Facile One-Step Method to Fabricate a Slippery Lubricant-Infused Surface (LIS) with Self-Replenishment Properties for Anti-Icing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LIS
2.3. Surface Characterization
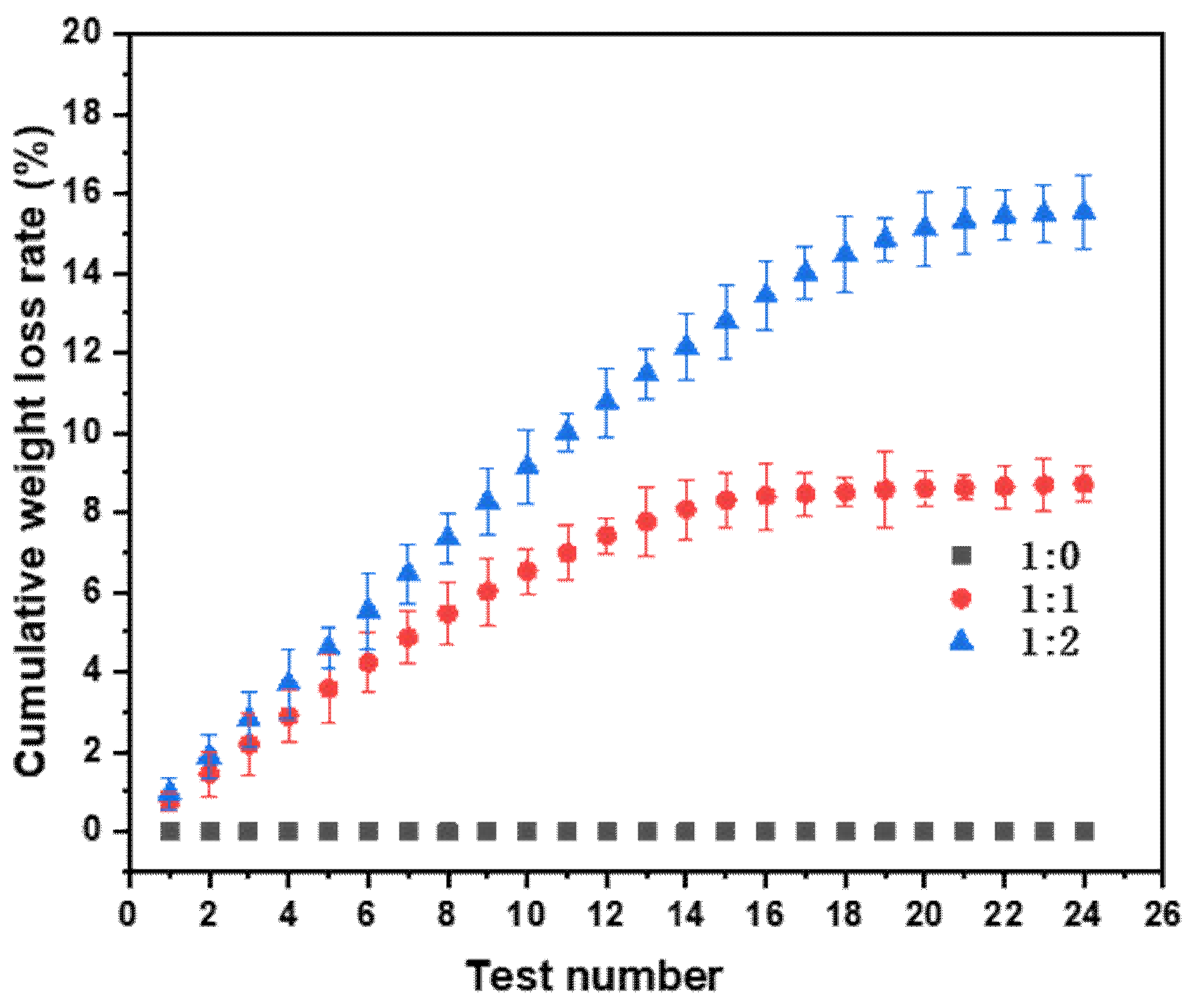
2.4. Oil Loss/Replenishment Cycle Tests
2.5. Compression Mechanical Property Tests
2.6. Anti-Icing Tests
3. Results
3.1. Preparation Process and Surface Morphology
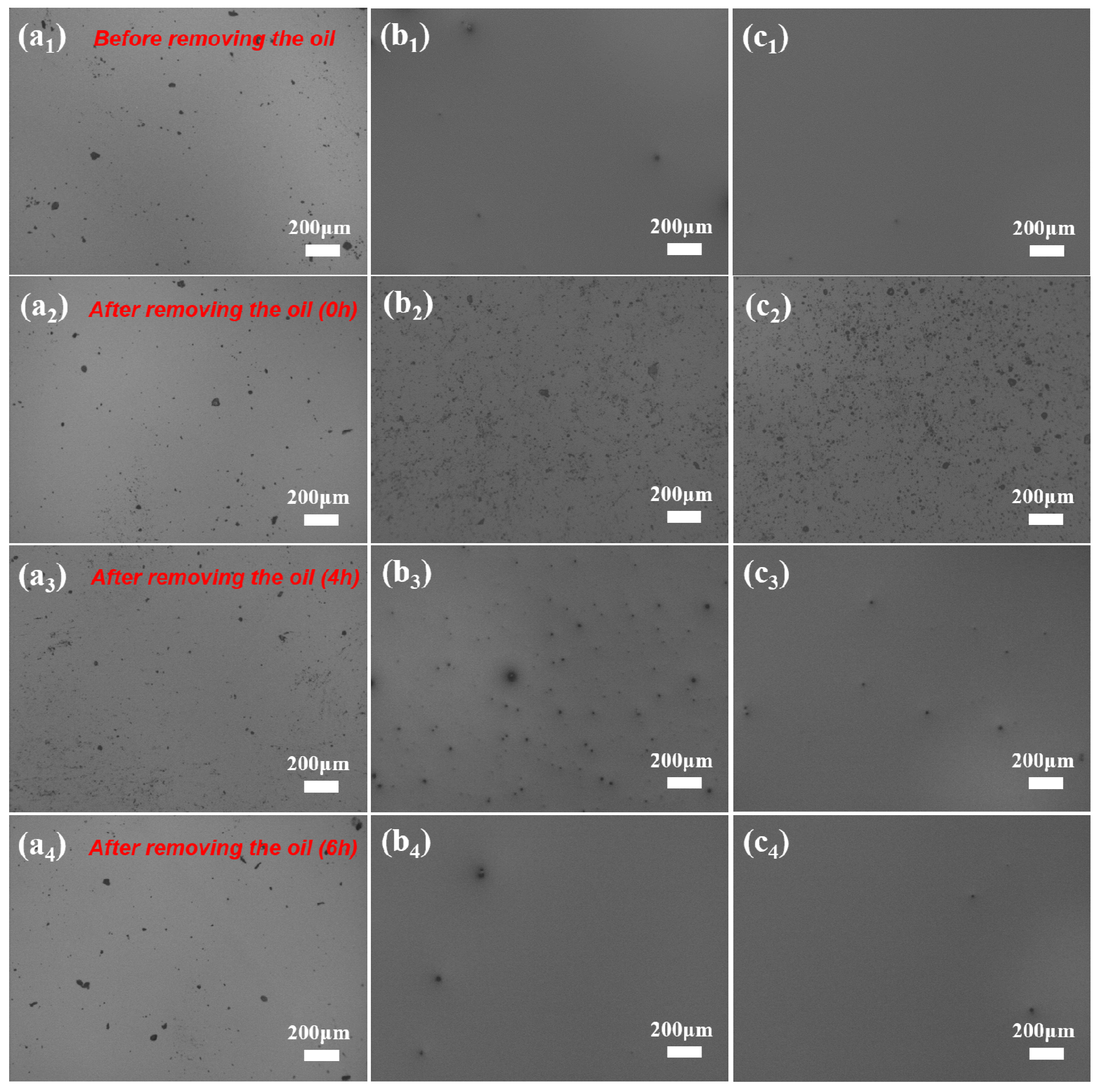
3.2. Self-Complementing Behavior
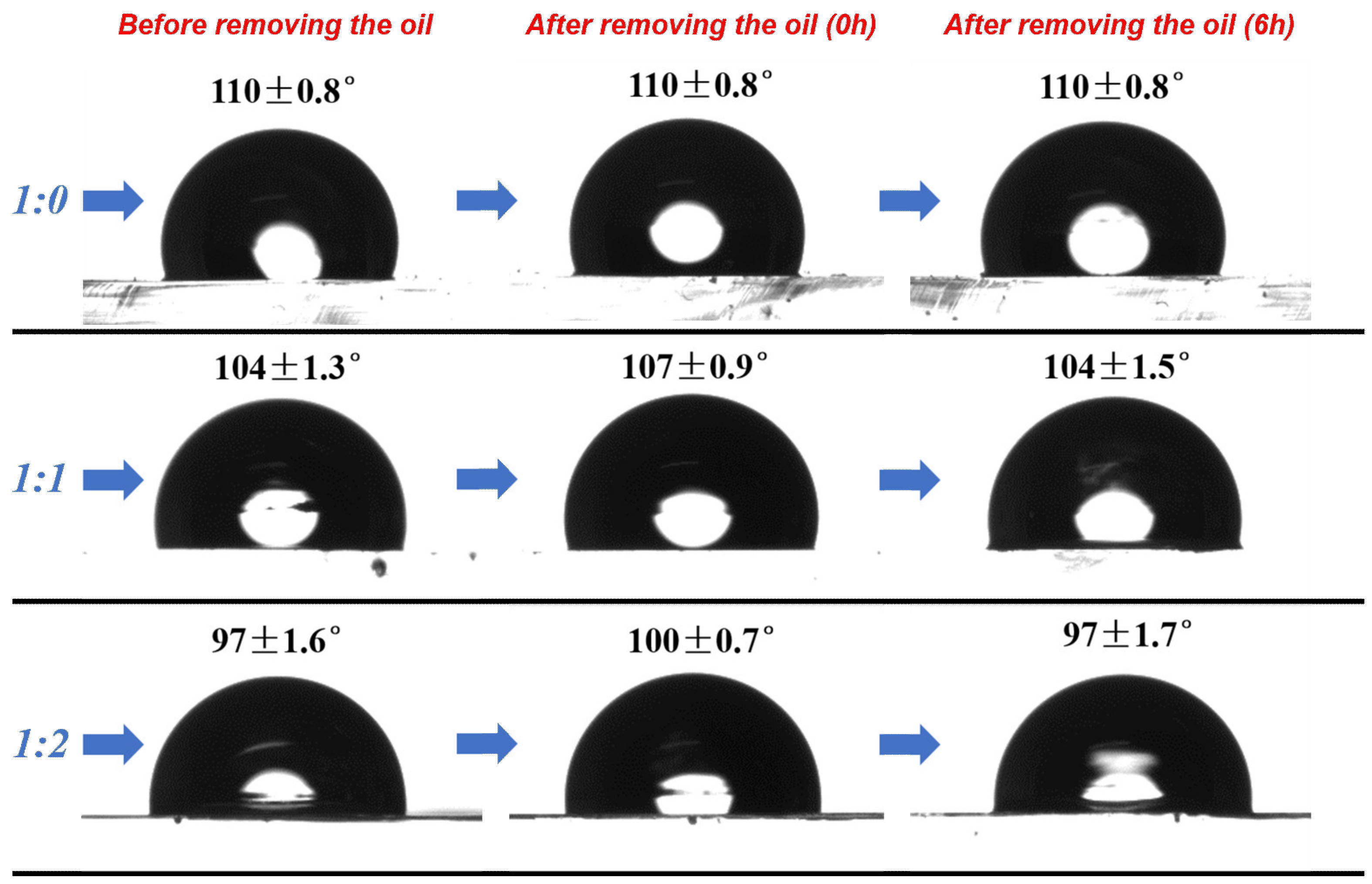
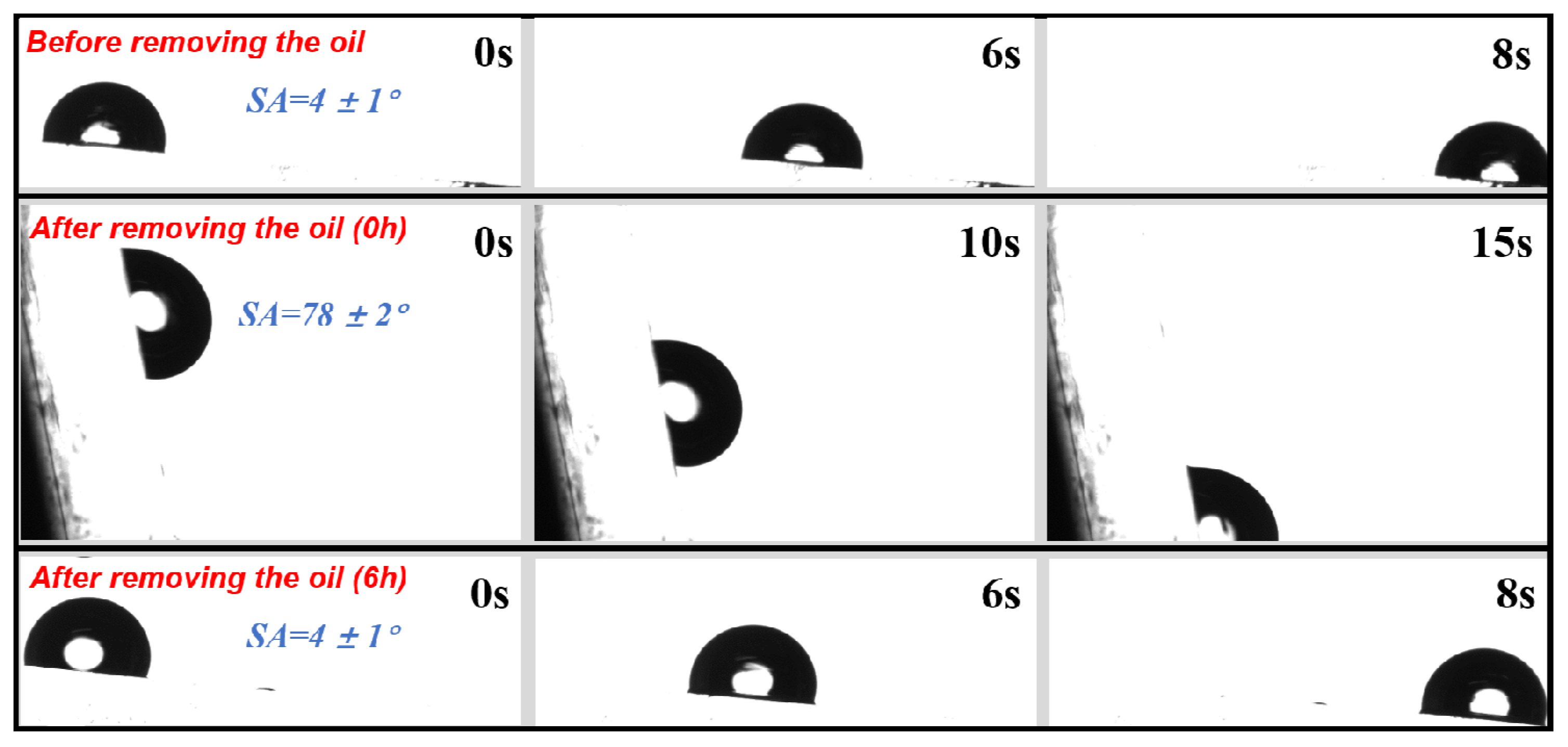
3.3. Surface Wettability
3.4. Mechanical Properties
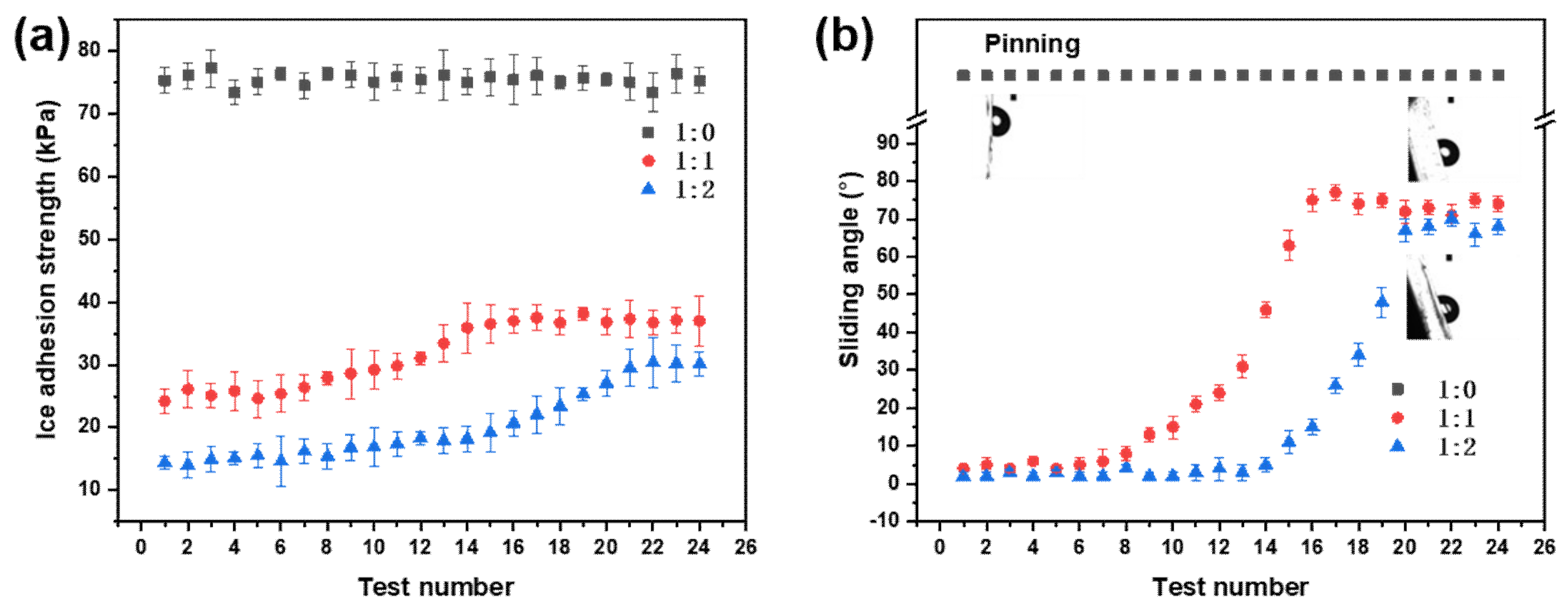
3.5. Anti-Icing Properties
4. Conclusions
- (1)
- The dimethyl silicone oil (PMX-200) showed good compatibility with PDMS resin. The addition of silicone oil in the PDMS matrix greatly reduced the surface water slide angle and improved the surface lubrication ability.
- (2)
- The presence of silicone oil endowed the LIS with a self-replenishment ability to the surface oil film. With the increase of silicone oil content in the PDMS matrix, the self-replenishment rate and amount were enhanced significantly, which was beneficial to the recovery of surface lubrication.
- (3)
- The LIS exhibited low ice adhesion strength because of the presence of the oil film. Moreover, the ice adhesion strength on the LIS was still very low after 24 cycles of the icing/deicing tests. The LIS showed excellent and durable anti-icing performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Lv, J.Y.; Song, Y.L.; Jiang, L.; Wang, J.J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.M.; Chen, J.; Zhang, Y.F.; Wang, X.P.; Cui, D.P.; Song, Y.L.; Jiang, L.; Wang, J. Anti-icing coating with an aqueous lubricating layer. ACS Appl. Mater. Inter. 2014, 6, 6998–7003. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.F.; Wang, Y.M.; Zhang, Z.M.; Liu, Y.L. Application of anti-icing coating based on adsorption of functional substances by microporous sphere. Prog. Org. Coat. 2019, 137, 105320. [Google Scholar] [CrossRef]
- Zuo, Z.P.; Liao, R.J.; Guo, C.; Yuan, Y.; Zhao, X.T.; Zhuang, A.Y.; Zhang, Y.Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Zhang, S.N.; Huang, J.Y.; Cheng, Y.; Yang, H.; Chen, Z.; Lai, Y.K. Bioinspired surfaces with superwettability for anti-Icing and ice-phobic application: Concept, mechanism, and design. Small 2017, 13, 1701867. [Google Scholar] [CrossRef]
- Pan, S.; Wang, N.; Xiong, D.S.; Deng, Y.L.; Shi, Y. Fabrication of superhydrophobic coating via spraying method and its applications in anti-icing and anti-corrosion. Appl. Surf. Sci. 2016, 389, 547–553. [Google Scholar] [CrossRef]
- Cheng, T.T.; He, R.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Magnetic particle-based super-hydrophobic coatings with excellent anti-icing and thermoresponsive deicing performance. J. Mater. Chem. A 2015, 3, 21637–21646. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.C.; Wang, L.T.; Wang, Z.; Du, C.W.; Li, X.G.; Zhang, D.W. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Tian, X.L.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef]
- Qian, H.C.; Li, M.L.; Li, Z.; Lou, Y.T.; Huang, L.Y.; Zhang, D.W.; Xu, D.K.; Du, C.W.; Lu, L.; Gao, J. Mussel-inspired superhydrophobic surfaces with enhanced corrosion resistance and dual-action antibacterial properties. Mater. Sci. Eng. C 2017, 80, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Qian, H.C.; Zhang, D.W.; Deng, L.P.; Huang, L.Y.; Xu, D.K.; Du, C.W.; Li, X.G. The role of surface morphology in the barrier properties of epoxy coatings in different corrosion environments. Prog. Org. Coat. 2017, 104, 199–209. [Google Scholar] [CrossRef]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Lee, C.; Choi, C.H.; Kim, C.J. Superhydrophobic drag reduction in laminar flows: A critical review. Exp. Fluids 2016, 57, 176. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al-Deyab, S.S.; Zhang, K.Q.; Chen, T.; et al. Robust superhydrophobic TiO2@ fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Qian, H.C.; Xu, D.K.; Du, C.W.; Zhang, D.W.; Li, X.G.; Huang, L.Y.; Deng, L.P.; Tu, Y.C.; Mol, J.M.C.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically robust superhydrophobic steel surface with anti-icing, UV-durability, and corrosion resistance properties. ACS Appl. Mater. Inter. 2015, 7, 6260–6272. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Maslakov, K.I. Anti-icing properties of a superhydrophobic surface in a salt environment: An unexpected increase in freezing delay times for weak brine droplets. Phys. Chem. Chem. Phys. 2016, 18, 3131–3136. [Google Scholar] [CrossRef]
- Zhang, D.W.; Qian, H.C.; Wang, L.T.; Li, X.G. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.H.; Zhang, L.Z.; Yan, Y.; Zhu, J. Mechanical durability of superhydrophobic surfaces: The role of surface modification technologies. Appl. Surf. Sci. 2017, 392, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S.H.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhao, Z.; Yan, Y.; Zhang, D. Slippery liquid-infused porous electric heating coating for anti-icing and de-icing applications. Surf. Coat. Technol. 2019, 374, 889–896. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Lu, Z. Slippery liquid-infused porous surface bio-inspired by pitcher plant for marine anti-biofouling application. Colloid. Surf. B 2015, 136, 240–247. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, H.; Chen, W.; Liu, X. Corrosion protection application of slippery liquid-infused porous surface based on aluminum foil. Appl. Surf. Sci. 2017, 423, 365–374. [Google Scholar] [CrossRef]
- Semprebon, C.; McHale, G.; Kusumaatmaja, H. Apparent contact angle and contact angle hysteresis on liquid infused surfaces. Soft Matter 2017, 13, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Qiu, R.; Xiao, Y.; Zheng, J.; Lin, C. Slippery liquid-infused porous surface fabricated on CuZn: A barrier to abiotic seawater corrosion and microbiologically induced corrosion. Appl. Surf. Sci. 2018, 457, 468–476. [Google Scholar] [CrossRef]
- Xiang, T.F.; Zhang, M.; Sadig, H.R.; Li, Z.C.; Zhang, M.X.; Dong, C.D.; Yang, L.; Chan, W.M.; Li, C. Slippery liquid-infused porous surface for corrosion protection with self-healing property. Chem. Eng. J. 2018, 345, 147–155. [Google Scholar] [CrossRef]
- Manna, U.; Raman, N.; Welsh, M.A.; Zayas-Gonzalez, Y.M.; Blackwell, H.E.; Palecek, S.P.; Lynn, D.M. Slippery liquid-infused porous surfaces that prevent microbial surface fouling and kill non-adherent pathogens in surrounding media: A controlled release approach. Adv. Funct. Mater. 2016, 26, 3599–3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Inter. 2013, 5, 6704–6711. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Zhang, D.W.; Ye, Y.W.; Ma, L.W.; Minhas, B.; Liu, B.; Terryn, H.A.; Mol, J.M.C.; Li, X.G. Durable lubricant-infused anodic aluminum oxide surfaces with high-aspect-ratio nanochannels. Chem. Eng. J. 2019, 368, 138–147. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Inter. 2017, 9, 11247–11257. [Google Scholar] [CrossRef] [PubMed]
- Heale, F.L.; Parkin, I.P.; Carmalt, C.J. Slippery liquid infused porous TiO2/SnO2 nanocomposite thin films via aerosol assisted chemical vapour deposition with anti-icing and fog retardant properties. ACS Appl. Mater. Inter. 2019, 11, 41804–41812. [Google Scholar] [CrossRef]
- Zhang, M.L.; Chen, R.R.; Liu, Q.; Liu, J.Y.; Yu, J.; Song, D.L.; Liu, P.L.; Gao, L.T.; Wang, J. Long-term stability of a liquid-infused coating with anti-corrosion and anti-icing potentials on Al alloy. ChemElectroChem 2019, 6, 3911–3919. [Google Scholar] [CrossRef]
- Wei, C.Q.; Zhang, G.F.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Silicone oil-infused slippery surfaces based on Sol–Gel process-induced nanocomposite coatings: A facile approach to highly stable bioinspired surface for biofouling resistance. ACS Appl. Mater. Inter. 2016, 8, 34810–34819. [Google Scholar] [CrossRef]
- Zhang, P.F.; Chen, H.W.; Zhang, L.W.; Zhang, D.Y. Anti-adhesion effects of liquid-infused textured surfaces on high-temperature stainless steel for soft tissue. Appl. Surf. Sci. 2016, 385, 249–256. [Google Scholar] [CrossRef]
- Erbil, H.Y. Improvement of lubricant-infused surfaces for anti-icing applications. Surf. Innov. 2016, 4, 214–217. [Google Scholar] [CrossRef]
- Lu, D.X.; Zhang, Y.L.; Han, D.D.; Wang, H.; Xia, H.; Chen, Q.D.; Ding, H.; Sun, H.B. Solvent-tunable PDMS microlens fabricated by femtosecond laser direct writing. J. Mater. Chem. C 2015, 3, 1751–1756. [Google Scholar] [CrossRef]
- Zang, S.S.; Zhang, R.; Chen, H.; Lu, Y.D.; Zhou, J.H.; Chang, X.; Qiu, G.X.; Wu, Z.H.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mat. Sci. Eng. C 2015, 46, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.C.; Yang, J.Z.; Lou, Y.T.; ur Rahman, O.; Li, Z.Y.; Ding, X.; Gao, J.; Du, C.W.; Zhang, D.W. Mussel-inspired superhydrophilic surface with enhanced antimicrobial properties under immersed and atmospheric conditions. Appl. Surf. Sci. 2019, 465, 267–278. [Google Scholar] [CrossRef]
- Jin, M.M.; Shen, Y.Z.; Luo, X.Y.; Tao, J.; Xie, Y.H.; Chen, H.F.; Wu, Y. A combination structure of microblock and nanohair fabricated by chemical etching for excellent water repellency and icephobicity. Appl. Surf. Sci. 2018, 455, 883–890. [Google Scholar] [CrossRef]
- Wang, C.Y.; Nair, S.S.; Veeravalli, S.; Moseh, P.; Wynne, K.J. Sticky or slippery wetting: Network formation conditions can provide a one-way street for water flow on platinum-cured silicone. ACS Appl. Mater. Inter. 2016, 8, 14252–14262. [Google Scholar] [CrossRef]
- Zhuo, Y.Z.; Wang, F.; Xiao, S.B.; He, J.Y.; Zhang, Z.L. One-step fabrication of bioinspired lubricant-regenerable icephobic slippery liquid-infused porous surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Zhu, X.T.; Lu, J.W.; Li, X.M.; Wang, B.; Song, Y.M.; Miao, X.; Wang, Z.J.; Ren, G.N. A simple way to a slippery lubricant impregnated coating with ultra-stability and self-replenishment property. Ind. Eng. Chem. Res. 2019, 58, 8148–8153. [Google Scholar] [CrossRef]
- Crisp, A.; de Juan, E.; Tiedeman, J. Effect of silicone oil viscosity on emulsification. Arch. Ophthalmol. 1987, 105, 546–550. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly (dimethylsiloxane)(PDMS) surfaces. J. Colloid. Interf. Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.X.; Liu, Y.Y.; Liang, X.D. Durability of a lubricant-infused Electrospray Silicon Rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, B.; Tian, Y.; Wang, F.; Chen, Q.; Zhang, F.; Qian, H.; Ma, L. Facile One-Step Method to Fabricate a Slippery Lubricant-Infused Surface (LIS) with Self-Replenishment Properties for Anti-Icing Applications. Coatings 2020, 10, 119. https://doi.org/10.3390/coatings10020119
Zhang J, Liu B, Tian Y, Wang F, Chen Q, Zhang F, Qian H, Ma L. Facile One-Step Method to Fabricate a Slippery Lubricant-Infused Surface (LIS) with Self-Replenishment Properties for Anti-Icing Applications. Coatings. 2020; 10(2):119. https://doi.org/10.3390/coatings10020119
Chicago/Turabian StyleZhang, Juantao, Bei Liu, Yan Tian, Fushan Wang, Qingguo Chen, Fan Zhang, Hongchang Qian, and Lingwei Ma. 2020. "Facile One-Step Method to Fabricate a Slippery Lubricant-Infused Surface (LIS) with Self-Replenishment Properties for Anti-Icing Applications" Coatings 10, no. 2: 119. https://doi.org/10.3390/coatings10020119



