3.2. Cure in a Convection Oven
Once the co-monomer composition was set for the resin system under study, a DEA experiment was conducted in order to determine the optimal cure time for the resin.
Figure 2 shows the curve of log ion viscosity
vs. time for the cure carried out in a convection oven at 140 °C. As mentioned previously in the text, this temperature was chosen based on previously published data of related resins [
11,
30], and preliminary tests. According to the sample preparation method employed in this work, it is expected that polymer chain growth starts immediately after mixing the resin components with BF
3·OEt
2, following a cationic polymerization mechanism that predominates during the initial 30 min resting time that precedes heating. Because no effort has been made to suppress oxygen from the capped vials used, an auto-oxidation process triggered by the presence of oxygen at high temperatures cannot be excluded under the conditions employed [
39], especially during longer reaction times. As the temperature is increased to 140 °C, it is possible that both cationic and free radical reactions coexist in the system, contributing to the final cure of the thermoset. Indeed, the thermal polymerization of similar tung oil-based resins in the absence of cationic or free radical initiators has been demonstrated and addressed previously in the literature [
11].
Figure 2.
Dielectric analysis (DEA) of the cure of a tung oil-based thermosetting resin at 140 °C. The resin is composed of 70 wt % of tung oil, 15 wt % of divinylbenzene (DVB), 10 wt % of styrene (ST), 3 wt % of soybean oil, and 2 wt % of BF3·OEt2.
Figure 2.
Dielectric analysis (DEA) of the cure of a tung oil-based thermosetting resin at 140 °C. The resin is composed of 70 wt % of tung oil, 15 wt % of divinylbenzene (DVB), 10 wt % of styrene (ST), 3 wt % of soybean oil, and 2 wt % of BF3·OEt2.
After initial heating (first 45 min. not shown), the growth of polymer chains predominates in the system, and a steady increase in the ion viscosity, due to the lower mobility of the resin components, is noticed during the following 30 min (
Figure 2). After 1 h and 15 min, the increase in ion viscosity slows down gradually until the resin is fully cured and the ion viscosity plateaus. The complete polymerization of the resin is attained after 8 h. However, the change in ion viscosity between 6 and 8 h is negligible for practical purposes, and, as will be shown next with the DSC results, further cure of the system can’t be detected after 6 h.
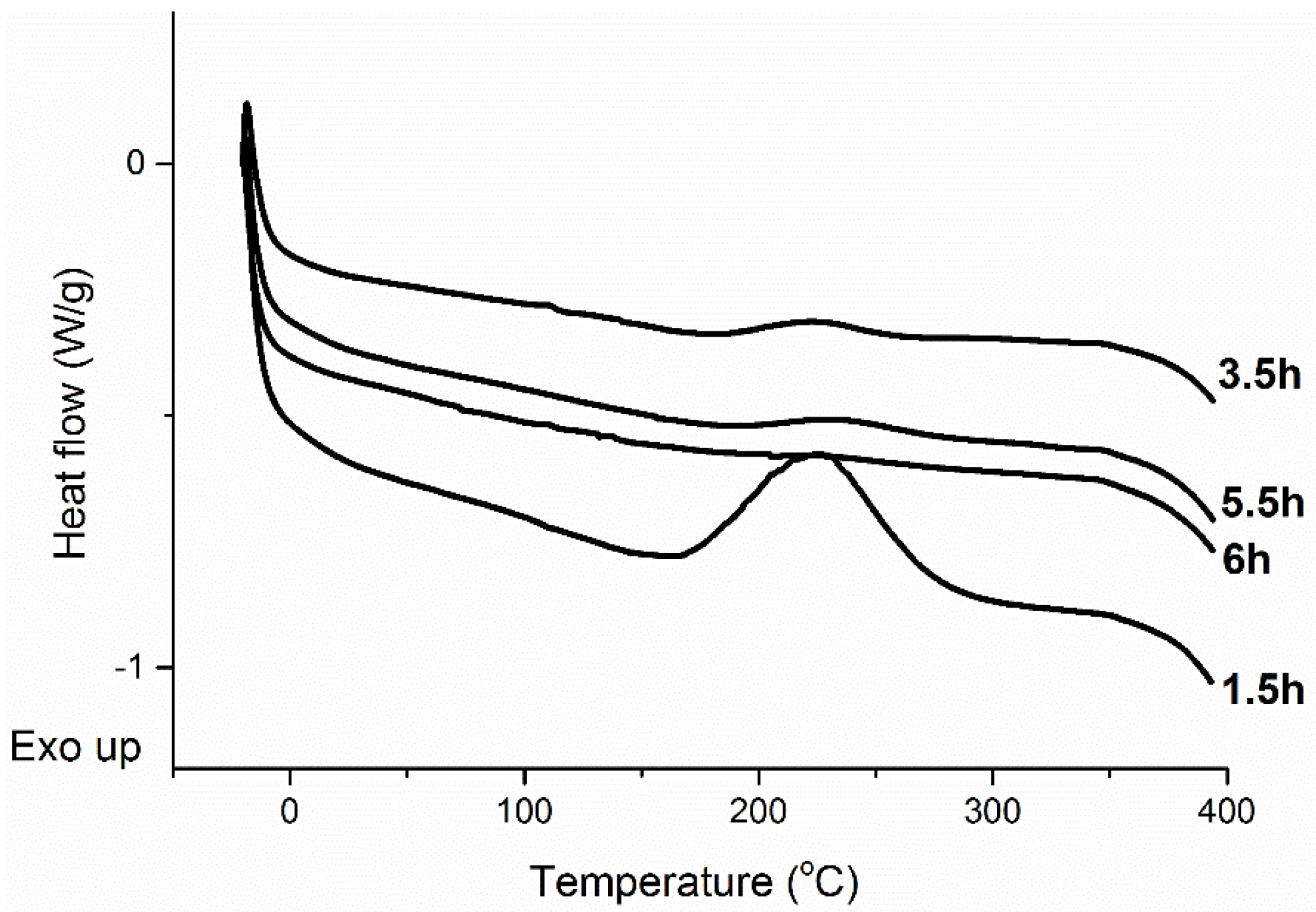
In order to confirm the ideal cure time, sample mixtures were prepared and heated at 140 °C in a convection oven, for different times. Each one of these samples was analyzed by DSC in order to check for residual cure of the polymer. Indeed, the eventual residual cure of a sample leads to an exotherm at approximately 230 °C.
Figure 3 presents the DSC curves of samples cured for 1.5 h, 3.5 h, 5.5 h, and 6 h. From the curve of the sample cured for 1.5 h, an obvious exothermic peak is seen at approximately 230 °C (
Figure 3). The area under that peak clearly decreases with increases in cure time. Indeed, a clearly lower area is observed, at the same temperature, for the sample cured for 3.5 h. The trend is maintained with the sample cured for 5.5 h, but when the resin is heated for 6 h, no exothermic peaks could be detected by the DSC. Therefore, the DSC data confirms the results obtained from the DEA, and 6 h was selected as the ideal heating time for the preparation of the tung oil thermosetting resins reported here. The more discrete transitions occurring at other temperatures will be discussed later in the text, when analyzing the DMA data. A quick analysis of
Figure 3 suggests that the cure behavior matches a self-catalyzed process. Further kinetic experiments are necessary in order to fully elucidate the cure mechanism.
Figure 3.
Differential scanning calorimetry (DSC) curves of tung oil-based thermosetting resins cured for 1.5 h, 3.5 h, 5.5 h, and 6 h at 140 °C. The resin is composed of 70 wt % of tung oil, 15 wt % of DVB, 10 wt % of ST, 3 wt % of soybean oil, and 2 wt % of BF3·OEt2.
Figure 3.
Differential scanning calorimetry (DSC) curves of tung oil-based thermosetting resins cured for 1.5 h, 3.5 h, 5.5 h, and 6 h at 140 °C. The resin is composed of 70 wt % of tung oil, 15 wt % of DVB, 10 wt % of ST, 3 wt % of soybean oil, and 2 wt % of BF3·OEt2.
3.3. Microwave Cure in the Presence of CNTs
One of the most common limitations for the preparation of good CNT composites is the tendency for agglomeration and precipitation of this carbon species in a variety of solvents. It can be seen in
Figure 4, however, that a negligible precipitation of CNTs is observed after 60 min of its suspension in tung oil and in the crude resin (
Figure 4). When DMSO is used as the solvent, CNTs start precipitating almost instantly after suspension, and the majority of CNTs have completely precipitated after 60 min (
Figure 4). Besides the higher viscosity of tung oil in comparison to DMSO, which helps to keep the CNTs suspended, it is believed that the polarity of the solvent greatly affects its interaction with the CNTs. In the case of triglycerides, the long chain fatty acids confer a strong non-polar character to the molecule, which favors van der Waals interactions with CNTs, helping to achieve a good dispersion for at least 60 min in the crude resin. In this project, achieving a good dispersion of CNTs in vegetable oil is crucial for maximizing the heat transfer from CNTs to the resin under microwaves. As indicated in
Figure 4 and as suggested by the examination of composites after the cure (
Figure 5—discussed later in the text), the CNT suspensions generated in this work are stable enough for sufficient time to allow for consolidation of the thermoset, potentially resulting in a composite with well-dispersed CNTs. When the initiator for the cationic polymerization is added to the crude mixture of co-monomers and CNTs, gellation happens within 30 min, potentially maintaining the original suspension of CNTs. During the subsequent cure stages, the polymer is consolidated and a final material, which most likely contains evenly dispersed CNTs, is obtained. Indications of a good CNT dispersion in the final composites can be seen in
Figure 5.
The extraordinary heat generated by the microwave irradiation of CNTs is the result of localized Joule heating from the interaction of RF electromagnetic waves and the nanostructured carbon material.
Table 1 shows the heating rates obtained for suspensions of CNTs in tung oil irradiated under different microwave powers. As expected, an increase in microwave power results in an increase of the heating rates, regardless of the CNT concentration (
Table 1). Moreover, for microwave powers between 100 and 500 W, increasing the CNT concentration results in an increase of the heating rates. It is interesting to notice, however, that a greater increase in the heating rate with CNT concentration, in proportional terms, is observed at lower powers. For instance, when irradiated at 100 W, the measured heating rate of a suspension with 0.25 wt % of CNT is approximately 1.8 times higher than that of pure tung oil (
Table 1). At 500 W, the heating rate of a suspension with 0.25 wt % of CNTs is only approximately 1.3 times higher than that of pure tung oil (
Table 1). The heating rates discussed here were determined in a short time interval in comparison to the cure of the tung oil resins discussed next. It is possible, therefore, that over the course of the extended cure times under microwaves, the average heating rates may differ from the ones reported in
Table 1. However, the results in
Table 1 are still representative of the effect of adding CNTs to triglyceride-based resins cured under microwaves.
Figure 4.
Settling tests comparing suspensions of carbon nanotubes (CNTs) in tung oil, dimethyl sulfoxide (DMSO), and the crude monomer mixture comprised of 1.4 g of tung oil, 0.2 g of divinylbenzene, and 0.3 g of styrene. (a) CNTs in DMSO after resting for 5 min; (b) CNTs in tung oil after resting for 5 min; (c) CNTs in the crude monomer mixture after resting for 5 min; (d) CNTs in DMSO after resting for 60 min; (e) CNTs in tung oil after resting for 60 min; and (f) CNTs in the crude monomer mixture after resting for 60 min.
Figure 4.
Settling tests comparing suspensions of carbon nanotubes (CNTs) in tung oil, dimethyl sulfoxide (DMSO), and the crude monomer mixture comprised of 1.4 g of tung oil, 0.2 g of divinylbenzene, and 0.3 g of styrene. (a) CNTs in DMSO after resting for 5 min; (b) CNTs in tung oil after resting for 5 min; (c) CNTs in the crude monomer mixture after resting for 5 min; (d) CNTs in DMSO after resting for 60 min; (e) CNTs in tung oil after resting for 60 min; and (f) CNTs in the crude monomer mixture after resting for 60 min.
Figure 5.
Tung oil-based resin bearing 0.25 wt % of CNTs and cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 1 h 30 min post-cure step at 140 °C in a convection oven analyzed by: (A) TEM; (B) SEM; and (C) optical microscopy at 10× magnification.
Figure 5.
Tung oil-based resin bearing 0.25 wt % of CNTs and cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 1 h 30 min post-cure step at 140 °C in a convection oven analyzed by: (A) TEM; (B) SEM; and (C) optical microscopy at 10× magnification.
Table 1.
Heating rates of carbon nanotubes (CNTs) in tung oil. Values are presented in °C s−1.
Table 1.
Heating rates of carbon nanotubes (CNTs) in tung oil. Values are presented in °C s−1.
| Power (W) | CNT Concentration |
|---|
| 0.0 wt % | 0.1 wt % | 0.25 wt % |
|---|
| 100 | 0.31 ± (0.04) | 0.35 ± (0.06) | 0.56 ± (0.02) |
| 200 | 0.65 ± (0.19) | 0.80 ± (0.02) | 1.17 ± (0.06) |
| 300 | 1.13 ± (0.04) | 1.24 ± (0.12) | 1.42 ± (0.10) |
| 400 | 1.53 ± (0.05) | 1.66 ± (0.06) | 1.89 ± (0.06) |
| 500 | 1.67 ± (0.12) | 1.68 ± (0.08) | 2.12 ± (0.03) |
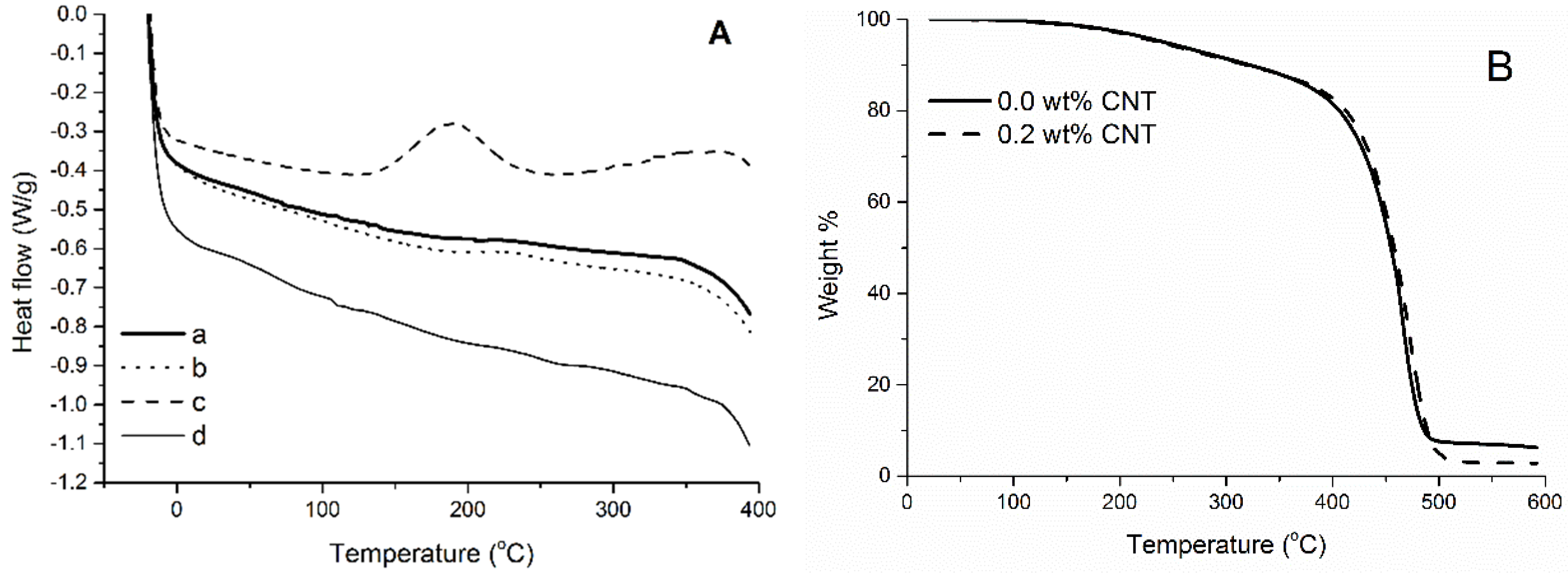
When considering materials cured solely in a convection oven, no significant difference in the thermal behavior between resins with and without CNTs is observed, as can be seen in
Figure 6. In
Figure 6A, the DSC curves of samples with and without CNTs exhibit a very similar trace, with only discrete transitions, indicating that both materials are completely cured after being heated at 140 °C in a convection oven for 6 h. Likewise, the TG curves of samples with and without CNTs (
Figure 6B) are very similar up until 498 °C, when most of the materials have been completely degraded. One possible reason for the lower residue content of the resin cured in the presence of CNTs may be the catalytic action of metals present in the CNTs during the final stages of the thermal decomposition (>470 °C), when high molecular weight and carbonized fragments experience further degradation in comparison to samples devoid of CNTs. Indeed, the CNTs have not been purified before being incorporated in the resin. They have a nominal ash content of 1.5 wt %, rich in Fe and Al. As will be discussed later in the text, before reaching temperatures >470 °C, the effects imparted by the use of CNTs are negligible in the thermal behavior of the system. Therefore it can be concluded that CNTs had little effect on the polymerization reaction carried out in a convection oven.
Figure 6.
(A) DSC curves of: (a) unreinforced tung oil-based resin cured for 6 h at 140 °C in a convection oven, (b) tung oil-based resin reinforced with 0.2 wt % of CNTs and cured for 6 h at 140 °C in a convection oven, (c) a tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and (d) a tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven; (B) TG curves of tung oil-based resins cured for 6 h at 140 °C in a convection oven with and without CNTs.
Figure 6.
(A) DSC curves of: (a) unreinforced tung oil-based resin cured for 6 h at 140 °C in a convection oven, (b) tung oil-based resin reinforced with 0.2 wt % of CNTs and cured for 6 h at 140 °C in a convection oven, (c) a tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and (d) a tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven; (B) TG curves of tung oil-based resins cured for 6 h at 140 °C in a convection oven with and without CNTs.
The situation is completely different when the cure is carried out under microwaves. With the goal of finding the appropriate microwave conditions that result in a fully cured sample in the shortest possible time, it was hypothesized that due to the rapid and uniform heating obtained by microwave irradiation of well dispersed CNTs in the tung oil-based resin, the cure time can be drastically reduced in comparison to the regular cure carried out in a convection oven. After preliminary tests, an ideal microwave step was established with irradiation at 100 W for 10 min, followed by irradiation at 200 W for 10 min. When heating the crude monomer mixture under microwaves, a fine control over the microwave power utilized is necessary. If excessive heat is applied over a short time interval, the monomers boil before the polymerization is completed, resulting in an undesirable rigid foam structure. If excessive time is applied during the microwave cure, signs of thermal degradation are observed in the samples. The ideal microwave schedule obtained allowed for full consolidation of the thermoset, without formation of bubbles or cracks. Samples submitted to that schedule, however, still exhibit a residual cure peak when analyzed by DSC. In the DSC curve of a microwaved CNT/tung oil-based sample, the prominent exothermic peak at 190 °C reveals that the microwave cure step alone did not result in a completely cured material (
Figure 6A). When testing alternative post-cure times, it has been found that after a post-cure step of 1 h 30 min, the resin is still not completely cured (
Figure S2). However, a completely cured sample is obtained when the material undergoes a 2 h 10 min post-cure at 140 °C in a convection oven after the microwave step (
Figure 6A). The absence of exothermic peaks in
Figure 6A (curve d) is indicative that the sample is fully cured. The post-cure of 2 h 10 min was arbitrarily selected so that the total reaction/cure time (microwave + convection oven) equals 2 h 30 min.
These results show that a great decrease in heating time, from 6 h, for the convection oven-only procedure, to 2 h 30 min total, for the microwave assisted step, is indeed successfully achieved. When one considers the final 2 h 10 min post-cure step as being common to both processes, the decrease in cure time is even more significant—3 h 50 min in the convection oven versus 20 min in the microwave. This significant decrease in cure time represents a great advantage for industrial applications where high throughput and shorter down times almost always translate into energy saving and process efficiency.
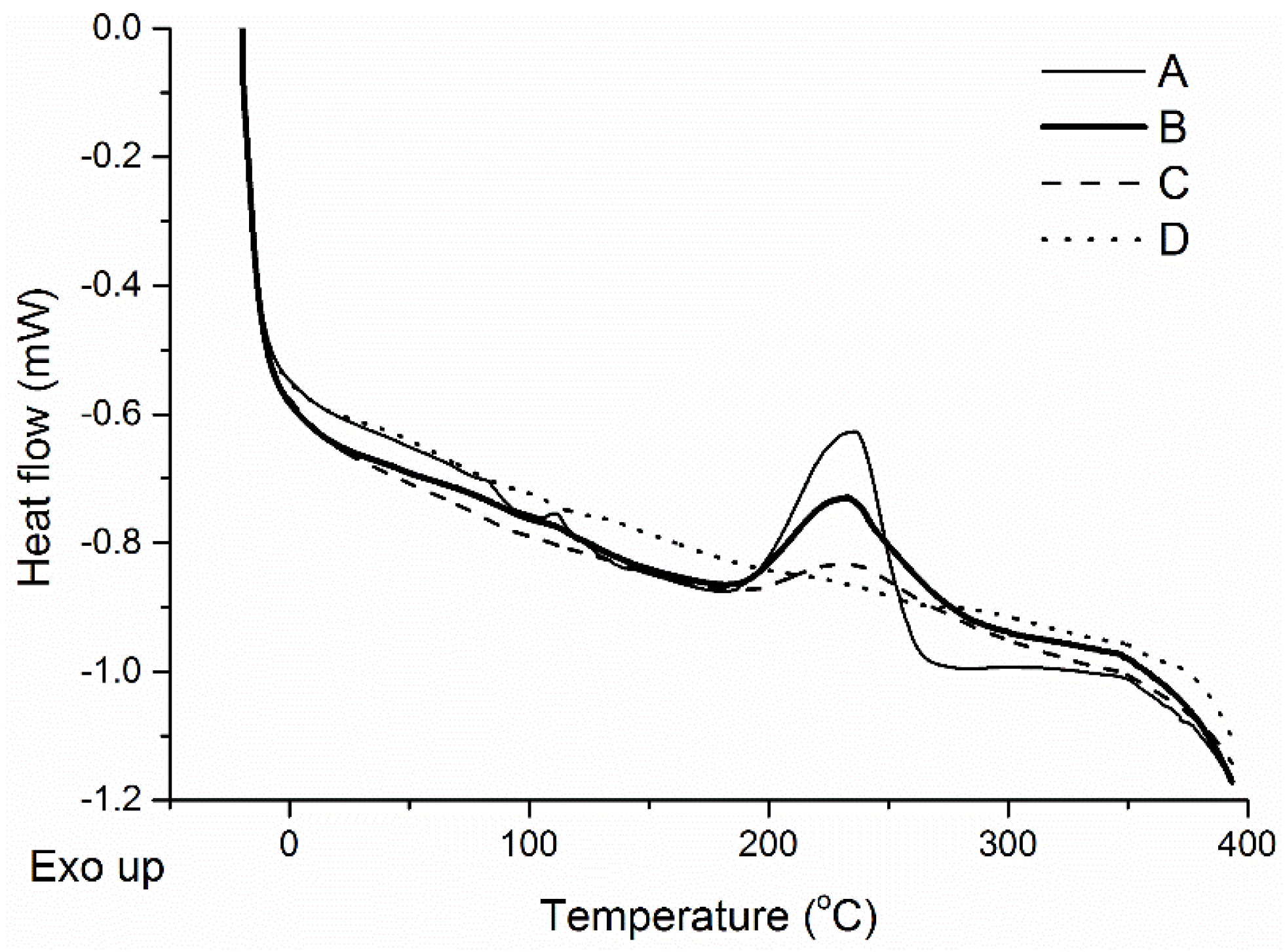
In
Figure 7, a decrease of the exothermic peak at approximately 230 °C can be seen as the CNT loading is increased from 0.02 wt % to 0.25 wt %. This phenomenon is consistent with the results in
Table 1, where increasing concentrations of CNT result in higher heating rates. Indeed, when the same cure schedule is applied to tung oil-based composites of different CNT loadings, higher temperatures are achieved, during the microwave stage of the curing process, for the samples with higher CNT loadings. The higher temperatures ensure a faster polymerization rate for the system, and according to the DSC curves presented in
Figure 7, it is possible to obtain a fully cured sample, with the optimized cure schedule, by introducing 0.25 wt % of CNTs. It is important to note that a higher temperature is observed for the exothermic peak of post-cured samples (
Figure 7A–C) in comparison to a non-post-cured sample (
Figure 6A). This occurs because, for partially cured samples, post-curing in the convection oven enables an increase of the crosslink density. Therefore, post-cured samples require higher temperatures to resume the polymerization, which translates into an exothermic peak at a higher temperature during the DSC analysis.
Figure 7.
DSC curves of CNT/tung oil-based composites with various CNT loading, microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven. The CNT loading in the composites is (A) 0.02 wt %; (B) 0.10 wt %; (C) 0.20 wt %; and (D) 0.25 wt %.
Figure 7.
DSC curves of CNT/tung oil-based composites with various CNT loading, microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven. The CNT loading in the composites is (A) 0.02 wt %; (B) 0.10 wt %; (C) 0.20 wt %; and (D) 0.25 wt %.
3.4. Dynamic Mechanical Analysis and Thermogravimetry
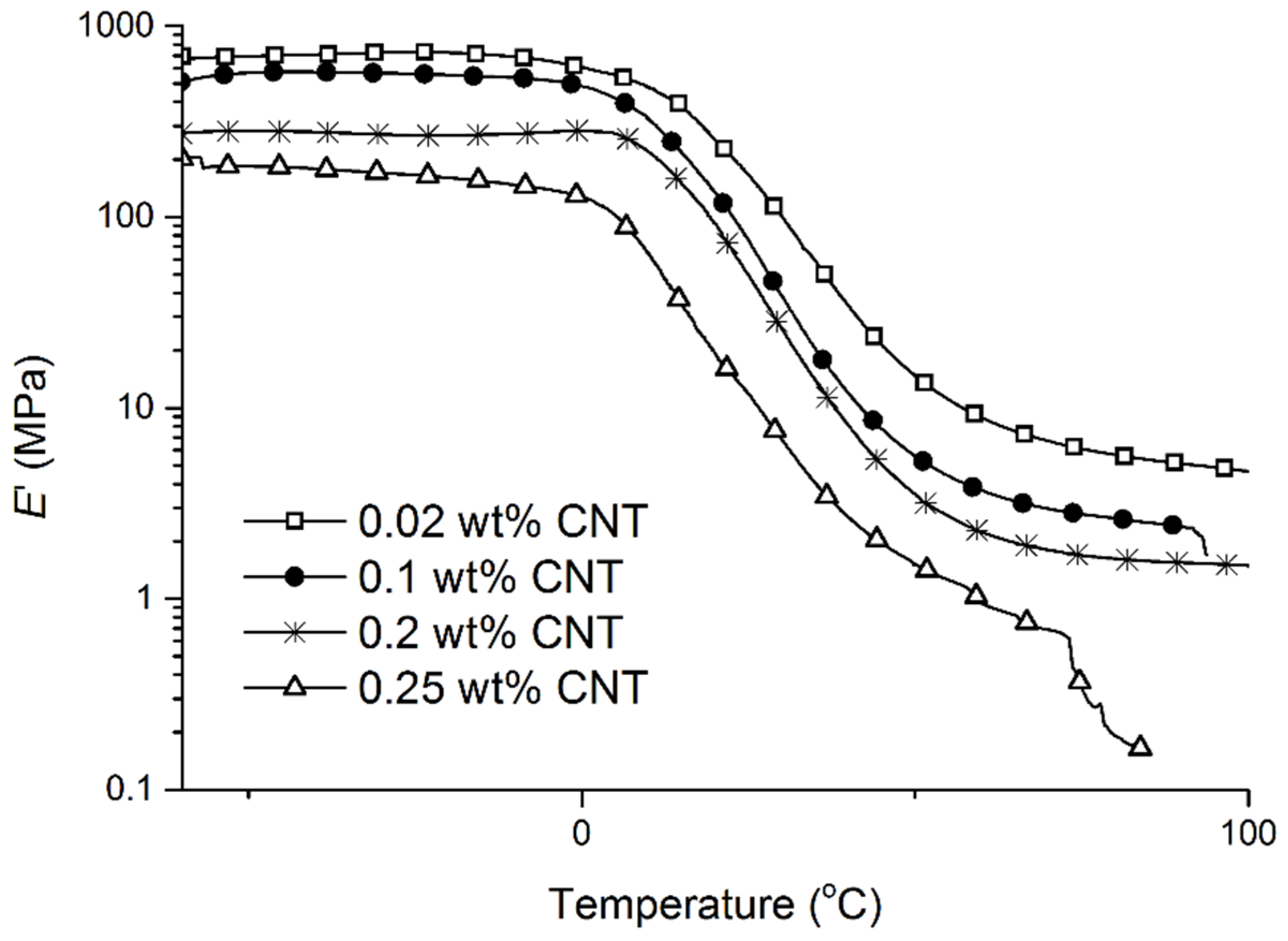
From a theoretical standpoint, there is no difference in the number of polymerization sites between samples, since the cure reaction proceeds mostly by cationic initiation, and for all experiments the same amount of BFE was used. Nevertheless, it is known that increasing temperatures increase the transfer reaction during the cationic polymerization, which may result in lower molecular weights. When samples containing evenly dispersed CNTs are microwaved, localized heat is instantly generated at each CNT particle and immediately dissipated to the surrounding medium. Overall, a quicker and more homogeneous heating of the system is obtained in comparison to conventional heating, or microwaving in the absence of CNTs. In seeking shorter cure times by combining CNTs and microwaves, it has been observed that an increase in CNT loading results in faster heating rates. As a consequence, more monomer molecules are simultaneously heated, which leads to faster polymerization rates and overall lower mechanical properties (
Figure 8). Furthermore, partial polymer degradation can also increase with increasing CNT loading, leading to poorer mechanical properties and higher soluble content, as will be discussed later in the text.
In
Table 2, a clear trend is observed for the decrease in storage modulus (
Eʹ) at 25 °C as the CNT loading is increased from 0.00 to 0.20 wt % (entries 1–4,
Table 2). When the CNT loading is increased from 0.20 to 0.25 wt % (entries 4 and 5,
Table 2), a slight increase in
E’ is observed. Indeed, an increase of only 0.05 wt % in CNTs is not sufficient to make an appreciable difference between the samples. The numbers are in fact very similar, with a difference of only 2.5 MPa. Consistent trends are observed when considering
Eʹ at
Tg + 50 °C.
Figure 8.
Storage modulus vs. temperature curves of a tung oil-based resin reinforced with different amounts of CNTs and cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven.
Figure 8.
Storage modulus vs. temperature curves of a tung oil-based resin reinforced with different amounts of CNTs and cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven.
Table 2.
Glass transition temperature (Tg) and Storage modulus (Eʹ) at 25 °C as determined by dynamic mechanical analysis (DMA), and thermogravimetric analysis (TGA) results for samples cured either in a convection oven, or under microwaves.
Table 2.
Glass transition temperature (Tg) and Storage modulus (Eʹ) at 25 °C as determined by dynamic mechanical analysis (DMA), and thermogravimetric analysis (TGA) results for samples cured either in a convection oven, or under microwaves.
| Entry | Cure Process | Post-Cure (140 °C) | CNT Loading (wt %) | Tg (°C) | Eʹ at 25 °C (MPa) | E’ at Tg+50 °C (MPa) | T90 d (°C) | T50 e (°C) |
|---|
| 1 | Microwave a | 2 h 10 min | 0.00 | 32.2 | 104.1 | 3.8 | 324 | 455 |
| 2 | Microwave a | 2 h 10 min | 0.02 | 33.6 | 99.6 | 3.3 | 269 | 419 |
| 3 | Microwave a | 2 h 10 min | 0.10 | 34.4 | 81.4 | 2.8 | 327 | 449 |
| 4 | Microwave a | 2 h 10 min | 0.20 | 33.2 | 48.9 | 1.5 | 369 | 449 |
| 5 | Microwave a | 2 h 10 min | 0.25 | 31.2 | 51.4 | 2.0 | 374 | 456 |
| 6 | Oven b | 2 h 10 min | 0.00 | 28.3 | 154.0 | -c | 320 | 455 |
| 7 | Oven b | 2 h 10 min | 0.25 | 38.1 | 200.8 | 5.1 | 317 | 458 |
When the sample is cured exclusively in a convection oven, in the absence of CNTs (entry 6,
Table 2), a storage modulus at 25 °C of 154 MPa is obtained. It is expected that the unique resin composition used in this work results in samples that exhibit mechanical properties different than similar systems, prepared by the cationic polymerization of vegetable oils and other comonomers. When one takes into consideration the relative concentrations of tung oil and divinylbenzene (DVB) in the resin composition, the observed values for storage modulus (
Eʹ) at 25 °C reported here are in line with previously published data from similar systems. For example, it has been reported that cationic resins containing 54 wt % of tung oil and 35 wt % of DVB exhibit
Eʹ at 25 °C of approximately 1050 MPa [
38]. When the relative amount of oil in the resin is increased to 70 wt %, while the amount of DVB is decreased to 15 wt %, a natural decrease in the mechanical properties is expected. Hence, the observed value of 154 MPa for
Eʹ at 25 °C for the unreinforced resin cured in the convection oven (
Table 2, entry 6) is considered in line with previous studies. Likewise, it has been reported that cationic samples containing 45 wt % of olive or canola oils and 15 wt % of DVB exhibit room temperature
E’ values of 220 MPa and 450 MPa, respectively [
40]. These values show the variability in mechanical properties with changes in the resin composition. Therefore, when the specific composition of the resin studied in this work is taken into account, the mechanical properties reported for the unreinforced resin cured in the convection oven can be considered in line with similar studied systems.
Eʹ at 25 °C for the unreinforced resin cured in the convection oven is significantly higher than that obtained by a similar sample, cured in the microwave (entry 1,
Table 2), corroborating the idea that the rapid cure experienced by the samples cured in the microwave compromises the mechanical properties. Additionally, during the DMA experiments, the sample consistently broke prematurely, before
Tg + 50 °C was attained (
Table 2, entry 6). Unlike samples cured in the microwave, when a tung oil-based resin reinforced with CNTs is cured in a convection oven, a significant increase in
Eʹ at 25 °C is observed (entries 6 and 7,
Table 2), showing the reinforcement character of CNTs. A comparison of the
Eʹ values at 25 °C and at
Tg + 50 °C for composites reinforced with 0.25 wt % of CNTs cured in the microwave and in a convection oven (entries 5 and 7,
Table 2) shows very clearly the detrimental effect of the expedite microwave cure to the mechanical properties of the material.
Despite the observed changes in storage modulus of samples cured in the microwave with different CNT loadings, the values measured for the
Tg of these samples are fairly consistent, varying only between 31.2 °C and 34.4 °C, with no regular pattern (entries 1–5,
Table 2). For samples cured exclusively in a convection oven, however, addition of CNTs results in a significant increase of the
Tg (entries 6 and 7,
Table 2). It is possible that covalent bonding occurs between defect sites on the CNTs and the resin components through possible free radical reactions during the conventional cure of the materials due to the extended heating at elevated temperatures, altering the dynamics of the regular matrix-reinforcement interactions for the system. Along the same lines, it is speculated that the slight difference in
Tg between the unreinforced resin cured in the oven and in the microwave lies in the cure mechanism being different in the two processes. A much deeper investigation of the cure mechanisms, which is beyond the intended scope of the current manuscript, is necessary in order to fully understand this phenomenon. Better suited, single-component systems might allow for a clearer analysis of the problem. It is important to highlight, however, that the difference in
Tg is only 3.9 °C (
Table 2, entries 1 and 6).
Thermogravimetric analysis was used to assess differences in thermal stability between samples cured in a convection oven and under microwaves with varying amounts of CNTs (
Table 2,
Figure 9). The temperatures at which 90 wt % (
T90) and 50 wt % (
T50) of the samples remain during the thermal degradation are presented in
Table 2. It can be observed that, for CNT composites cured under microwaves, increasing CNT loadings result in an increase in
T90 (entries 2–5,
Table 2), suggesting that the addition of CNTs result in materials that start degrading at a higher temperature when compared to their CNT-free counterparts. When
T50 is considered, a very similar trend is observed, with the only exception being the samples containing 0.1 wt % CNTs and 0.2 wt % CNTs (entries 3 and 4,
Table 2), for which no appreciable difference was detected. When a sample devoid of CNTs is cured under microwaves, its resulting thermal stability is very similar to that of a sample cured in a convection oven, with
T90 varying only by 4 °C, and
T50 being exactly the same (entries 1 and 6,
Table 2). The difference in the resulting thermal stability of samples cured under microwaves with and without CNTs, indicates that a different polymerization process occurs depending on the presence of CNTs. As discussed earlier, the localized heat generated by the absorption of microwaves by CNTs prompts the simultaneous growth of multiple polymer chains, which results in a matrix with lower molecular weight, and lower thermal stability. The addition of CNTs to the resin, for samples cured exclusively in a convection oven, does not impart a significant change in
T90 and
T50, with changes of only 3 °C in both cases (entries 6 and 7,
Table 2). This expected behavior confirms that CNTs have no effect in the heating process taking place in the convection oven, acting more as a reinforcement than as a heating agent. Due to the small CNT loading used, no significant difference was observed in the thermal stability of the two samples cured exclusively in a convection oven (entries 6 and 7,
Table 2).
Figure 9.
Thermogravimetric analysis (TGA) curves of tung oil-based resins, cured in a convection oven for 6 h at 140 °C, reinforced with (A) 0.00 wt % CNTs; and (B) 0.25 wt % CNTs, and tung oil-based resins, cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C, in a convection oven, reinforced with (C) 0.00 wt % CNTs; (D) 0.02 wt % CNTs; (E) 0.10 wt % CNTs; (F) 0.20 wt % CNTs; and (G) 0.25 wt % CNTs.
Figure 9.
Thermogravimetric analysis (TGA) curves of tung oil-based resins, cured in a convection oven for 6 h at 140 °C, reinforced with (A) 0.00 wt % CNTs; and (B) 0.25 wt % CNTs, and tung oil-based resins, cured under microwaves at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C, in a convection oven, reinforced with (C) 0.00 wt % CNTs; (D) 0.02 wt % CNTs; (E) 0.10 wt % CNTs; (F) 0.20 wt % CNTs; and (G) 0.25 wt % CNTs.
3.5. Soxhlet Extraction
After Soxhlet extraction with methylene chloride, the samples cured exclusively in a convection oven exhibited, on average, 17 wt % of soluble material, while samples cured under microwaves exhibit, on average, 19 wt % of soluble material. These numbers are in agreement with previously published data on similar tung oil-based resins [
38]. The percentage of soluble material extracted is inversely proportional to the crosslink density of the polymer, therefore, it can be concluded that samples cured under microwaves are only slightly less cross-linked than those cured exclusively in a convection oven.
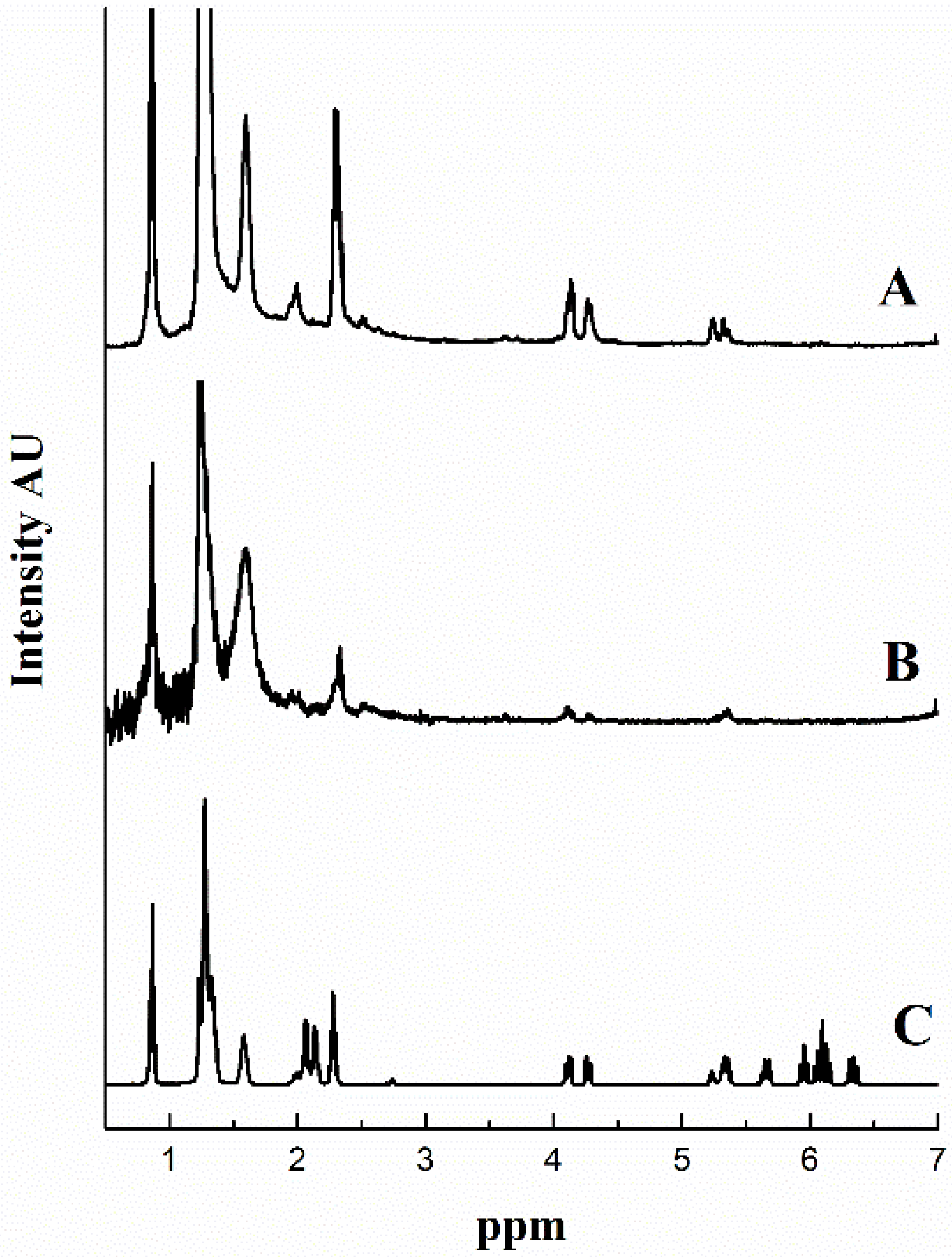
The soluble material recovered after Soxhlet extraction from each sample was analyzed by
1H NMR spectroscopy (
Figure 10) and the results reveal that the extracts correspond to partially reacted tung oil and/or tung oil oligomers.
Figure 10C shows a standard
1H NMR spectrum of a conjugated triglyceride with peaks at 4.00–4.40 ppm, corresponding to the glycerol methylenes, and the series of multiplets at 5.20–6.50 ppm, corresponding to the protons of conjugated sp
2 hybridized carbons. In
Figure 10A,B, the spectra match very closely that of tung oil, with the exception of peaks >5.5 ppm, denoting that at least some of the carbon-carbon double bonds in tung oil have reacted, most likely forming soluble oligomers.
Figure 10.
1H NMR spectra of (A) tung oil-based resin cured for 6 h at 140 °C in a convection oven without CNTs, (B) tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven, and (C) tung oil.
Figure 10.
1H NMR spectra of (A) tung oil-based resin cured for 6 h at 140 °C in a convection oven without CNTs, (B) tung oil-based resin reinforced with 0.25 wt % CNTs microwaved at 100 W for 10 min, followed by 200 W for 10 min, and a 2 h 10 min post-cure step at 140 °C in a convection oven, and (C) tung oil.