Fecal Microbiota Transplantation: Expanding Horizons for Clostridium difficile Infections and Beyond
Abstract
:1. Introduction
- Early Case Reports: The “discovery phase” stemmed from a number of literature reports, often expressing considerable surprise that FMT worked so well to dramatically cure terminally ill patients. The severe condition was thought to be a staphylococcal pseudomembranous enterocolitis [2,3,4,5], but after 1981 [6,7], when CDI toxin became detectable [3,4,5,6,7], clinicians questioned and minimized the role of Staphylococcus aureus in pseudomembranous intestinal lesions because they realized that most pseudomembranous enterocolitis diagnoses were positive for CDI toxin [8].
- Donor Selection: Half a century later, with FMT becoming used more frequently, a group experienced in utilising FMT published the first standardized donor selection guidelines [9]. More stringent selection criteria are already appearing, and there is every reason to believe that more refined guidelines will emerge as we incorporate additional exclusion criteria, such as family history of obesity, metabolic syndrome and diabetes [10], into FMT practice.
- Methodology Refinements: As FMT case reports publish from various parts of the world, summaries and comparisons of numerous methodological analyses are helping refine FMT practice [11,12,13,14]. Topics reviewed to date include history of FMT, the changing face of CDI and stool solvent, volume and delivery routes (via the upper GI tract versus the lower GI tract). For example, and perhaps significant for future FMT administration, a recent study reported 27 patients who underwent fixed volume FMT delivered via upper enteroscopy and colonoscopy at the same session to cover both the small and large intestine. In the largest study of its kind, all 27 patients cleared C. difficile toxin from their systems, resulting in 100% clinical efficacy with only minimal, transient adverse effects [15]. These cure rates contrast with that of 73%–92% for delivery via nasogastric/duodenal tubes and via upper endoscopy. This “dual coverage” method points to the importance of developing encapsulated microbiota to treat CDI, so that normal microbiota can be administered via oral delivery, thereby exposing the entire GI tract to healthy, diverse flora and potentially improving efficacy of CDI eradication.
- Long-Term Follow-up: Several articles have addressed the need for long-term follow up of patients receiving FMT since it is crucial to know about any untoward effects that may only develop long after the procedure [14,16]. At this time, short-term adverse effects have been remarkably infrequent following FMT [11]. In a long-term follow-up study of 77 patients after FMT, some new diseases developed in 4 subjects, although these had not been present in the donors and a clear relationship between the new disease and FMT was not evident [17]. Reflecting on the origin of the microbiota used in FMT, one could conclude that this “biologic” therapy derived from a healthy donor, say 35 years of age, has already undergone multiple decades of in vivo “testing” for adverse events within that healthy donor [18]. Long-term safety evidence to date [17] implies that microbiota from healthy, well-screened donors is currently the safest FMT product we have, but longitudinal studies to monitor and correlate gut microbiota with the health of donors and recipients have yet to be done. Indeed, in our own practice with 26 years follow-up of several thousand FMT recipients, there has been no donor-to recipient illness transfer. Ironically, the perceived safety of cultured consortia of defined microbiota, with their inherent potential for gene transfer and exchange, cannot rival the deep level of safety that comes from a healthy, well-screened human donor. Such data come from treating with non-toxigenic C. difficile to outcompete toxigenic strains to prevent colonization [19]. The C. difficile toxin-encoding PaLoc region from a toxigenic strain was recently shown to mobilize to non-toxigenic isolates, indicating that non-toxigenic strains can become toxigenic through horizontal gene transfer events [20]. Using spores of non-toxigenic strains, in this case Clostridia spores, in therapeutics may be risky, as evidenced by the fact that several placebo patients were found to be infected with non-toxigenic strains during clinical trials, apparently due to spore contamination of communal areas [21].
- Regrowth of Depleted Microbiota: Pre- and post-FMT microbiota composition of the recipient’s GI tract has also been of particular interest. While it is possible to achieve durable implantation of donor bacteria [22], it is also becoming clear that eradicating C. difficile by antagonistic, but non-pathogenic, Clostridium spores can lead to a recovered, functional microbiota population due to regrowth of occult “missing” components such as Firmicutes and Bacteroidetes [23].
- FDA Oversight: In March 2014, the United States Food and Drug Administration (FDA) announced its intention to exercise enforcement discretion regarding Investigational New Drug (IND) applications for use of FMT for recurrent CDI. An IND application is not required to treat recurrent CDI cases, but it is still necessary for non-CDI indications and for research situations. Understanding the FDA position is crucial both to clinicians and FMT research teams [24].
2. Expanding Use of FMT for CDI in Specialised Clinical Situations
- Elderly Patients: Elderly patients are the most susceptible to relapse after initial treatment of CDI with standard of care antibiotic therapy [25,26] and are the majority of patients currently treated for relapsing CDI. Consequently, it is important to know that FMT is safe and effective in this patient population. Using data collected from multiple centres, Agrawal et al. [27] reported on 146 patients, finding 83.5% and 95.2% primary and secondary cure rates in relapsing CDI. These rates associated with a short-term adverse effect rate of 3.4% either due to the CDI, the FMT or both.
- Patients with Severe CDI: The only published study with indices about this indication is a collection of 13 cases from a multicentre study of severe and/or complicated CDI in patients who had failed several courses of antibiotics and who were subsequently treated with FMT. Of these, 84% had severe CDI and 92% had complicated CDI, and their mean post-treatment follow-up was 15 months. Primary and secondary cure rates were 84% and 92%, respectively, with minimal adverse effects of abdominal bloating and cramping early post treatment [28]. Such data indicate that age and severity should not be a barrier when considering FMT as a treatment option in the elderly, even those with severe and complicated CDI.
- The Immunosuppressed: This unique patient group frequently contracts CDI, and concerns have arisen regarding the safety of FMT for immunosuppressed patients with IBD and non-IBD associated-CDI given the possibility that septicaemia could result from the FMT procedure. In a retrospective multicentre study, Kelly et al. [29] reported on 80 CDI patients, each immunosuppressed due to HIV, solid organ transplant or cancer, and 36 of whom also had IBD. All patients received FMT, resulting in an 89% cure rate of CDI without any infection resulting from the FMT. They recorded several treatment-related adverse effects, including sedation-related aspiration and worsening of IBD. Brandt et al. [30] reported on a smaller cohort of 13 immunosuppressed IBD patients who had no CDI but were being treated for IBD with FMT. Apart from transient abdominal distension and bloating in 2 patients, there were no other adverse effects. Such data reinforce the conclusion that immunosuppressed patients have no increased risk of infection from the FMT treatment itself relative to non-immunosuppressed patients.
- Patients after Sub-total/total Colectomy: There are, as yet, few publications to indicate whether patients with partial or total colectomy suffering CDI are more difficult to cure with FMT than other types of CDI patients. At the Centre for Digestive Diseases (CDD), we have had a total of 3 patients with sub-total colectomy for whom FMT failed to cure their CDI, even with combined, repeated nasojejunal and colonic transcolonoscopic infusions; these patients have continued to have CDI for up to 7 years. There has been one publication [31], however, in which FMT via nasoduodenal administration cured a patient after total proctocolectomy of CDI in the remaining small bowel.
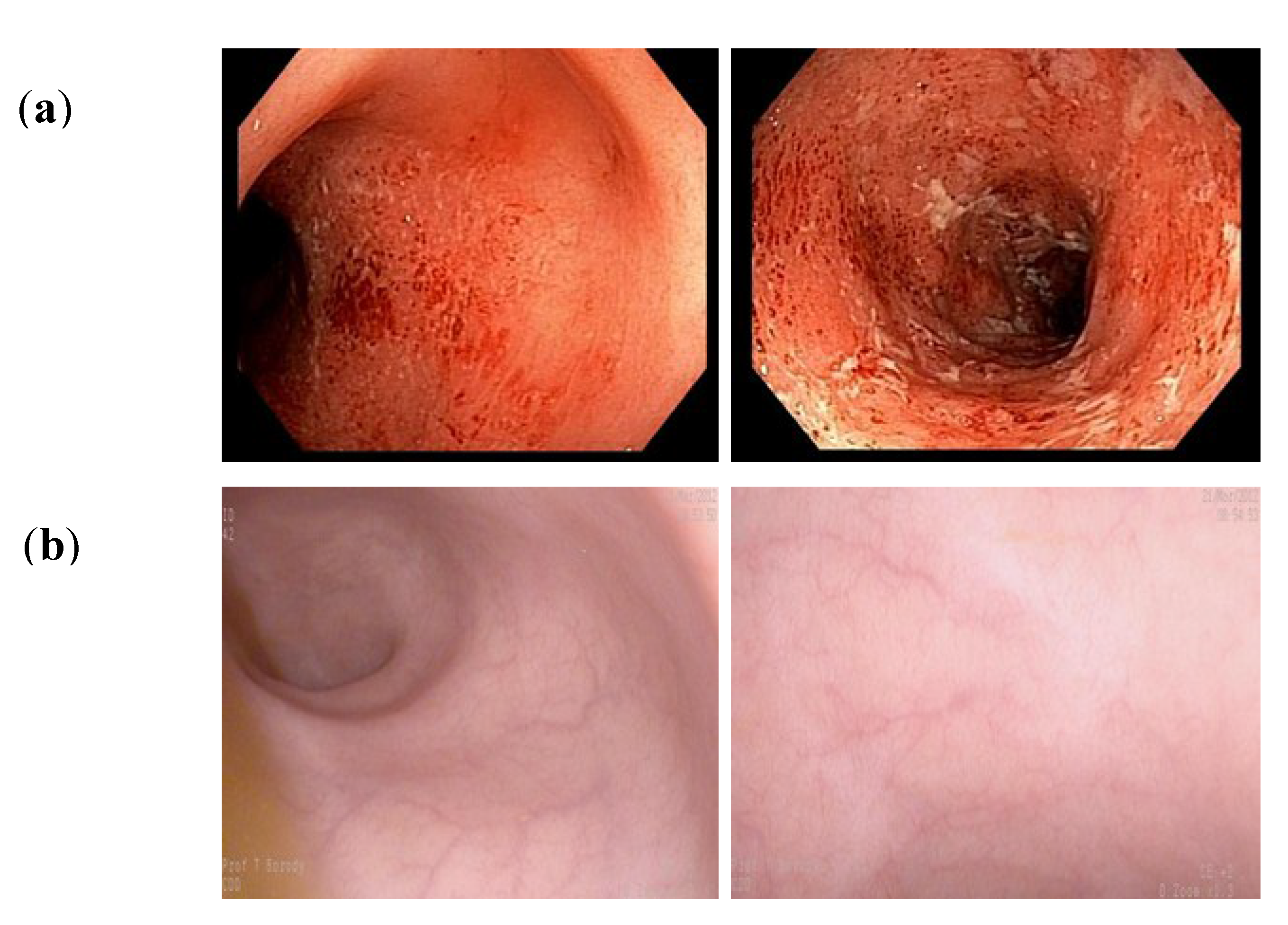
- Patients with IBD and CDI: A proportion of patients with ulcerative colitis (UC) and Crohn’s disease (CD) are co-infected with Clostridium difficile. We have reported treating such patients with FMT, noting efficient eradication of the CDI, but there are several outcomes for the IBD [32,33]. IBD symptoms may improve initially in a sub-group for the first few weeks/month, followed by symptom recurrence. In a larger proportion of patients, symptoms remain unchanged, and, in a small percentage, the IBD symptoms worsen, perhaps due to the withdrawal of antibiotics in patients who receive FMT (to avoid interfere with the implanted bacteria). Others have reported similar observations [34].
3. Expanding the Use of FMT to Non-CDI Colitis
| Clinicaltrials.gov Identifier | Indication being Trialed | Phase of Trial |
|---|---|---|
| NCT01790061 | UC | Phase 2/Phase 3 |
| NCT01793831 | CrD | Phase 2/Phase 3 |
| NCT01847170 | IBD | Phase 1 |
| NCT01896635 | UC | Phase 2 |
| NCT01947101 | UC | Phase 1 |
| NCT02016469 | IBD | NP |
| NCT02033408 | IBD | Phase 4 |
| NCT02049502 | UC-associated Pouchitis | Phase 2 |
| NCT02058524 | UC | Phase 1 |
| NCT02092402 | IBS | NP |
| NCT02108821 | IBD | Phase 1 |
| NCT02154867 | IBS | Phase 2 |
| NCT02199561 | CrD | Phase1/Phase 2 |
| NCT02227342 | UC | Phase 1/Phase 2 |
| NCT02291523 | UC | NP |
| NCT02299973 | IBS | NP |
| NCT02328547 | IBS | Phase 2 |
| NCT02330211 | Crohn’s Colitis | Phase1/Phase 2 |
| NCT02330653 | UC | Phase1/Phase 2 |
| NCT02335281 | IBD | Phase 2 |
| NCT02391012 | IBD | Phase 1 |
| NCT02417974 | CrD | Phase 2 |
| NCT02390726 | UC | Phase 0 |
- Number and frequency of FMTs
- Use of frozen stool vs. fresh stool vs. a selected consortium of organisms
- Pre-treatment with antibiotics, including which antibiotics, how many antibiotics and how long to pre-treat with antibiotics
- Determining whether there are more/less efficacious donor microbiota and what compositional differences affect efficacy
- Determining whether to administer FMT during active mucosal inflammation or to heal the mucosa with anti-inflammatory medications, e.g., immunosuppressive therapy, and then administer FMT
- Determining whether to maintain mucosal healing therapy after FMT, e.g., with 6-mercaptopurine or azathioprine for prolonged periods of time, while waiting for the transplanted microbiota to effect the “healing process” and, perhaps, to improve implantation? In a study by Borody et al. [37], patient follow up at 33 months revealed that 57% of the patients achieved mucosal healing when some were maintained on 6-mercaptopurine or azathioprine after FMT. This suggests that long-term follow-up of these patients, some of whom could be kept on anti-inflammatory agents, could be yet another mechanism by which to increase the success rate of FMT for UC patients.
4. FMT Use in Non-CDI Single Infections
5. Microbiome-Derived FMT Therapies Moving to Mainstream Medicine
| Product Candidate | Route of Administration | Company |
|---|---|---|
| MB-101 | Oral delivery | Assembly Biosciences1 |
| Full-spectrum MicrobiotaTM | Oral, colonoscopic delivery | CIPAC Limited |
| RBX2660 | Enema delivery | Rebiotix Inc |
| Ecobiotic® SER-109 | Oral delivery | Seres Health |
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Collins, D.C. Pseudomembranous enterocolitis. Further observations on the value of donor fecal enemata as an adjunct in the treatment of pseudomembranous enterocolitis. Am. J. Proctol. 1960, 2, 389–391. [Google Scholar]
- Cutolo, L.C.; Kleppel, N.H.; Freund, H.R.; Holker, J. Fecal feedings as a therapy in Staphylococcus enterocolitis. NY State J. Med. 1959, 59, 3831–3833. [Google Scholar]
- Fenton, S.; Stephenson, D.; Weder, C. Pseudomembranous colitis associated with antibiotic therapy—An emerging entity. Can. Med. Assoc. J. 1974, 111, 1110–1111, 1114. [Google Scholar]
- Bowden, T.A., Jr.; Mansberger, A.R., Jr.; Lykins, L.E. Pseudomembraneous enterocolitis: Mechanism for restoring floral homeostasis. Am. Surg. 1981, 47, 178–183. [Google Scholar]
- Schwan, A.; Sjolin, S.; Trottestam, U.; Aronsson, B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 1983, 2, 845. [Google Scholar] [CrossRef]
- Froberg, M.K.; Palavecino, E.; Dykoski, R.; Gerding, D.N.; Peterson, L.R.; Johnson, S. Staphylococcus aureus and Clostridium difficile cause distinct pseudomembranous intestinal diseases. Clin. Infect. Dis. 2004, 39, 747–750. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef]
- Borody, T.; Leis, S.; Pang, G.; Wettstein, A. Fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Available online: http://www.uptodate.com/contents/fecal-microbiota-transplantation-in-the-treatment-of-recurrent-clostridium-difficile-infection (accessed on 26 April 2015).
- Landy, J.; Al-Hassi, H.O.; McLaughlin, S.D.; Walker, A.W.; Ciclitira, P.J.; Nicholls, R.J.; Clark, S.K.; Hart, A.L. Review article: Faecal transplantation therapy for gastrointestinal disease. Aliment. Pharmacol. Ther. 2011, 34, 409–415. [Google Scholar] [CrossRef]
- Brandt, L.J. American journal of gastroenterology lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am. J. Gastroenterol. 2013, 108, 177–185. [Google Scholar] [CrossRef]
- Dutta, S.K.; Girotra, M.; Garg, S.; Dutta, A.; von Rosenvinge, E.C.; Maddox, C.; Song, Y.; Bartlett, J.G.; Vinayek, R.; Fricke, W.F. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2014, 12, 1572–1576. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef]
- Blumberg, R.; Powrie, F. Microbiota, disease, and back to health: A metastable journey. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Borody, T.J.; Peattie, D.; Kapur, A. Could fecal microbiota transplantation cure all Clostridium difficile infections? Future Microbiol. 2014, 9, 1–3. [Google Scholar] [CrossRef]
- Longstreth, W.T., Jr.; Meschke, J.S.; Davidson, S.K.; Smoot, L.M.; Smoot, J.C.; Koepsell, T.D. Hypothesis: A motor neuron toxin produced by a clostridial species residing in gut causes ALS. Med. Hypotheses 2005, 64, 1153–1156. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Grehan, M.J.; Borody, T.J.; Leis, S.M.; Campbell, J.; Mitchell, H.; Wettstein, A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J. Clin. Gastroenterol. 2010, 44, 551–561. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.; Pindar, C.; Kammer, D.; McKenney, J.; Vuliv, M.; Lombardo, M.; Henn, M.; Aunins, J.; et al. Clinical evaluation of ser-109, a rationally designed, oral microbiome-based therapeutic for the treatment of recurrent Clostridium difficile. Available online: http://www.gastro.org/pressroom/James_W._Freston_Abstract_Orally_Delivered_Microbes.pdf (accessed on 26 April 2015).
- Kelly, C.R.; Kunde, S.S.; Khoruts, A. Guidance on preparing an investigational new drug application for fecal microbiota transplantation studies. Clin. Gastroenterol. Hepatol. 2014, 12, 283–288. [Google Scholar] [CrossRef]
- Pepin, J.; Alary, M.E.; Valiquette, L.; Raiche, E.; Ruel, J.; Fulop, K.; Godin, D.; Bourassa, C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 2005, 40, 1591–1597. [Google Scholar] [CrossRef]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef]
- Agrawal, M.; Aroniadis, O.C.; Brandt, L.J.; Kelly, C.; Freeman, S.; Surawicz, C.; Broussard, E.; Stollman, N.; Giovanelli, A.; Smith, B.A. A long-term follow-up study of the efficacy and safety of fecal microbiota transplant (FMT) for recurrent/severe/complicated C. difficile infection (CDI) in the elderly. Gastroenterology 2014, 146. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Greenberg, A.; Borody, T.J.; Kelly, C.; Mellow, M.; Surawicz, C.; Cagle, L.A.; Neshatian, L. Long-term follow-up study of fecal microbiota transplantation (FMT) for severe or complicated Clostridium difficile infection (CDI). Gastroenterology 2013, 144, S185. [Google Scholar] [CrossRef]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef]
- Brandt, L.; Aroniadis, O.; Greenberg, A.; Borody, T.; Finlayson, S.; Furnari, V.; Kelly, C. Safety of fecal microbiota transplantation (FMT) in immunocompromised (IC) patients with inflammatory bowel disease (IBD). Am. J. Gastroenterol. 2013, 108, S556–S556. [Google Scholar]
- Miao, C.L.; Mowery, A.D.; Khara, H.S.; Shellenberger, M.J.; Komar, M. C. Difficile small bowel enteritis after total proctocolectomy successfully treated with fecal transplant. In Presented at the American College of Gastroenterologists, Philadelphia, PA, USA, 20 October 2014.
- Borody, T.J.; Wettstein, A.R.; Leis, S.; Hills, L.A.; Campbell, J.; Torres, M. Clostridium difficile complicating inflammatory bowel disease: Pre-and post-treatment findings. Gastroenterology 2008, 134, A361. [Google Scholar] [CrossRef]
- Borody, T.; Wettstein, A.; Nowak, A. Fecal microbiota transplantation (FMT) eradicates Clostridium difficile infection (CDI) in inflammatory bowel disease (IBD). UEG J. 2013, 1, A57. [Google Scholar]
- Singh, N.; Suskind, D.; Wahbeh, G. Fecal bacteriotherapy in a 6 year old patient with ulcerative colitis and Clostridium difficile: P‐136 yi. Inflamm. Bowel. Dis. 2012, 18, S69. [Google Scholar] [CrossRef]
- Borody, T.J.; George, L.; Andrews, P.; Brandl, S.; Noonan, S.; Cole, P.; Hyland, L.; Morgan, A.; Maysey, J.; Moore-Jones, D. Bowel-flora alteration: A potential cure for inflammatory bowel disease and irritable bowel syndrome? Med. J. Aust. 1989, 150, 604. [Google Scholar]
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: Techniques, applications, and issues. Gastroenterol. Clin. North Am. 2012, 41, 781–803. [Google Scholar] [CrossRef]
- Borody, T.; Wettstein, A.; Campbell, J.; Leis, S.; Torres, M.; Finlayson, S.; Nowak, A. Fecal microbiota transplantation in ulcerative colitis: Review of 24 years experience. Am. J. Gastroenterol. 2012, 107, S665–S665. [Google Scholar]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015, 149, 102–106. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015, 149, 110–118. [Google Scholar] [CrossRef]
- Zhang, F.M.; Wang, H.G.; Wang, M.; Cui, B.T.; Fan, Z.N.; Ji, G.Z. Fecal microbiota transplantation for severe enterocolonic fistulizing crohn’s disease. World J. Gastroenterol. 2013, 19, 7213–7216. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Kump, P.; Gröchenig, H.; Spindelböck, W.; Hoffmann, C.; Gorkiewicz, G.; Wenzl, H. Preliminary clinical results of repeatedly fecal microbiota transplantation (FMT) in chronic active ulcerative colitis. United European Gastroenterol. J. 2013, 1, A57. [Google Scholar]
- Kump, P.K.; Grochenig, H.P.; Lackner, S.; Trajanoski, S.; Reicht, G.; Hoffmann, K.M.; Deutschmann, A.; Wenzl, H.H.; Petritsch, W.; Krejs, G.J.; et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm. Bowel. Dis. 2013, 19, 2155–2165. [Google Scholar] [CrossRef]
- Singh, R.; van Nood, E.; Nieuwdorp, M.; van Dam, B.; ten Berge, I.J.; Geerlings, S.E.; Bemelman, F.J. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin. Microbiol. Infect. 2014, 20, O977–O978. [Google Scholar] [CrossRef]
- Freedman, A. Use of Stool Transplant to Clear Fecal Colonization with Carbapenem-Resistant Enterobacteraciae (cre): Proof of Concept; Infectious Diseases Society of America: Philadelphia, PA, USA, 2014. [Google Scholar]
- Hamilton, M.J.; Weingarden, A.R.; Sadowsky, M.J.; Khoruts, A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 761–767. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef]
- Smits, L.P.; Bouter, K.E.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borody, T.J.; Peattie, D.; Mitchell, S.W. Fecal Microbiota Transplantation: Expanding Horizons for Clostridium difficile Infections and Beyond. Antibiotics 2015, 4, 254-266. https://doi.org/10.3390/antibiotics4030254
Borody TJ, Peattie D, Mitchell SW. Fecal Microbiota Transplantation: Expanding Horizons for Clostridium difficile Infections and Beyond. Antibiotics. 2015; 4(3):254-266. https://doi.org/10.3390/antibiotics4030254
Chicago/Turabian StyleBorody, Thomas J., Debra Peattie, and Scott W. Mitchell. 2015. "Fecal Microbiota Transplantation: Expanding Horizons for Clostridium difficile Infections and Beyond" Antibiotics 4, no. 3: 254-266. https://doi.org/10.3390/antibiotics4030254





