Influence of Magnesium Aluminate Nanoparticles on Epoxy-Based Intumescent Flame Retardation Coating System
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
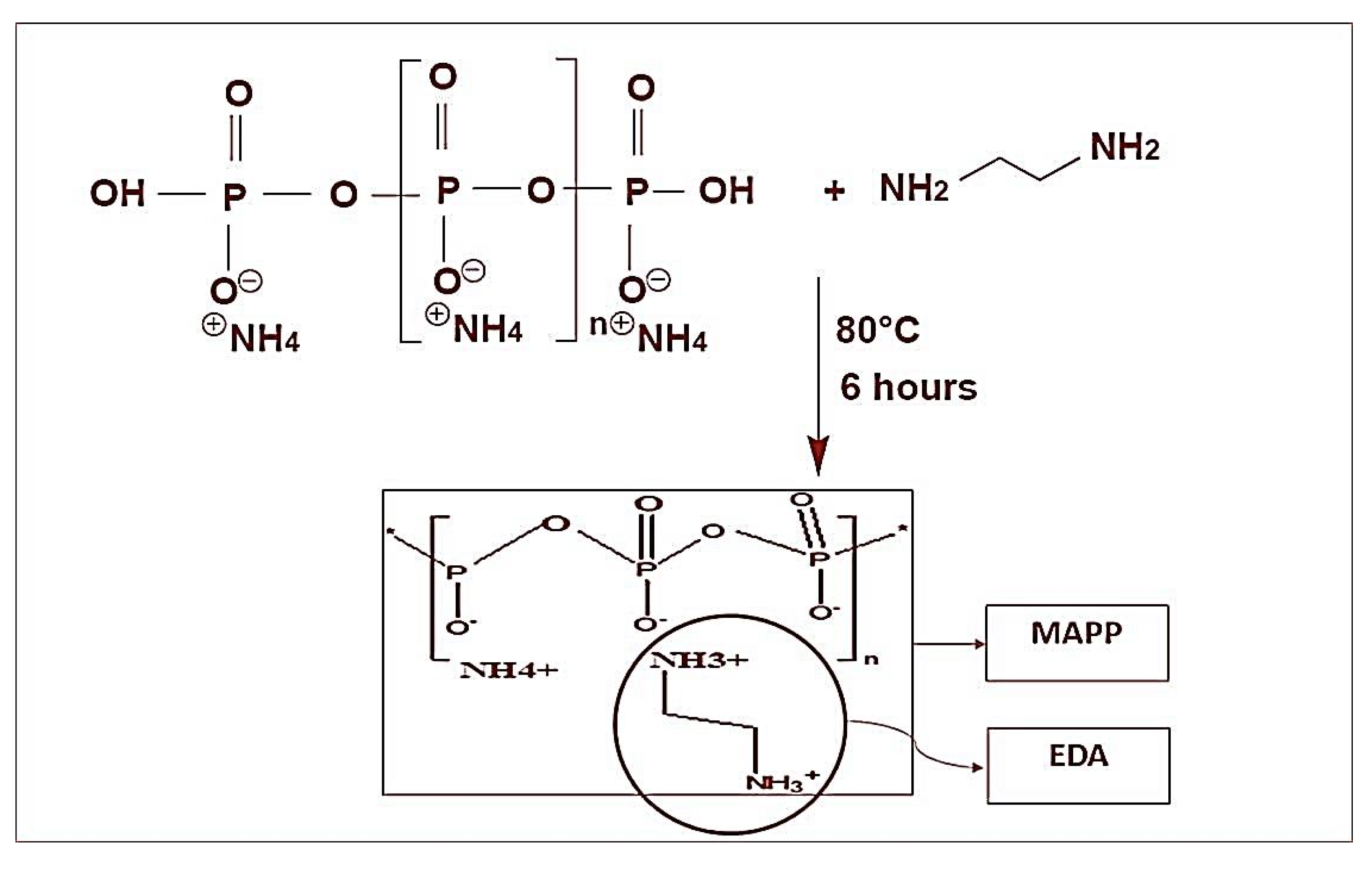
2.2.1. Preparation of MAPP
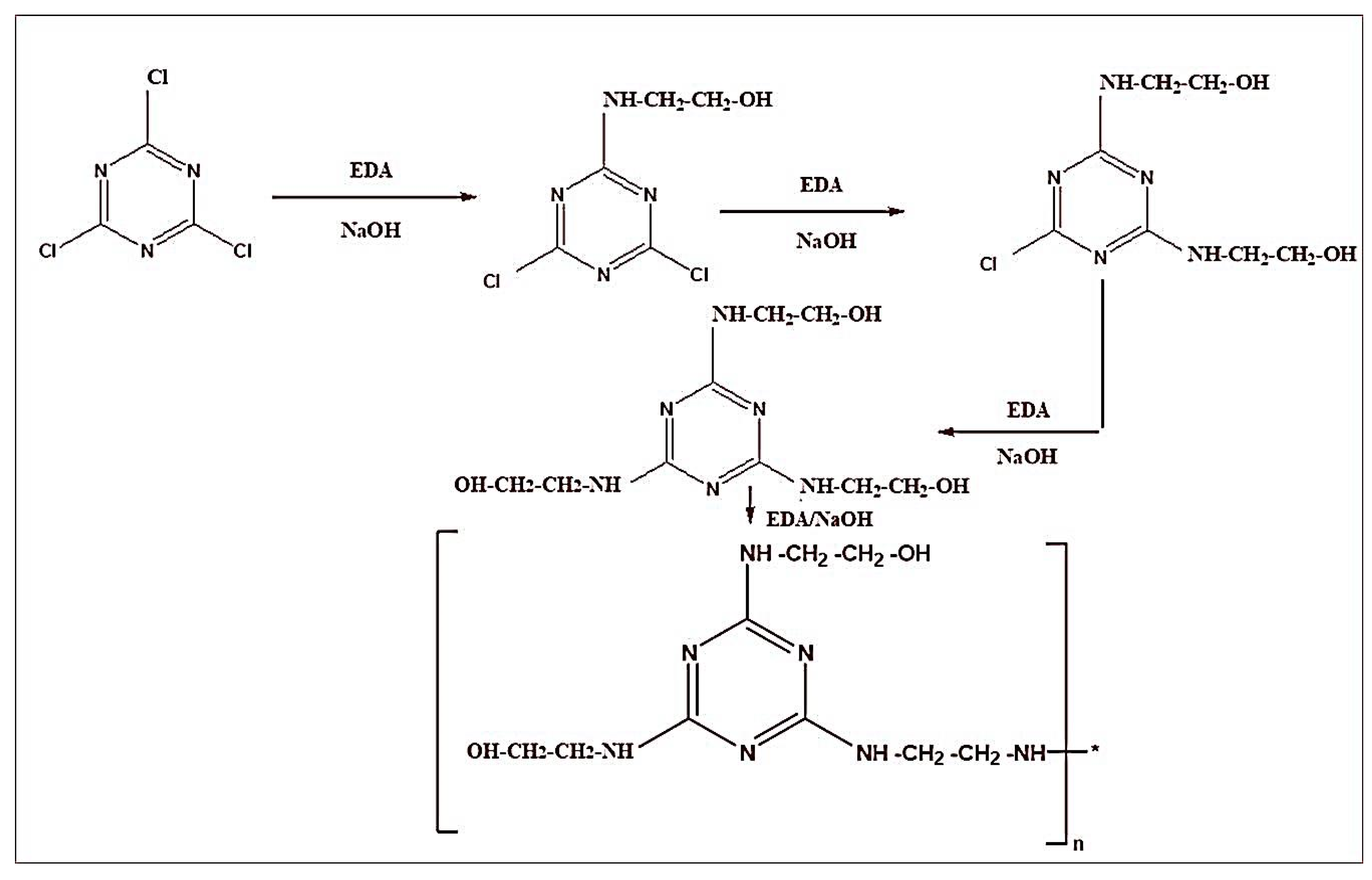
2.2.2. Preparation of CFA
2.2.3. Synthesis of MgAl2O4 NPs
2.2.4. Formulation Sample Preparation
2.2.5. Characterization MgAl2O4 NPs
2.2.6. Characterization Methods for Coatings
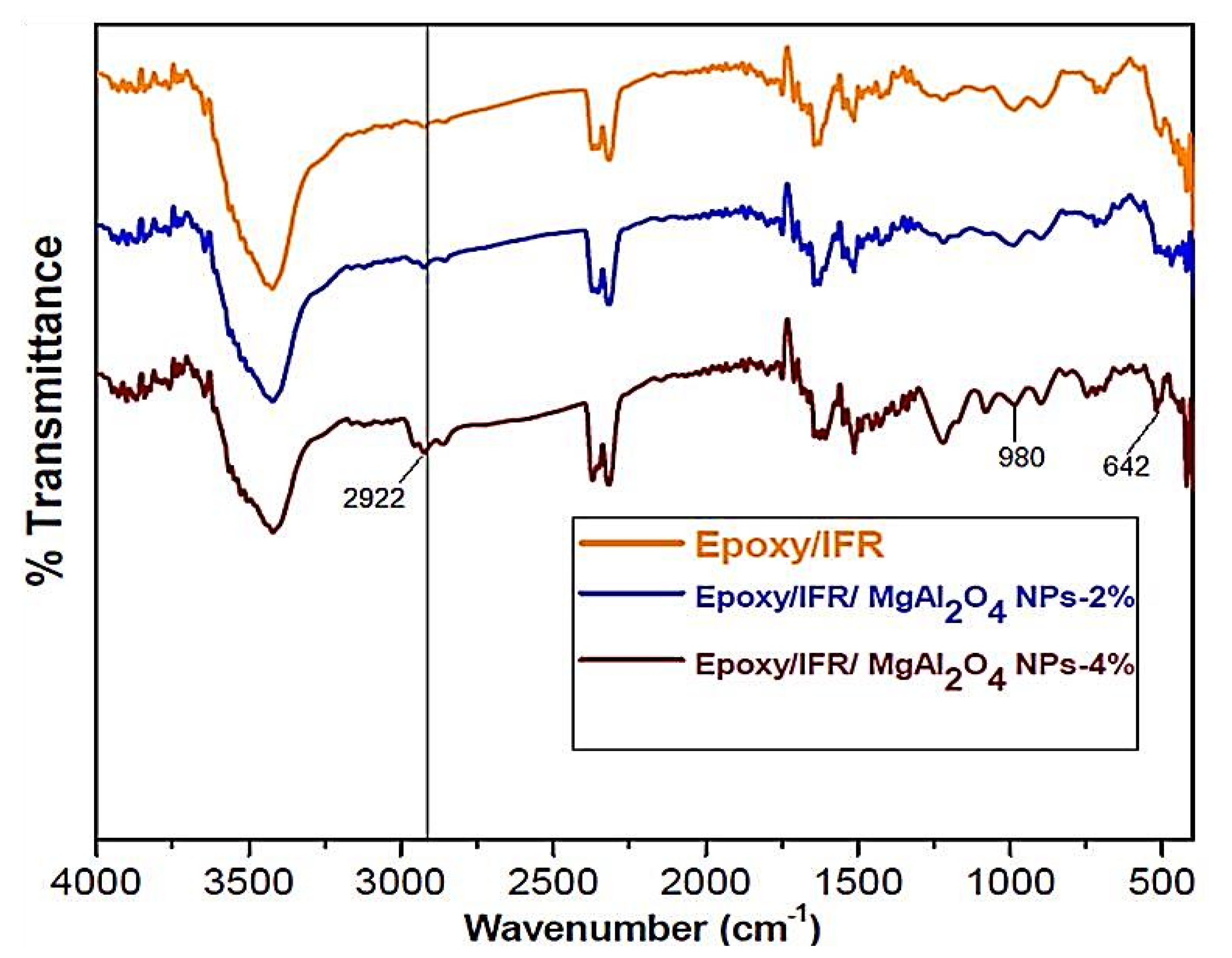
FTIR Test
X-ray Diffraction Study
Flame Retardancy Test
Thermogravimetric Analysis Test
Combustion Test
3. Result and Discussion
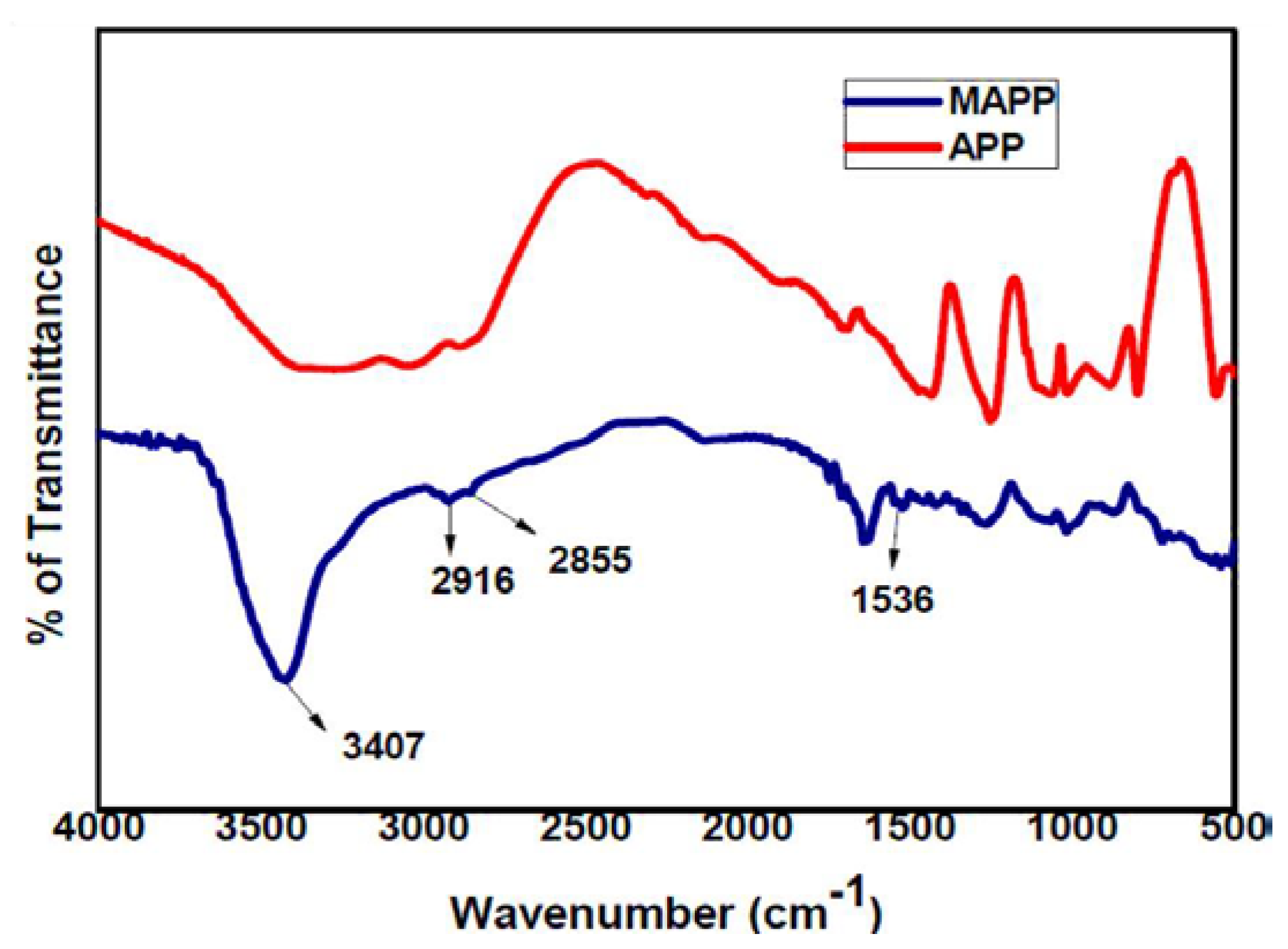
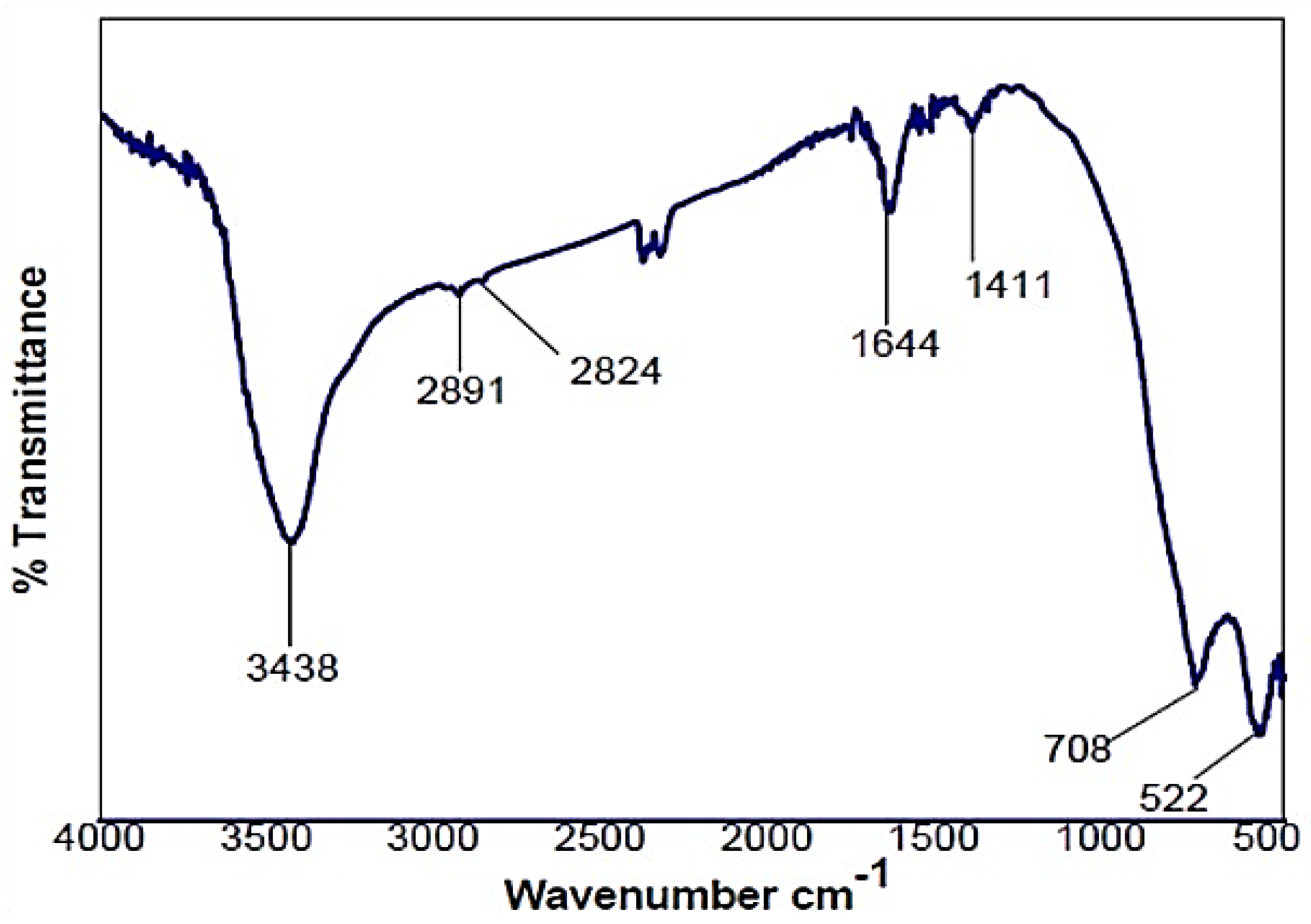
3.1. FTIR of MAPP
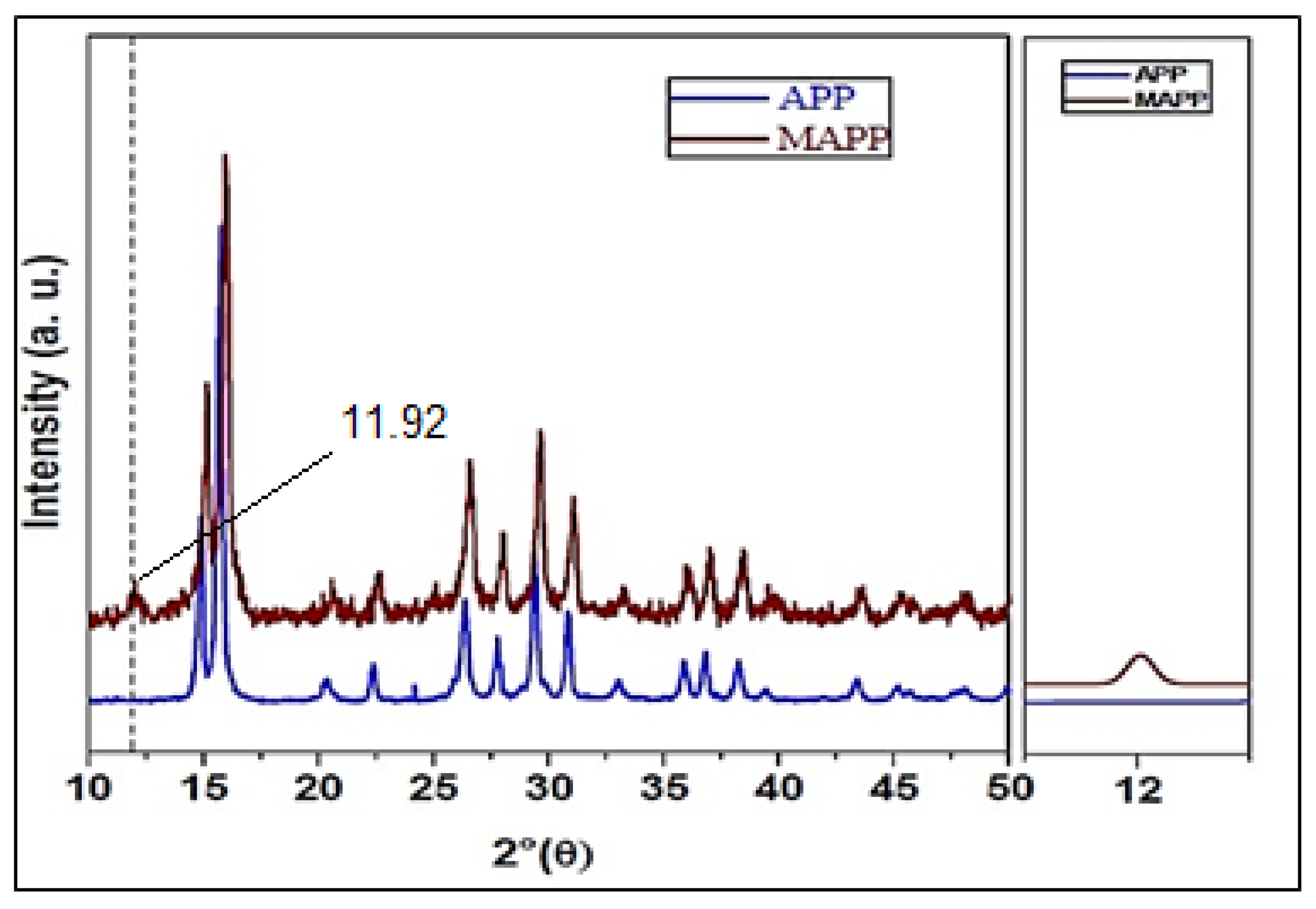
3.2. XRD of MAPP
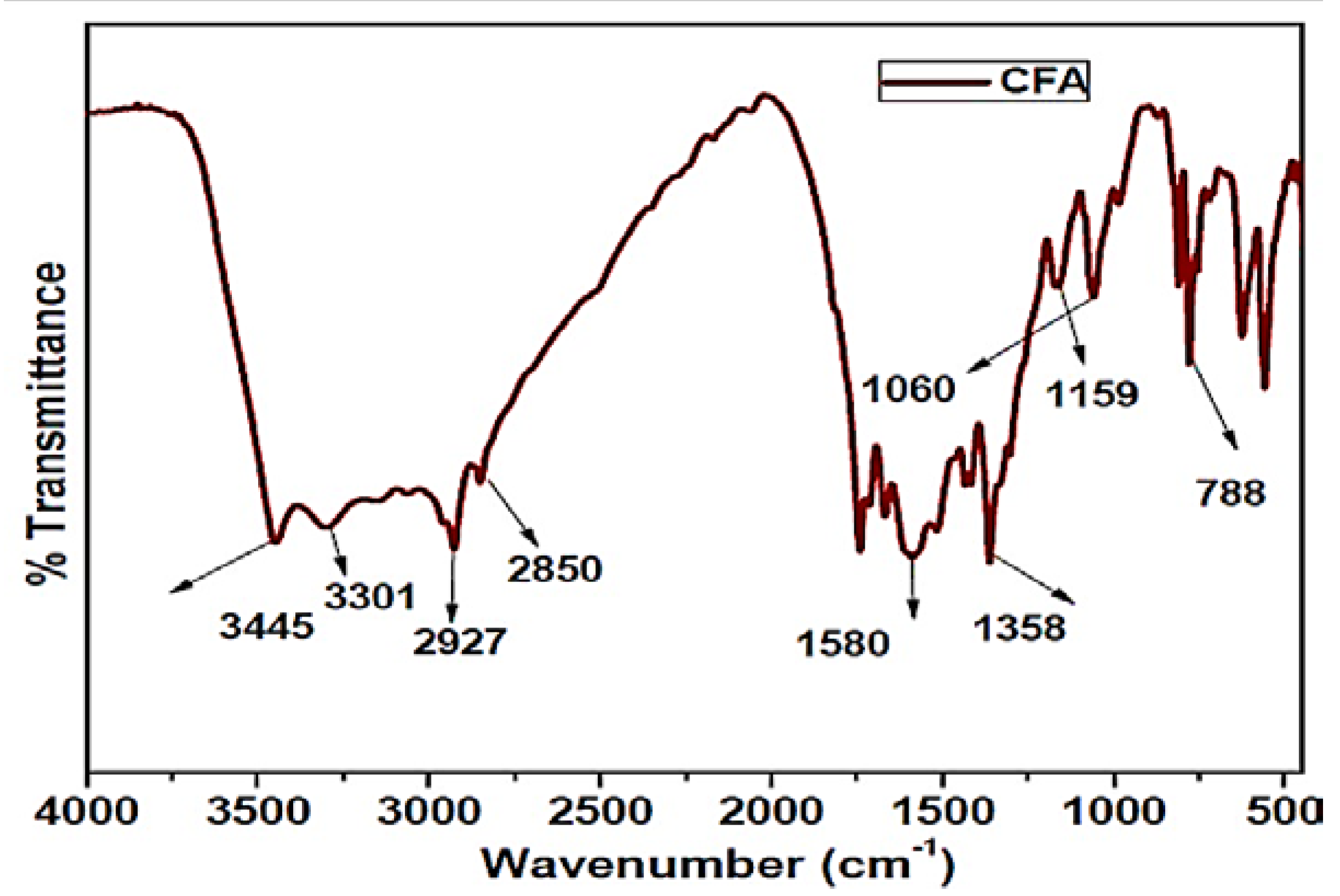
3.3. FTIR of CFA
3.4. Thermogravimetric Analysis of CFA
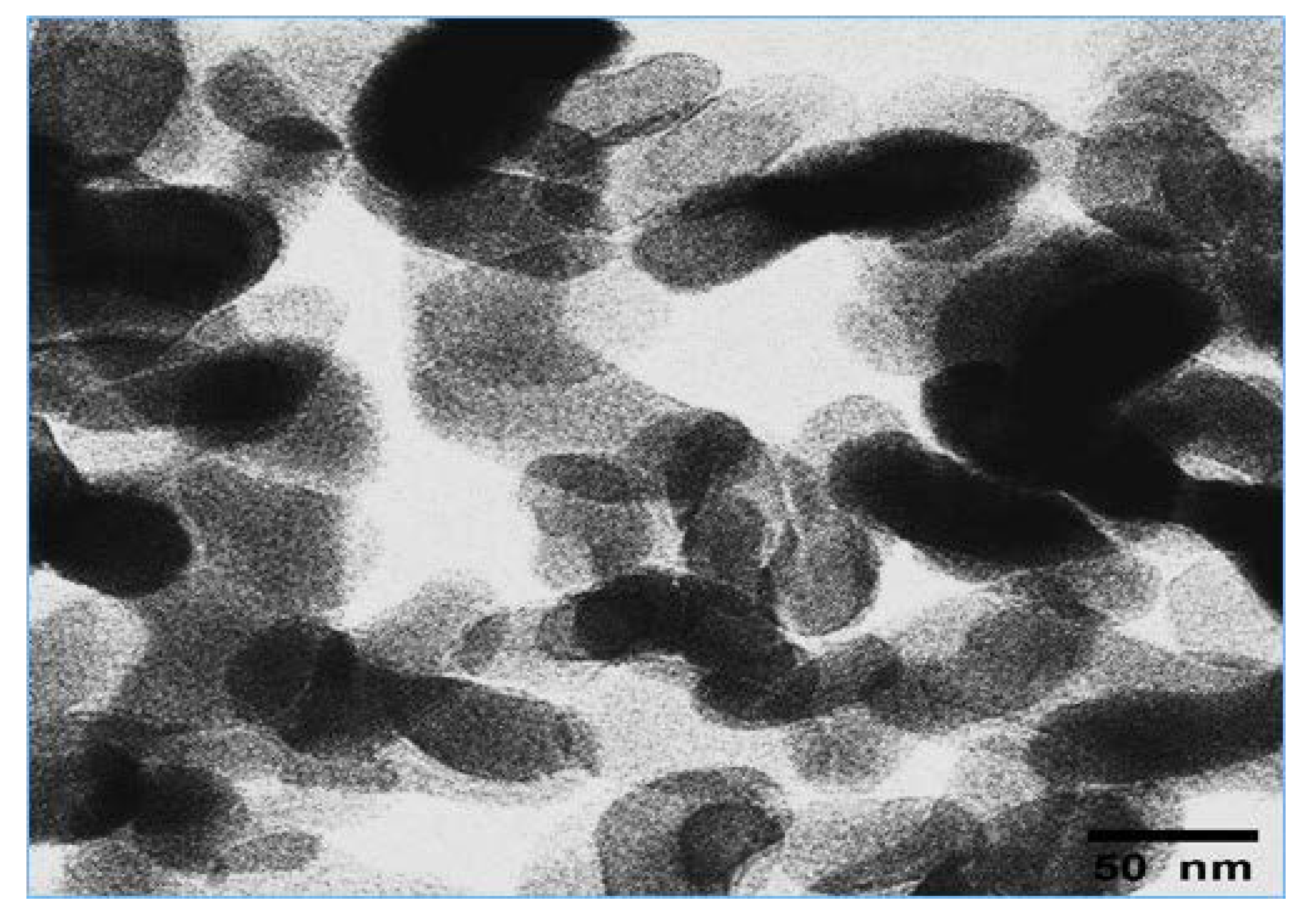
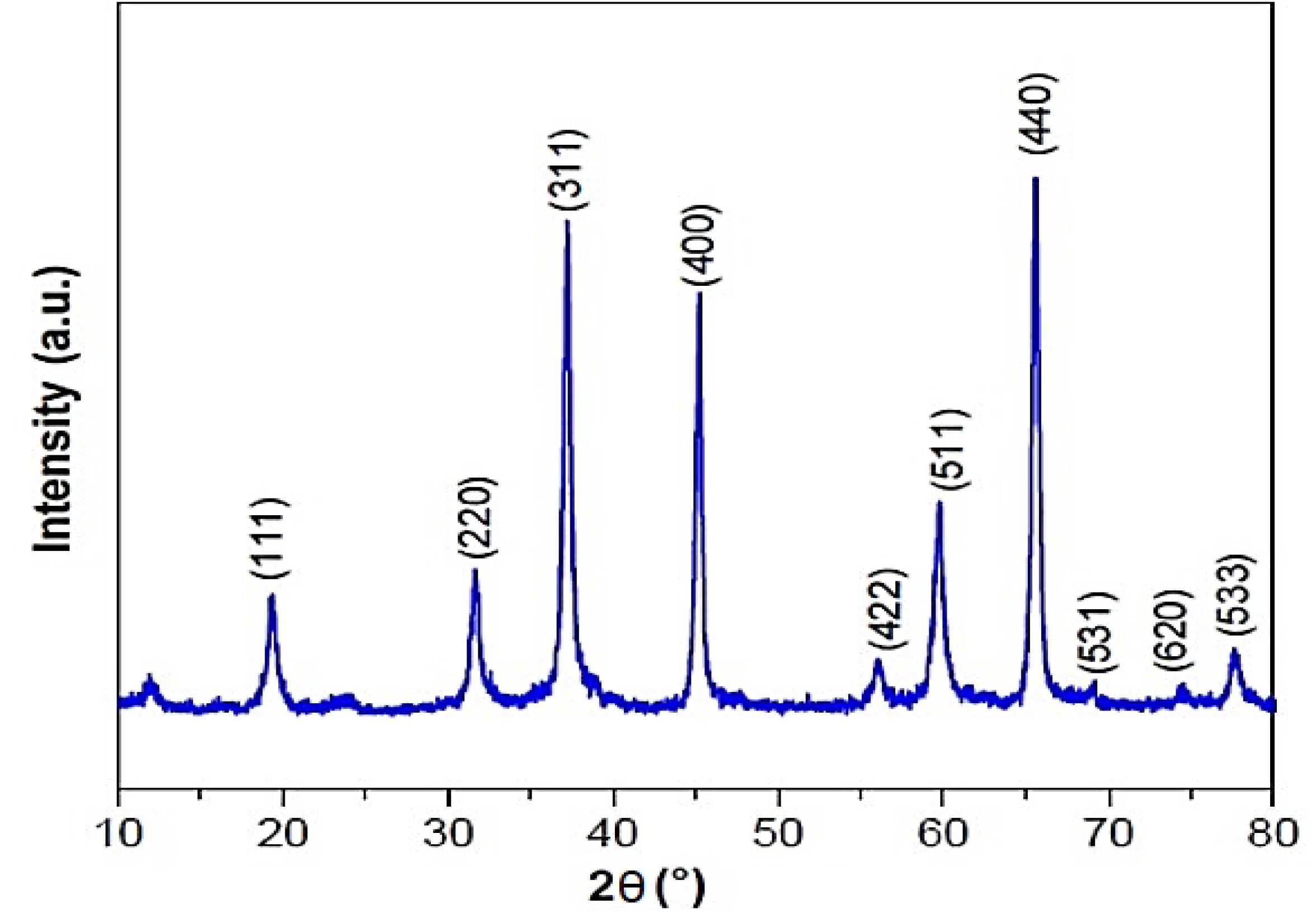
3.5. Characterization of MgAl2O4 NPs
3.6. Flame Retardancy Test
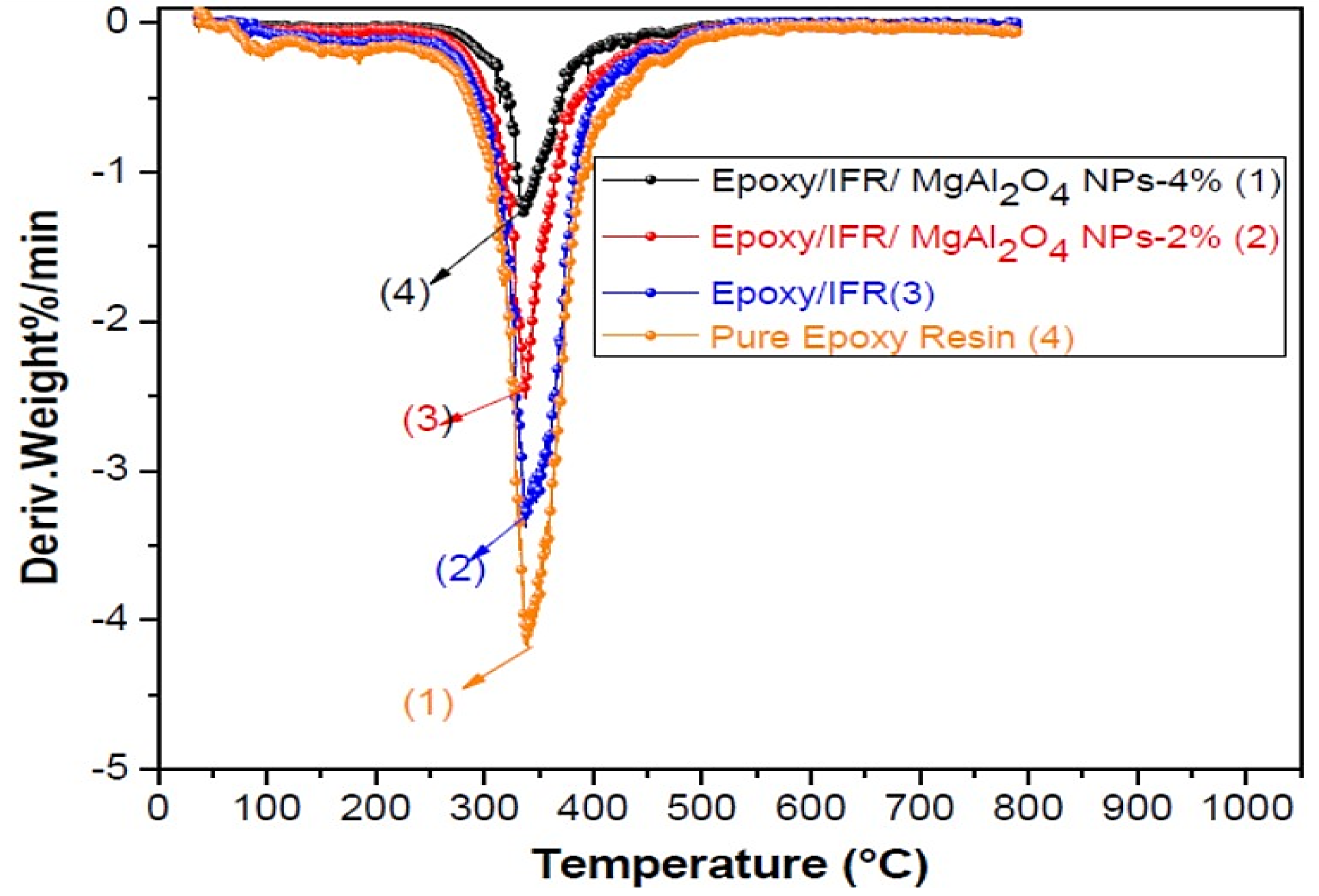
3.7. Thermogravimetric Analysis of Coatings
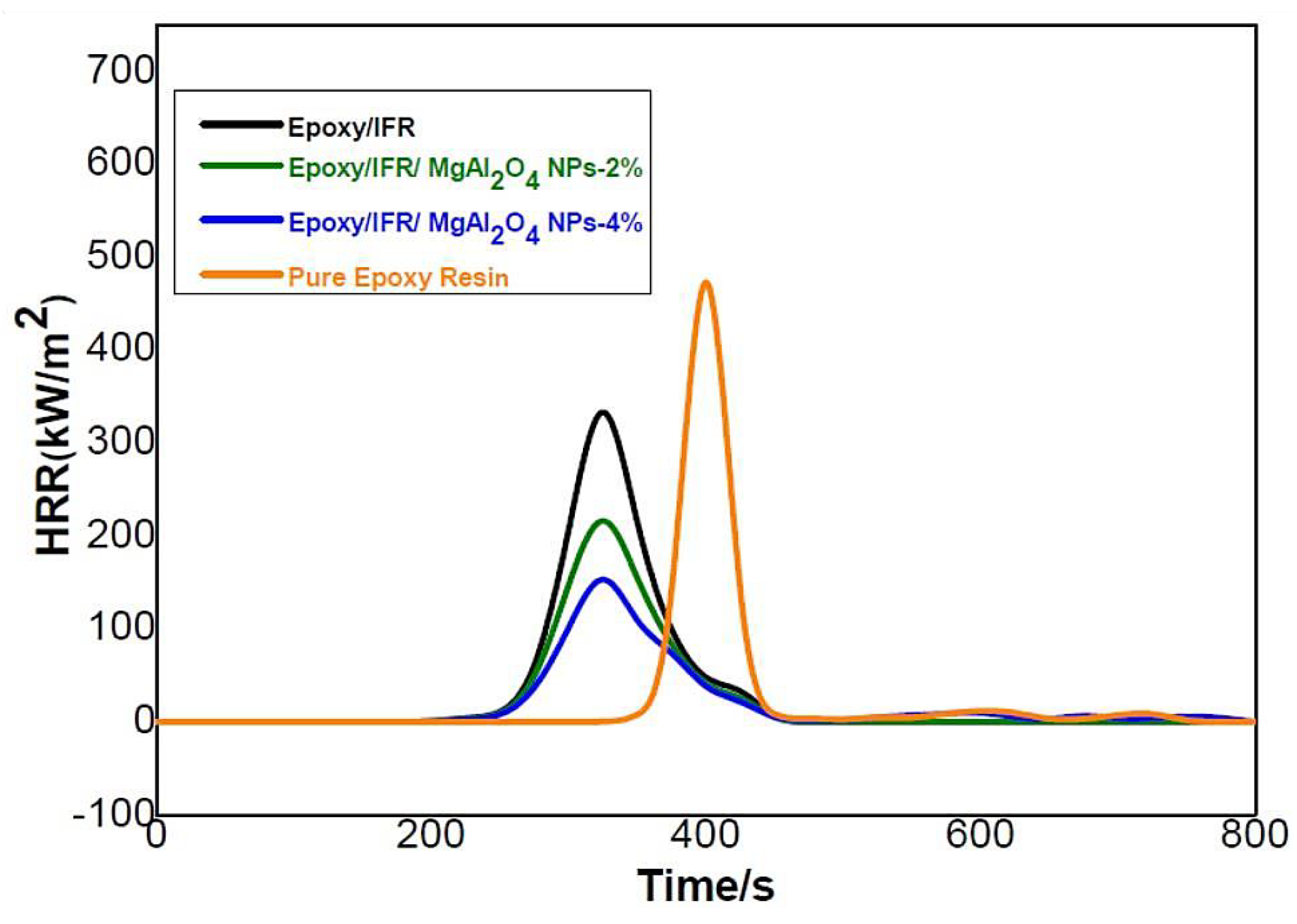
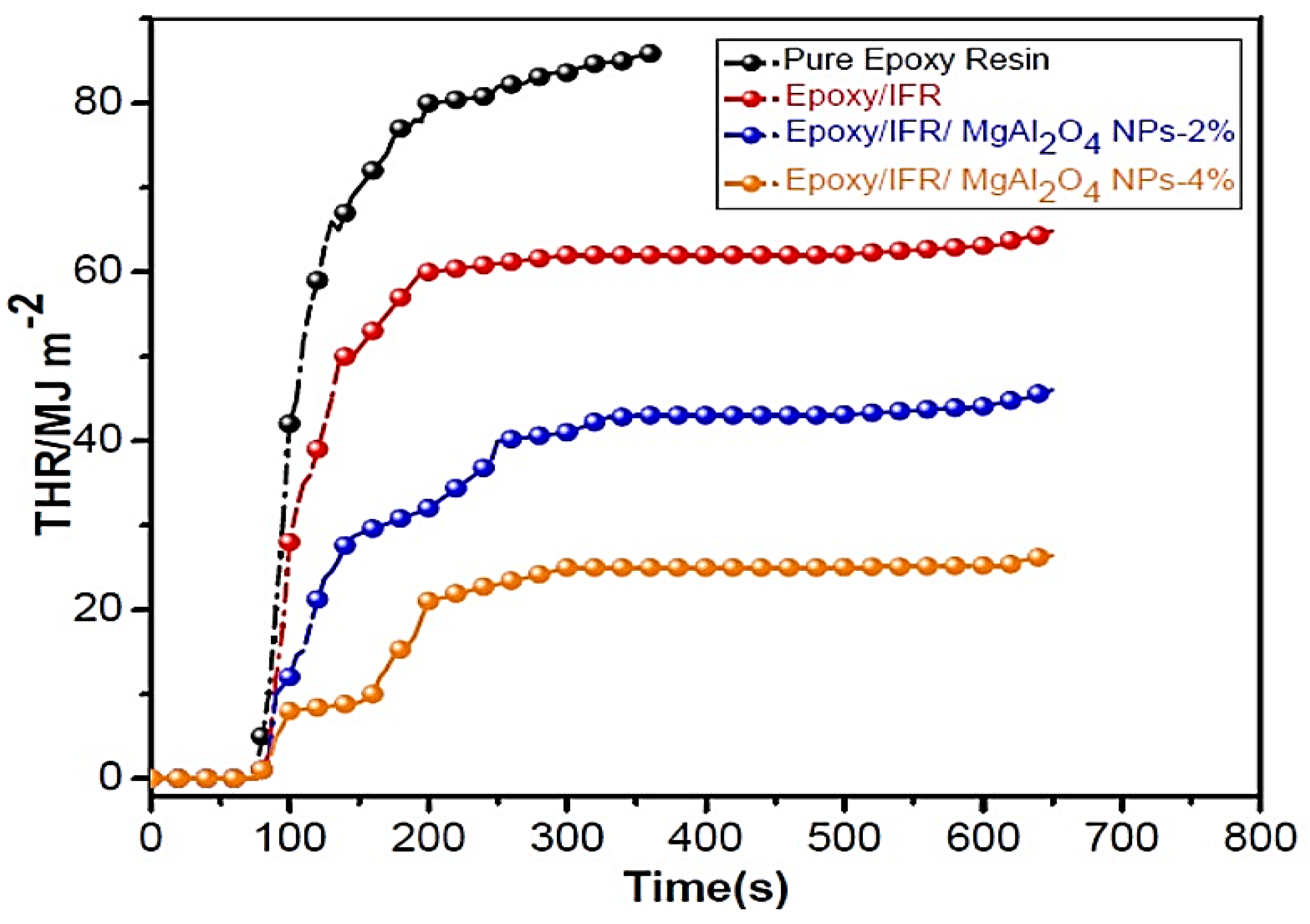
3.8. Cone-Calorimetric Analysis
3.9. Compositions of Charred Layers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dittrich, B.; Wartig, K.-A.; Mülhaupt, R.; Schartel, B. Flame-retardancy properties of intumescent ammonium poly(phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 2014, 6, 2875–2895. [Google Scholar]
- Bourbigot, S.; Bras, M.L.; Delobel, R. Carbonization mechanisms resulting from intumescence association with the ammonium polyphosphate-pentaerythritol fire retardant system. Carbon N. Y. 1993, 31, 1219–1230. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Trémillon, J.M. Synergistic effect of zeolite in an intumescence process: Study of the interactions between the polymer and the additives. J. Chem. Soc. Faraday Trans. 1996, 92, 3435–3444. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Bowbigot, S.; Patrice, B. 4A zeolite synergistic agent in new flame retardant intumescent formulations of polyethylenic polymers-study of the effect of the constituent monomers. Polym. Degrad. Stab. 1996, 3910, 275–287. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Kim, K.H. Effect of precursor ratios on the synthesis of MgAl2O4 nanoparticles by a reverse microemulsion method. J. Ceram. Process. Res. 2010, 11, 96–99. [Google Scholar]
- Composites, P.; Aldalbahi, A.; Alotaibi, B.; El-faham, A. Their E ff ects on Flame Retardancy of polypropylene nanocomposites. J. Thermoplast. Compos. Mater. 2020, 1–11. [Google Scholar] [CrossRef]
- Dai, J.; Li, B. Synthesis, Thermal Degradation, and Flame Retardance of Novel Triazine Ring-Containing Macromolecules for Intumescent Flame Retardant Polypropylene. J. Appl. Polym. Sci. 2010, 116. [Google Scholar] [CrossRef]
- Delobel, R.; Le Bras, M.; Ouassou, N.; Alistiqsa, F. Thermal Behaviours of Ammonium Polyphosphate-Pentaerythritol and Ammonium Pyrophosphate-Pentaerythritol Intumescent Additives in Polypropylene Formulations. J. Fire Sci. 1990, 8, 85–108. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Li, J. Effects of a semi-bio-based triazine derivative on intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2019, 30, 1259–1268. [Google Scholar] [CrossRef]
- Édelobel, R.; Bourbigot, S.; Le Bras, M.; Schmidt, Y.; Marie Leroy, J. Invariant values of kinetic parameters—Evaluation of fire retardancy application to the PP-APP/PER system. Makromol. Chemie. Macromol. Symp. 1993, 74, 59–69. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Lang, D.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Flame retardant mechanism of a novel intumescent flame retardant polypropylene. Procedia Eng. 2013, 52, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.P.; Li, W.Y.; Wang, Y.Z. Synthesis and characterization of a novel nitrogen-containing flame retardant. J. Appl. Polym. Sci. 2004, 94, 1556–1561. [Google Scholar] [CrossRef]
- Li, G.; Liang, G.; He, T.; Yang, Q.; Song, X. Effects of EG and MoSi2 on thermal degradation of the intumescent coating. Polym. Degrad. Stab. 2007, 92. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Dai, J.; Jia, H.; Gao, S. Synergistic effects of lanthanum oxide on a novel intumescent flame retardant polypropylene system. Polym. Degrad. Stab. 2008, 93, 9–16. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.S.; Deng, C.L.; Wang, D.Y.; Song, Y.P.; Wang, Y.Z. The synergistic flame-retardant effect of O-MMT on the intumescent flame-retardant PP/CA/APP systems. Polym. Adv. Technol. 2010, 21, 789–796. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Z.; Hu, K.; Fan, W. Flammability and thermal degradation of flame retarded polypropylene composites containing melamine phosphate and pentaerythritol derivatives. Polym. Degrad. Stab. 2005, 90, 523–534. [Google Scholar] [CrossRef]
- Wu, Q.; Qu, B. Synergistic effects of silicotungistic acid on intumescent flame-retardant polypropylene. Polym. Degrad. Stab. 2001, 74, 255–261. [Google Scholar] [CrossRef]
- Nikolic, M.; Lawther, J.M.; Sanadi, A.R. Use of nanofillers in wood coatings: A scientific review. J. Coat. Technol. Res. 2015, 12, 445–461. [Google Scholar] [CrossRef]
- Patricia, C.; Lovergine, N.; Prete, P. Progress in Crystal Growth and Characterization of Materials: Preface. Prog. Cryst. Growth Charact. Mater. 2003, 47, 63–64. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Zhang, L.; Zhang, J.; Xiong, W.; Wu, Y.; Song, B.; Mai, Y. Enhanced Flame Retardancy and Mechanical Properties of Intumescent Flame-Retardant Polypropylene with Triazine Derivative-Modified Nano-SiO2. Polym. Sci. Ser. B 2020. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Saini, P.; Dhawan, S.K. Poly (3,4-ethylenedioxythiophene) γ-Fe2O3 polymer composite–super paramagnetic behavior and variable range hopping 1D conduction mechanism–synthesis and characterization. Polym. Adv. Technol. 2008, 229–236. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, R.; Song, Y.; He, J. Investigations of flame-retarded thermoplastic poly(imide-urethane)s with intumescent flame retardants. Ind. Eng. Chem. Res. 2014, 53, 9728–9737. [Google Scholar] [CrossRef]
- Tang, W.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B. Intumescent flame retardant behavior of charring agents with different aggregation of piperazine/triazine groups in polypropylene. Polym. Degrad. Stab. 2019, 169. [Google Scholar] [CrossRef]
- Tian, J.; Tian, P.; Ning, G.; Pang, H.; Song, Q.; Cheng, H.; Fang, H. Synthesis of porous MgAl2O4 spinel and its superior performance for organic dye adsorption. RSC Adv. 2015, 5, 5123–5130. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Wen, P.; Zhan, J.; Hong, N.; Cai, W.; Gui, Z.; Hu, Y. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2017, 144, 454–463. [Google Scholar] [CrossRef]
- Emir, H.; Arkiş, E.; Balköse, D.; Ülkü, S. Synergistic effect of natural zeolites on flame retardant additives. Polym. Degrad. Stab. 2005, 89, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Ravadits, I.; Tóth, A.; Marosi, G.; Márton, A.; Szép, A. Organosilicon surface layer on polyolefins to achieve improved flame retardancy through an oxygen barrier effect. Polym. Degrad. Stab. 2001, 74, 419–422. [Google Scholar]
- Xiao, Z.; Liu, S.; Zhang, Z.; Mai, C.; Xie, Y.; Wang, Q. Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylate phosphate and melamine-urea-formaldehyde resin. Prog. Org. Coat. 2018, 121, 64–72. [Google Scholar] [CrossRef]
- Xu, B.; Ma, W.; Wu, X.; Qian, L.; Jiang, S. Flame retardancy and thermal behavior of intumescent flame-retardant EVA. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- ASTM D2863-13 Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index); ASTM International: West Conshohocken, PA, USA, 2013.
- ISO 5660-1 Reaction-to-Fire Tests-Heat Release, Smoke Production and Mass Loss Rate-Part 1: Heat Release Rate (Cone Calorimeter Method); International Standard Organization: Geneva, Switzerland, 2002.
- Yang, K.; Xu, M.J.; Li, B. Synthesis of N-ethyl triazine-piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2013, 98, 1397–1406. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Nageswara Rao, T.; Manohra Naidu, T.; Kim, M.S.; Parvatamma, B.; Prashanthi, Y.; Bon Heun, K. Influence of Zinc Oxide Nanoparticles and Char Forming Agent Polymer on Flame Retardancy of Intumescent Flame Retardant Coatings. Nanomaterials 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]


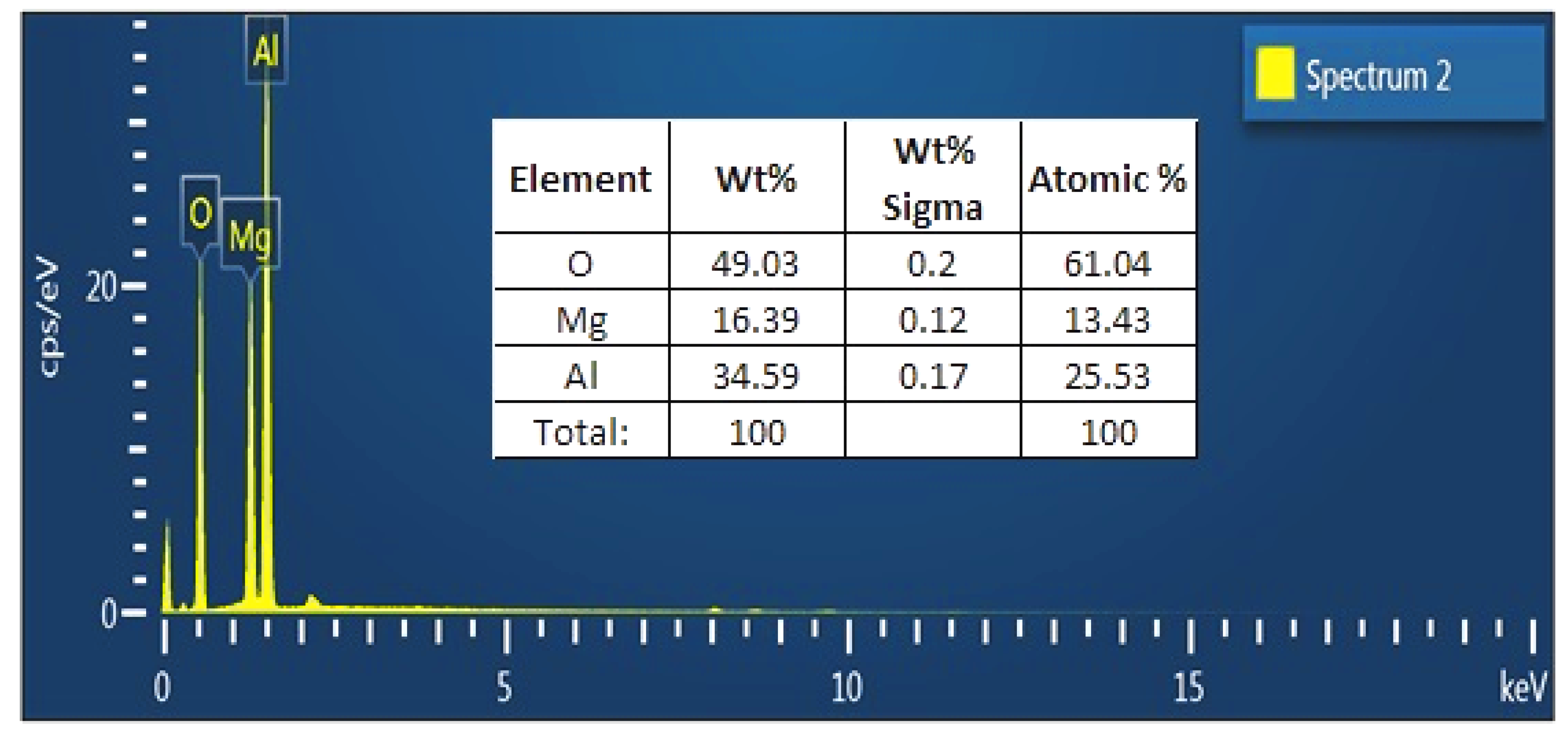

| Sample | Resin (%) | MAPP (%) | CFA (%) | MgAl2O4 NPs |
|---|---|---|---|---|
| Pure Epoxy Resin | 100 | 0 | 0 | 0 |
| Epoxy/IFR | 70 | 20 | 10 | 0 |
| Epoxy/IFR/MgAl2O4 NPs-2% | 68 | 20 | 10 | 2 |
| Epoxy/IFR/MgAl2O4 NPs-4% | 66 | 20 | 10 | 4 |
| Sample | LOI (%) | UL-94V |
|---|---|---|
| Pure Epoxy Resin | 25.2 | No rating |
| Epoxy/IFR | 31.2 | V-0 |
| Epoxy/IFR/MgAl2O4 NPs-2% | 31.9 | V-0 |
| Epoxy/IFR/MgAl2O4 NPs-4% | 32.6 | V-0 |
| STDEV | 3.40 |
| Sample | Tonset (°C) | MLR%/min | Char Residue (%) at 800 °C |
|---|---|---|---|
| Pure Epoxy Resin | 192 | 15.2 | 0.00 |
| Epoxy/IFR | 187 | 12.3 | 23.12 |
| Epoxy/IFR/MgAl2O4 NPs-2% | 165 | 11.2 | 31.05 |
| Epoxy/IFR/MgAl2O4 NPs-4% | 122 | 8.3 | 41.14 |
| Sample | TTI (S) | PHRR (kW m−2) | THR (MJ m−2 kg−1) |
|---|---|---|---|
| Pure Epoxy Resin | 60 | 453 | 82 |
| Epoxy/IFR | 55 | 321 | 63 |
| Epoxy/IFR/MgAl2O4 NPs-2% | 43 | 203 | 42 |
| Epoxy/IFR/MgAl2O4 NPs-4% | 43 | 105 | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuhimd, H.; Nageswara Rao, T.; Song, J.-i.; Yarasani, P.; Ahmed, F.; Parvatamma, B.; Alothman, A.A.; Mana AL-Anazy, M.; Ifseisi, A.A. Influence of Magnesium Aluminate Nanoparticles on Epoxy-Based Intumescent Flame Retardation Coating System. Coatings 2020, 10, 968. https://doi.org/10.3390/coatings10100968
Abuhimd H, Nageswara Rao T, Song J-i, Yarasani P, Ahmed F, Parvatamma B, Alothman AA, Mana AL-Anazy M, Ifseisi AA. Influence of Magnesium Aluminate Nanoparticles on Epoxy-Based Intumescent Flame Retardation Coating System. Coatings. 2020; 10(10):968. https://doi.org/10.3390/coatings10100968
Chicago/Turabian StyleAbuhimd, Hatem, Tentu Nageswara Rao, Jung-il Song, Prashanthi Yarasani, Faheem Ahmed, Botsa Parvatamma, Asma A. Alothman, Murefah Mana AL-Anazy, and Ahmad A. Ifseisi. 2020. "Influence of Magnesium Aluminate Nanoparticles on Epoxy-Based Intumescent Flame Retardation Coating System" Coatings 10, no. 10: 968. https://doi.org/10.3390/coatings10100968





