Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biological Materials
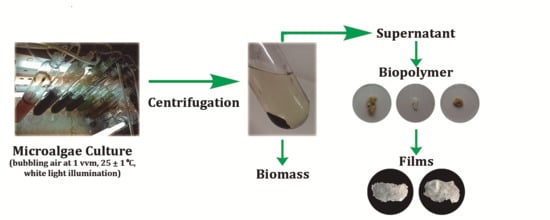
2.3. Microalgae Culture
2.4. Polymer Precipitation Capacity
2.5. Film-Forming Capacity
- The fresh polymer precipitate was dissolved in 10 mL of 5% NaCl with gentle agitation on a hot plate, until the temperature reached 60 °C. The plasticizer (1.0 mL of glycerol) was added, and the agitation continued for two more minutes. Then, the viscous solution was precipitated with 0.5% cetrimide in a ratio of 1:1 (v/v). The precipitate was recovered, laminated and dried at 25 °C for 24 h.
- The fresh polymer precipitate was dissolved in 10 mL of 5% NaCl with gentle agitation on a hot plate, until the temperature reached 60 °C. The plasticizer (0.5 g of D-sorbitol) was added, and the agitation continued for two more minutes. Then, the viscous solution was precipitated with 0.5% cetrimide in a ratio of 1:1 (v/v). The precipitated was recuperated, laminated and dried at 25 °C for 24 h.
- The fresh polymer precipitate was dissolved in 10 mL of 5% NaCl with gentle agitation on a hot plate, until the temperature reached 60 °C. The plasticizer (1.0 mL of glycerol) was added, and the agitation continued for two more minutes. The viscous solution obtained was poured in a petri dish and dried at 30 °C for 12 h.
- The fresh polymer precipitate was dissolved in 20 mL of 0.1 M NaOH with gentle agitation on a hot plate, until the temperature reached 80 °C. The plasticizer (1.0 mL of glycerol) was added, and the agitation continued for 20 min. The viscous solution was emptied in a petri dish and dried at 30 °C for 12 h.
- The fresh polymer precipitate was dissolved in 20 mL of 0.1 M NaOH with gentle agitation on a hot plate, until the temperature reached 80 °C. The plasticizer (1.0 mL of glycerol) was added, and the agitation continued for 20 min. The viscous solution obtained was precipitated with 0.5% cetrimide in a ratio of 1:1 (v/v). The precipitated was later recuperated, laminated and dried at 25 °C for 24 h.
2.6. Microstructure Analysis of the Biofilms
2.6.1. SEM
2.6.2. Atomic Force Microscopy (AFM)
2.7. Bioactivity of Microalgae Polymers
2.7.1. Biopolymer Clean-up
2.7.2. Antifungal Activity
2.7.3. Antibacterial Activity
2.7.4. Antioxidant Activity
2.8. Emulsification Index Test of the Extracellular Medium
2.9. Protein and Sugar Analysis
2.10. FT-IR Analysis of the Clean Biopolymers
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microalgal Growth and Biomass Productivity
3.2. Polymer Precipitation Capacity
3.3. Film-Forming Capacity
3.4. Clean Biopolymer
3.5. Antifungal Activity
3.6. Antibacterial Activity
3.7. Antioxidant Activity
3.8. Emulsification Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Sousa-Gallagher, M.J.; Macedo, I.; Rodriguez-Aguilera, R.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of “Regional” cheese. J. Food Eng. 2010, 97, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, M.A.; Souza, B.W.S.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Seed extracts of Gleditsia triacanthos: Functional properties evaluation and incorporation into galactomannan films. Food Res. Int. 2010, 43, 2031–2038. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocoll. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Pereira, J.; Simões, M.; Silva, J.L. Microalgal assimilation of vitamin B12 toward the production of a superfood. J. Food Biochem. 2019, 43, 1–15. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; Sousa, J.M.; Moura, P.; Bandarra, N.M. Microalgae—Source of natural bioactive molecules as functional ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Gouveia, L.; Coutinho, C.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional biscuits with PUFA-ω3 from Isochrysis galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Gouveia, L.; Raymundo, A.; Batista, A.P.; Sousa, I.; Empis, J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur. Food Res. Technol. 2006, 222, 362–367. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Vonshak, A. Microalgae: Laboratory growth techniques and the biotechnology of biomass production. In Photosynthesis and Production in a Changing Environment; Springer: Dordrecht, The Netherlands, 1993; pp. 337–355. [Google Scholar]
- Stanier, R.Y.; Deruelles, J.; Rippka, R.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar]
- Arzate-Vázquez, I.; Chanona-Pérez, J.J.; Calderón-Domínguez, G.; Terres-Rojas, E.; Garibay-Febles, V.; Martínez-Rivas, A.; Gutiérrez-López, G.F. Microstructural characterization of chitosan and alginate films by microscopy techniques and texture image analysis. Carbohydr. Polym. 2012, 87, 289–299. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, H.C.; Chen, Y.; Li, H.X.; Chen, C.J.; Zhou, M.G. Multiple resistance of Botrytis cinerea from vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China. Plant Dis. 2010, 94, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat Ebrahimi, M.M.; Dohm, N.; Müller, M.; Jansen, B.; Schönherr, H. Self-reporting hydrogels rapidly differentiate among enterohemorrhagic Escherichia coli (EHEC) and non-virulent Escherichia coli (K12). Eur. Polym. J. 2016, 81, 257–265. [Google Scholar] [CrossRef]
- Volkmer, B.; Heinemann, M. Condition-dependent cell volume and concentration of Escherichia coli to facilitate data conversion for systems biology modeling. PLoS ONE 2011, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Techaoei, S.; Lumyong, S.; Prathumpai, W.; Santiarwar, D.; Leelapornp, P. Screening characterization and stability of biosurfactant produced by Pseudomonas aeruginosa SCMU106 isolated from soil in northern Thailand. Asian J. Biol. Sci. 2011, 4, 340–351. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Barrocal, V.M.; García-Cubero, M.T.; González-Benito, G.; Coca, M. Production of biomass by Spirulina maxima using sugar beet vinasse in growth media. N. Biotechnol. 2010, 27, 851–856. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Ciapponi, R.; Turri, S.; Levi, M. Mechanical reinforcement by microalgal biofiller in novel thermoplastic biocompounds from plasticized gluten. Materials 2019, 12, 1476. [Google Scholar] [CrossRef] [Green Version]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of microalgae addition on active biodegradable starch film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Fabra, M.J.; Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Gavara, R.; López-Rubio, A. Structural and physicochemical characterization of thermoplastic corn starch films containing microalgae. Carbohydr. Polym. 2018, 186, 184–191. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, R.; Sajjad, W.; Nabi, G.; Hayat, K.M.; Duan, P.; Yao, L. Biodiesel production from algae to overcome the energy crisis. HAYATI J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Gloaguen, V.; Ruiz, G.; Morvan, H.; Mouradi-Givernaud, A.; Maes, E.; Krausz, P.; Strecker, G. The extracellular polysaccharide of Porphyridium sp.: An NMR study of lithium-resistant oligosaccharidic fragments. Carbohydr. Res. 2004, 339, 97–103. [Google Scholar] [CrossRef]
- Andhare, P.; Goswami, D.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Edifying the strategy for the finest extraction of succinoglycan from Rhizobium radiobacter strain CAS. Appl. Biol. Chem. 2017, 60, 339–348. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Dias, A.M.A.; De Sousa, H.J.C.; Braga, M.E.M.; Moraes, Â.M. Control of the properties of porous chitosan-alginate membranes through the addition of different proportions of Pluronic F68. Mater. Sci. Eng. C 2014, 44, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Demirci, F.; Yildirim, K.; Kocer, H.B. Antimicrobial open-cell polyurethane foams with quaternary ammonium salts. J. Appl. Polym. Sci. 2018, 135, 16–20. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; Westin, C.B.; Moraes, Â.M. Comparison of the properties of membranes produced with alginate and chitosan from mushroom and from shrimp. Int. J. Biol. Macromol. 2016, 91, 496–504. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J. New trends in biopolymer-based membranes for pervaporation. Molecules 2019, 24, 3584. [Google Scholar] [CrossRef] [Green Version]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Memb. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Chalermthai, B.; Chan, W.Y.; Bastidas-Oyanedel, J.R.; Taher, H.; Olsen, B.D.; Schmidt, J.E. Preparation and characterization of whey protein-based polymers produced from residual dairy streams. Polymers 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oromiehi, A.R. Biodegradable biocomposite films based on whey protein and zein: Barrier, mechanical properties and AFM analysis. Int. J. Biol. Macromol. 2008, 43, 209–215. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Selim, M.S.; Amer, S.K.; Mohamed, S.S.; Mounier, M.M.; Rifaat, H.M. Production and characterisation of exopolysaccharide from Streptomyces carpaticus isolated from marine sediments in Egypt and its effect on breast and colon cell lines. J. Genet. Eng. Biotechnol. 2018, 16, 23–28. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Jin, M.; Haobin, Z.; Li, Q.; Shao, D.; Jiang, C.; Huang, Q.; Yang, H.; Shi, J.; Hussain, N. Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk. LWT Food Sci. Technol. 2018, 89, 638–647. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y.; Li, H.; Zhao, C.; Yang, Y.; Hou, Y.; et al. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef]
- Han, P.P.; Shen, S.G.; Wang, H.Y.; Sun, Y.; Dai, Y.J.; Jia, S.R. Comparative metabolomic analysis of the effects of light quality on polysaccharide production of cyanobacterium Nostoc flagelliforme. Algal Res. 2015, 9, 143–150. [Google Scholar] [CrossRef]
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J. Biotechnol. 2003, 102, 143–152. [Google Scholar] [CrossRef]
- Grünewald, N.; Alban, S. Optimized and standardized isolation and structural characterization of anti-inflammatory sulfated polysaccharides from the red alga Delesseria sanguinea (Hudson) Lamouroux (Ceramiales, Delesseriaceae). Biomacromolecules 2009, 10, 2998–3008. [Google Scholar] [CrossRef]
- Anvari, M.; Tabarsa, M.; Cao, R.; You, S.; Joyner Melito, H.S.; Behnam, S.; Rezaei, M. Compositional characterization and rheological properties of an anionic gum from Alyssum homolocarpum seeds. Food Hydrocoll. 2016, 52, 766–773. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [Green Version]
- Geresh, S.; Mamontov, A.; Weinstein, J. Sulfation of extracellular polysaccharides of red microalgae: Preparation, characterization and properties. J. Biochem. Biophys. Methods 2002, 50, 179–187. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- De Faria, A.F.; Teodoro-Martinez, D.S.; De Oliveira Barbosa, G.N.; Gontijo Vaz, B.; Serrano Silva, Í.; Garcia, J.S.; Tótola, M.R.; Eberlin, M.N.; Grossman, M.; Alves, O.L.; et al. Production and structural characterization of surfactin (C 14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar]
- Souza, E.C.; Vessoni-Penna, T.C.; De Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Microalgae | Microalgae Biomass Extract | Crude Extracellular Medium | ||
|---|---|---|---|---|
| Sugar (1)* | Protein (2)* | Sugar (3)* | Protein (4)* | |
| Pp | 9.6 ± 0.1 b | 9.5 ± 0.0 c | 0.09 ± 0.0 a | 0.03 ± 0.0 d |
| No | 8.6 ± 0.1 c | 31.8 ± 1.3 a | 0.03 ± 0.0 c | 0.10 ± 0.0 c |
| Sm | 2.6 ± 0.0 f | 16.8 ± 0.2 b | 0.02 ± 0.0 d | 0.34 ± 0.0 a |
| Sy | 7.4 ± 0.1 d | Not detected | 0.04 ± 0.0 b | 0.15 ± 0.0 b |
| So | 10.3 ± 0.1 a | Not detected | 0.02 ± 0.0 d | Not detected |
| Sp | 5.0 ± 0.0 e | Not detected | 0.05 ± 0.0 b | Not detected |
| Formulation | Solvent/Plasticizer | Preparation/Drying | Biofilm Characteristics | ||
|---|---|---|---|---|---|
| No | Pp | Sy | |||
| I | 5% NaCl/Glycerol | Agitation at 60 °C for 2 min precipitation and lamination at 25 °C for 24 h | Thin, transparent and flexible. | Thin, transparent and with mobility. | Does not form film |
| II | 5% NaCl/D-sorbitol | Agitation at 60 °C for 2 min, precipitation and lamination at 25 °C for 24 h | Fragile | Thin, transparent and with mobility. | Does not form film |
| III | 5% NaCl/Glycerol | Agitation at 60 °C for 2 min and emptying in petri dish at 30 °C for 12 h | Does not form film | Does not form film | Does not form film |
| IV | 0.1 M NaOH/Glycerol | Agitation at 80 °C for 20 min and emptying in petri dish at 30 °C for 12 h | Does not form film | Does not form film | Does not form film |
| V | 0.1 M NaOH/Glycerol | Agitation at 80 °C for 20 min and precipitation and lamination at 25 °C for 24 h | Thin, transparent, rough and flexible | Gross, rough and fragile | Does not form film |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings 2020, 10, 120. https://doi.org/10.3390/coatings10020120
Morales-Jiménez M, Gouveia L, Yáñez-Fernández J, Castro-Muñoz R, Barragán-Huerta BE. Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings. 2020; 10(2):120. https://doi.org/10.3390/coatings10020120
Chicago/Turabian StyleMorales-Jiménez, Mónica, Luisa Gouveia, Jorge Yáñez-Fernández, Roberto Castro-Muñoz, and Blanca Estela Barragán-Huerta. 2020. "Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film" Coatings 10, no. 2: 120. https://doi.org/10.3390/coatings10020120