli, G.; Priola, A.; Sangermano, M.; Amerio, E.; Zini, E.; Fabbri, E. Hybrid nanocomposites containing silica and PEO segments: Preparation through dual-curing process and characterization. Polymer 2005, 46, 2872–2879. [Google Scholar] [CrossRef]
- Amerio, E.; Sangermano, M.; Malucelli, G.; Priola, A.; Voit, B. Preparation and characterization of hybrid nanocomposite coatings by photopolymerization and sol-gel process. Polymer 2005, 46, 11241–11246. [Google Scholar] [CrossRef]
- Innocenzi, P.; Brusatin, G. A comparative FTIR study of thermal and photo-polymerization processes in hybrid sol-gel films. J. Non-Cryst. Solids 2004, 333, 137–142. [Google Scholar] [CrossRef]
- Chou, T.P.; Chandrasekaran, C.; Cao, G.Z. Sol-gel-derived hybrid coatings for corrosion protection. J. Sol-Gel Sci. Technol. 2003, 26, 321–327. [Google Scholar] [CrossRef]
- Van Bommel, M.J.; ten Wolde, P.M.C.; Bernards, T.N.M. The influence of methacryloxypropyltrimethoxysilane on the sol-gel process of TEOS. J. Sol-Gel Sci. Technol. 1994, 2, 167–170. [Google Scholar] [CrossRef]
- Flores, M.; Foix, D.; Serra, A.; Ramis, X.; Sangermano, M. A versatile thiol-ene/sol-gel two-stage curing process based on a hyperbranched polyester with different degrees of 10-undecenoyl modification. Macromol. Mater. Eng. 2014, 299, 495–503. [Google Scholar] [CrossRef]
- Chiang, C.; Ma, C.M.; Wu, D.L.; Kuan, H.C. Preparation, characterization, and properties of novolac-type phenolic/SiO2 hybrid organic-inorganic nanocomposite materials by sol-gel method. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 905–913. [Google Scholar] [CrossRef]
- Mammeri, F.; Le Bourhis, E.; Rozesa, L.; Sanchez, C. Mechanical properties of hybrid organic-inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]
- Cakmakci, E.; Altıntas, Z.; Kahraman, M.V.; Apohan, N.K. Fluorine-containing photocurable hybrid coatings via anhydrous sol-gel method. J. Vinyl Additive Technol. 2015, 21, 272–277. [Google Scholar] [CrossRef]
- Copuroglu, M.; Sen, M. Synthesis and characterization of a Zr-containing silicate-based epoxy-functional polymer nanocomposite system. Polym. Eng. Sci. 2015, 55, 792–798. [Google Scholar] [CrossRef]
- Ni, L.; Chemtob, A.; Croutxé-Barghorn, C.; Moreau, N.; Bouder, T.; Chanfreau, S.; Pébère, N. Direct-to-metal UV-cured hybrid coating for the corrosion protection of aircraft aluminium alloy. Corros. Sci. 2014, 89, 242–249. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F. Cotton and polyester surface modification by methacrylic silane and fluorinated alkoxysilane via sol-gel and UV-curing coupled process. Surf. Coat. Technol. 2015, 271, 165–173. [Google Scholar] [CrossRef]
- ISO 105-C01:1989 Textiles—Tests for Colour Fastness—Part C01: Colour Fastness to Washing: Test 1; ISO: Geneva, Switzerland, 1989.
- Sangermano, M.; El Sayed, H.; Voit, B. Ethoxysilyl-modified hyperbranched polyesters as multifunctional coupling agents for epoxy-silica hybrid coatings. Polymer 2011, 52, 2103–2109. [Google Scholar] [CrossRef]
- Gigot, A.; Sangermano, M.; Capozzi, L.C.; Dietliker, K. In-situ synthesis of organic-inorganic coatings via a photolatent base catalyzed Michael-addition reaction. Polymer 2015, 68, 195–201. [Google Scholar] [CrossRef]
- Belon, C.; Schmitt, M.; Bistac, S.; Croutxé-Barghorn, C.; Chemtob, A. Friction and wear properties of hybrid sol-gel nanocomposite coatings against steel: Influence of their intrinsic properties. Appl. Surf. Sci. 2011, 257, 6618–6625. [Google Scholar] [CrossRef]
- Xie, H.; Shi, W. Polymer/SiO2 hybrid nanocomposites prepared through the photoinitiator-free UV curing and sol-gel processes. Compos. Sci. Technol. 2014, 93, 90–96. [Google Scholar] [CrossRef]
- Basturk, E.; Inan, T.; Güngor, A. Flame retardant UV-curable acrylated epoxidized soybean oil based organic–inorganic hybrid coating. Prog. Org. Coat. 2013, 76, 985–992. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Frigione, M. Novel hydrophobic free-solvent UV-cured hybrid organic–inorganic methacrylic-based coatings for porous stones. Prog. Org. Coat. 2014, 77, 803–812. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Frigione, M. Organic–inorganic UV-cured methacrylic-based hybrids as protectivecoatings for different substrates. Prog. Org. Coat. 2014, 77, 1117–1125. [Google Scholar] [CrossRef]
- Sen, F.; Kahraman, M.V. Hybrid dual-curable cyanate ester/boron phosphate composites via sequential thiol-ene photopolymerization and thermal polymerization. Prog. Org. Coat. 2014, 77, 1053–1062. [Google Scholar] [CrossRef]
- Li, C.; Cheng, J.; Yang, F.; Chang, W.; Nie, J. Preparation and Characterization of UV-Cured Hybrid Coatings by Triethoxysilane-Modified Dimethacrylate Based on Bisphenol-S Epoxy. J. Appl. Polym. Sci. 2013, 129, 2189–2195. [Google Scholar] [CrossRef]
- Roppolo, I.; Messori, M.; Perruchas, S.; Gacoin, T.; Boilot, J.P.; Sangermano, M. Multifunctional luminescent organic/inorganic hybrid films. Macromol. Mater. Eng. 2012, 297, 680–688. [Google Scholar] [CrossRef]
- Bi, Y.T.; Li, Z.J.; Liang, W. Preparation and characterization of epoxy/SiO2 nanocomposites by cationic photopolymerization and sol-gel process. Polym. Adv. Technol. 2014, 25, 173–178. [Google Scholar] [CrossRef]
- Chibac, A.L.; Melinte, V.; Buruiana, T.; Mangalagiu, I.; Buruiana, E.C. Preparation of photocrosslinked sol-gel composites based on urethane- acrylic matrix, silsesquioxane sequences, TiO2, and Ag/Au nanoparticles for use in photocatalytic applications. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1189–1204. [Google Scholar] [CrossRef]
| Formulation of the Organic Part | Inorganic Precursors | Highlights | Applications | Ref. |
|---|---|---|---|---|
| Hexanediol diacrylate; Aliphatic polyester urethane diacrylate; Trifunctional polyester acrylate; Fluoroacrylate | Methacryloxymethyltriethoxysilane | -increased flame retardancy; -hydrophobicity; -good optical properties | Transparent protective coatings for flammable thermoplastic substrates | [26] |
| Methacrylic acid | 3-glycidyloxypropyltrimethoxysilane; Zirconium (IV) n-propoxide; Tetraethoxysilane; Dimethyldiethoxysilane | -enhanced mechanical properties; -high thermal stability | Dental restoration/adhesion | [27] |
| Diglycidyl ether of bisphenol A | n-alkyltrimethoxysilane precursors | -enhanced corrosion protection | Protective coatings | [28] |
| Perfluoropolyether ethoxysilane-terminated | 3-(trimethoxysilyl)propyl methacrylate | -hydrorepellency and oleorepellency | Hydro and oil repellent finishing treatment for fabrics (cotton and polyester) | [29] |
| Ethoxysilyl-modified hyperbranched aliphatic-aromatic polyesters | Tetraethoxysilane | -improved thermo-mechanical behavior; -increased surface hardness; -high transparency | Toughened protective coatings | [30] |
| Hexafunctional aliphatic polyester-acrylate | Tetraethoxysilane; 3-acryloxypropyl-trimethoxysilane | -improved scratch resistance | Protective coatings | [31] |
| Diglycidyl ether bisphenol A | 3-(glycidyloxypropyl)trimethoxysilane [2-3,4-Epoxycyclohexylethyl]trimethoxysilane | -improved wear resistance of steel substrates; -improved mechanical properties | Protective coatings | [32] |
| 2-Hydroxyethyl acrylate; 1,6-Hexanediol diacrylate; Bisphenol A epoxy acrylate (containing 25 wt.%; tripropyleneglycol diacrylate) | 3-triethoxysilylpropylamine; Tetraethoxysilane | -high transparency; -enhanced thermal and mechanical properties | Protective coatings | [33] |
| Hexanediol diacrylate; Acrylated soybean oil | Methacryloxypopyltrimethoxysilane; Tetraethoxysilane | -hydrophobicity; -improved mechanical properties; -enhanced thermal stability; -enhanced flame retardancy | Protective coatings | [34] |
| Trimethylolpropane trimethacrylate; Polydimethylsiloxane | Trimethoxypropyl silane methacrylate; 3-mercaptopropyltriethoxysilane | -water repellency; -high transpirability | Cultural heritage protection | [35,36] |
| 2,2-Diallylbisphenol A, trimethylolpropane tris(3-mercaptopropionate) | 3-mercaptopropyltrimethoxysilane | -improved thermal stability; -enhanced flame retardancy; -hydrophobicity | Protective coatings | [37] |
| Bisphenol-S epoxy dimethacrylate | 3-isocyanatopropyltriethoxysilane | -enhancements of hardness and gloss; -improved thermal stability | Protective coatings | [38] |
| Bisphenol A ethoxylatediacrylate | Tetraethoxysilane | -enhanced scratch resistance; -high transparency | Light-emitting coatings | [39] |
| Bisphenol A epoxy resin | 3-isocyanatopropyltriethoxysilane; Tetraethoxysilane | -improved thermal stability; -enhanced impact strength and dynamic-mechanical behavior | Protective coatings | [40] |
| 2-hydroxyethylmethacrylate; Oligodimethacrylate based on poly(ethylene glycol); 3-(acryloyloxy)-2-hydroxy-propyl methacrylate | 3-trimethoxysilylpropyl methacrylate; Titanium (IV) butoxide | -increased thermal stability; -improved tensile strength; -efficiency in removing phenolic compounds from water | Protective photocatalytic coatings | [41] |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malucelli, G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings 2016, 6, 10. https://doi.org/10.3390/coatings6010010
Malucelli G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings. 2016; 6(1):10. https://doi.org/10.3390/coatings6010010
Chicago/Turabian StyleMalucelli, Giulio. 2016. "Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives" Coatings 6, no. 1: 10. https://doi.org/10.3390/coatings6010010






