Preparation and Microbiological Evaluation of Amphiphilic Kanamycin-Lipoamino Acid Ion-Pairs
Abstract
:1. Introduction
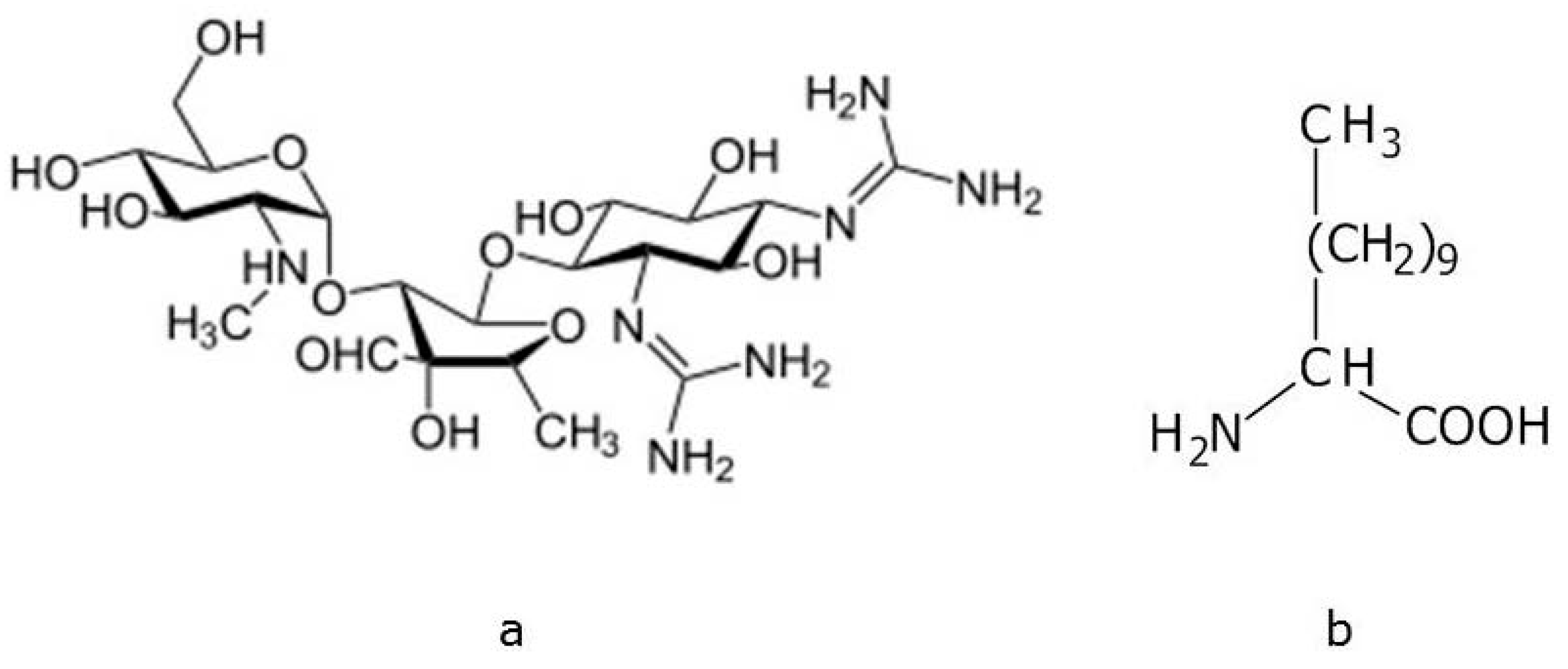
2. Results and Discussion
| Solvent | KANbase | KAN sulfate | KAN-LAA12 1:1 | KAN-LAA12 1:2 | KAN-LAA12 1:4 |
|---|---|---|---|---|---|
| Water | soluble a | 10–50 | 7.5 | 7.5 | 7.5 |
| Phosphate buffer (0.13 M, pH 7.4) | soluble | n.a. | 5 | 7.5 | 15 |
| Ethanol | slightly soluble | insoluble b | 10 | 15 | >20 |
| Acetone | slightly soluble | n.a. | 2.5 | 7.5 | >20 |
| Ethyl acetate | insoluble | n.a. | 1 | 2.5 | 2.5 |
| Dichloromethane | insoluble | insoluble | 5 | 15 | >20 |


| LAA12 | KAN | KAN/LAA12 | |||||
|---|---|---|---|---|---|---|---|
| 1:1 coev. | 1:1 PhM | 1:2 coev. | 1:2 PhM | 1:4 coev. | 1:4 PhM | ||
| 3500–3350 | 3350 | 3500–3350 | 3350–3215 | 3350–3215 | 3350 | 3500–3350 | |
| 3400 | |||||||
| 1750 | |||||||
| 1730 | |||||||
| 1720 | 1720 | 1720 | 1715 | 1715 | |||
| 1660 | 1660 | 1660 | 1660 | 1660 | 1660 | 1665 | 1665 |
| 1655 | 1655 | 1655 | |||||
| 1637 | 1637 | 1637 | 1635 | 1637 | 1635 | ||
| 1630 | 1630 | ||||||
| 1615 | 1618 | 1615 | 1610 | 1615 | 1615 | ||
| 1592 | 1595 | ||||||
| 1583 | 1583 | 1583 | 1580 | 1585 | 1585 | ||
| 1525 | 1525 | 1525 | |||||
| 1510 | 1505 | 1513 | |||||
| 1340 | |||||||
| 1310 | 1310 | 1310 | 1310 | 1310 | |||
| 1290 | |||||||
| 1265 | 1260 | ||||||
| 1250 | |||||||
| 1230 | 1230 | 1230 | |||||
| 1225 | |||||||
| 1215 | 1215 | 1215 | 1213 | ||||
| 1194 | 1194 | 1200 | |||||
| 1160 | |||||||
| 1154 | 1154 | 1150 | 1154 | 1154 | 1156 | 1156 | |
| 1093 | |||||||
| 1031 | 1031 | 1040 | 1031 | 1031 | 1031 | 1025 | |
| Compound | E. coli * ATCC 25922 | E. faecalis * ATCC 29212 | S. pneumoniae (No. 5) | L. fermentum (No. 2) |
|---|---|---|---|---|
| KAN sulfate | 4 | ≥16 | ≥16 | ≥16 |
| LAA12 | growth | growth | growth | growth |
| KAN-LAA12, 1:1 | 4 | ≥16 | ≥16 | ≥16 |
| 1:1 PhM | 4 | ≥16 | ≥16 | ≥16 |
| KAN-LAA12, 1:2 | 2 | ≥16 | ≥16 | ≥16 |
| 1:2 PhM | 4 | ≥16 | ≥16 | ≥16 |
| KAN-LAA12, 1:4 | 2 | ≥16 | ≥16 | ≥16 |
| 1:4 PhM | 4 | ≥16 | ≥16 | ≥16 |
3. Experimental
3.1. Ion-Pair Preparation
3.2. Physical Mixtures
3.3. Solubility Determination
3.4. Bacterial Strains
3.5. Susceptibility Test Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gilbert, D.N.; Leggett, J.E. Aminoglycosides. In Principles and Practice of Infectious Diseases, 7th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 2010; Volume 1, pp. 359–384. [Google Scholar]
- EMEA. The European Agency for the Evaluation of Medicinal Products—Veterinary Medicines and Inspections. Committee for Veterinary Medicinal Products—Kanamycin: Summary Report 1999 EMEA/MRL/514/98-FINAL. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014535.pdf (accessed on 1 February 2014).
- Lynch, S.R.; Puglisi, J.D. Structure of a eukaryotic decoding region A-site RNA. J. Mol. Biol. 2001, 306, 1023–1035. [Google Scholar]
- Lynch, S.R.; Puglisi, J.D. Structural origins of aminoglycoside specificity for prokaryotic ribosomes. J. Mol. Biol. 2001, 306, 1037–1058. [Google Scholar] [CrossRef]
- Shaw, K.J.; Rather, P.N.; Hare, R.S.; Miller, G.H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 1993, 57, 138–163. [Google Scholar]
- Pagès, J.M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef]
- Bera, S.; Zhanel, G.G.; Schweizer, F. Antibacterial activity of guanidinylated neomycin B- and kanamycin A-derived amphiphilic lipid conjugates. J. Antimicrob. Chemother. 2010, 65, 1224–1227. [Google Scholar] [CrossRef]
- Yang, L.; Ye, X.S. Development of aminoglycoside antibiotics effective against resistant bacterial strains. Curr. Top. Med. Chem. 2010, 10, 1898–1826. [Google Scholar] [CrossRef]
- Takemi, K.; Masayuki, Y.; Tetsutaro, N.; Kazuko, M.; Takashi, T.; Shigeharu, I. An Aminoglycosidic Antibiotic Salt. European Patent Office. EP 0011400, 28 May 1980. [Google Scholar]
- Neubert, R. Ion-pair transport across membranes. Pharm. Res. 1989, 6, 743–747. [Google Scholar] [CrossRef]
- Anderberg, E.K.; Lindmark, T.; Artursson, P. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm. Res. 1993, 10, 857–864. [Google Scholar] [CrossRef]
- Dai, W.G.; Dong, L.C. Characterization of physiochemical and biological properties of an insulin/lauryl sulfate complex formed by hydrophobic ion-pairing. Int. J. Pharm. 2007, 336, 58–66. [Google Scholar] [CrossRef]
- Gaudana, R.; Parenky, A.; Vaishya, R.; Samanta, S.K.; Mitra, A.K. Development and characterization of nanoparticulate formulation of a water soluble prodrug of dexamethasone by HIP complexation. J. Microencapsul. 2011, 28, 10–20. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Kawashima, Y.; Xia, D.; Cui, F. Hydrophobic ion-pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int. J. Nanomed. 2011, 6, 3049–3056. [Google Scholar]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Peng, Q.; Cao, X.; Gong, T.; Zhang, Z. A novel submicron emulsion system loaded with vincristine—Oleic acid ion-pair complex with improved anticancer effect: In vitro and in vivo studies. Int. J. Nanomed. 2013, 8, 1185–1196. [Google Scholar]
- Matschiner, S.; Neubert, R.; Wohlrab, W.; Matschiner, F. Influence of ion-pairing on ex vivo penetration of erythromycin into sebaceous follicles. Skin Pharmacol. 1996, 9, 270–273. [Google Scholar] [CrossRef]
- Dave, R.N.; Joshi, H.M.; Venugopalan, V.P. Novel biocatalytic polymer-based antimicrobial coatings as potential ureteral biomaterial: Preparation and in vitro performance evaluation. Antimicrob. Agents Chemother. 2011, 55, 845–853. [Google Scholar] [CrossRef]
- Infante, M.R.; Pinazo, A.; Seguer, J. Non-conventional surfactants from amino acids and glycolipids: Structure, preparation and properties. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 49–70. [Google Scholar] [CrossRef]
- Clapés, P.; Infante, M.R. Amino acid-based surfactants: Enzymatic synthesis, properties and potential applications. Biocatal. Biotransform. 2002, 20, 215–233. [Google Scholar]
- Toth, I. A novel chemical approach to drug delivery: Lipidic amino acid conjugates. J. Drug Target. 1994, 2, 217–239. [Google Scholar] [CrossRef]
- Pignatello, R.; Guccione, S.; Castelli, F.; Sarpietro, M.G.; Giurato, L.; Lombardo, M.; Puglisi, G.; Toth, I. Enhancement of drug affinity for cell membranes by conjugation with lipoamino acids II. Experimental and computational evidence using biomembrane models. Int. J. Pharm. 2006, 310, 53–63. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Micieli, D.; Pignatello, R.; Liang, M.T.; Toth, I.; Castelli, F. Effect of variation in the chain length and number in modulating the interaction of an immunogenic lipopeptide with biomembrane models. Thermochim. Acta 2008, 471, 14–19. [Google Scholar] [CrossRef]
- Valkò, K.; Toth, I.; Ward, P.; Slegel, P.; Gibbons, W.A. Lipidic peptides. XI. Quantitative structure-activity relationship of a series of lipidic amino acid conjugates of β-lactam antibiotics. Int. J. Pharm. 1992, 79, 123–230. [Google Scholar] [CrossRef]
- Toth, I.; Hughes, R.A.; Ward, P.; McColm, A.M.; Cox, D.M.; Anderson, G.J.; Gibbons, W.A. Fatty peptides. VI. Penicillin and cephalosporin esters with increased lipophilic character. Int. J. Pharm. 1991, 77, 13–20. [Google Scholar] [CrossRef]
- Ross, B.P.; DeCruz, S.E.; Lynch, T.B.; Davis-Goff, K.; Toth, I. Design, synthesis, and evaluation of a liposaccharide drug delivery agent: Application to the gastrointestinal absorption of gentamicin. J. Med. Chem. 2004, 47, 1251–1258. [Google Scholar] [CrossRef]
- Pignatello, R.; Pantò, V.; Salmaso, S.; Bersani, S.; Pistarà, V.; Kepe, V.; Barrio, J.R.; Puglisi, G. Flurbiprofen derivatives in Alzheimer’s disease: Synthesis, pharmacokinetic and biological assessment of lipoamino acid prodrugs. Bioconjug. Chem. 2008, 19, 349–357. [Google Scholar] [CrossRef]
- Pignatello, R.; Paolino, D.; Pantò, V.; Pistarà, V.; Calvagno, M.G.; Russo, D.; Puglisi, G.; Fresta, M. Lipoamino acid prodrugs of paclitaxel: Synthesis and cytotoxicity evaluation on human anaplastic thyroid carcinoma cells. Curr. Cancer Drug Targets 2009, 9, 202–213. [Google Scholar] [CrossRef]
- Pignatello, R.; Mangiafico, A.; Ruozi, B.; Puglisi, G.; Furneri, P.M. Amphiphilic erythromycin-lipoamino acid ion-pairs: Characterization and in vitro microbiological evaluation. AAPS PharmSciTech 2011, 12, 468–475. [Google Scholar] [CrossRef]
- Pignatello, R.; Mangiafico, A.; Basile, L.; Ruozi, B.; Furneri, P.M. Amphiphilic ion-pairs of tobramycin with lipoamino acids. Eur. J. Med. Chem. 2011, 46, 1665–1671. [Google Scholar] [CrossRef]
- VSDB: Veterinary Substances DataBase at University of Hertfordshire. Kanamycin Environmental Fate—Ecotoxicology—Human Health [updated 2013 May 26]. Available online: http://sitem.herts.ac.uk/aeru/vsdb/Reports/1921.htm/ (accessed on 1 March 2013).
- Chemspider by Royal Society of Chemistry Kanamycin. Available online: http://www.chemspider.com/Chemical-Structure.5810.html/ (accessed on 2 April 2013).
- Cron, M.J.; Fardig, O.B.; Johnson, D.L.; Palermiti, F.M.; Schmitz, H.; Hooper, I.R. The basic and clinical research of the new antibiotic, kanamycin. Ann. NY Acad. Sci. 1958, 76, 2–30. [Google Scholar]
- National Center for Biotechnology Information. Kanamycin—Compound Summary. PubChem Compound [updated 2005 June 24]. Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6032#x27/ (accessed on 1 March 2013).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Gibbons, W.A.; Hughes, R.A.; Charalambous, M.; Christodoulou, M.; Szeto, A.; Aulabaugh, A.E.; Mascagni, P.; Toth, I.; Lipidic peptides, I. Synthesis, resolution and structural elucidation of lipidic amino acids and their homo- and hetero-oligomers. Liebigs Ann. Chem. 1990, 1990, 1175–1183. [Google Scholar] [CrossRef]
- Puglisi, G.; Fresta, M.; Mazzone, G.; Furneri, P.M.; Tempera, G. Formulation parameters of fluoroquinolones-loaded liposomes and in vitro antimicrobial activity. Int. J. Pharm. 1995, 118, 65–76. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Tzemach, D.; Horowitz, A.T.; Shmeeda, H.; Yeh, J.; Zalipsky, S. Reduced toxicity and superior therapeutic activity of a mitomycin C lipid-based prodrug incorporated in pegylated liposomes. Clin. Cancer Res. 2006, 12, 1913–1920. [Google Scholar] [CrossRef]
- Stancampiano, A.H.S.; Puglisi, G.; Pignatello, R. Effect of lipophilicity of dispersed drugs on the physicochemical and technological properties of solid lipid nanoparticles. Open Drug Deliv. J. 2008, 2, 26–32. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, K.S.; Sung, K.C.; Tsai, C.Y.; Fang, J.Y. Skin permeation of buprenorphine and its ester prodrugs from lipid nanoparticles: Lipid emulsion, nanostructured lipid carriers and solid lipid nanoparticles. J. Microencapsul. 2009, 26, 734–747. [Google Scholar] [CrossRef]
- Kuznetsova, N.R.; Sevrin, C.; Lespineux, D.; Bovin, N.V.; Vodovozova, E.L.; Mészáros, T.; Szebeni, J.; Grandfils, C. Hemocompatibility of liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the lipid bilayer. J. Control. Release 2012, 160, 394–400. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pignatello, R.; Leonardi, A.; Petronio, G.P.; Ruozi, B.; Puglisi, G.; Furneri, P.M. Preparation and Microbiological Evaluation of Amphiphilic Kanamycin-Lipoamino Acid Ion-Pairs. Antibiotics 2014, 3, 216-232. https://doi.org/10.3390/antibiotics3020216
Pignatello R, Leonardi A, Petronio GP, Ruozi B, Puglisi G, Furneri PM. Preparation and Microbiological Evaluation of Amphiphilic Kanamycin-Lipoamino Acid Ion-Pairs. Antibiotics. 2014; 3(2):216-232. https://doi.org/10.3390/antibiotics3020216
Chicago/Turabian StylePignatello, Rosario, Antonio Leonardi, Giulio Petronio Petronio, Barbara Ruozi, Giovanni Puglisi, and Pio Maria Furneri. 2014. "Preparation and Microbiological Evaluation of Amphiphilic Kanamycin-Lipoamino Acid Ion-Pairs" Antibiotics 3, no. 2: 216-232. https://doi.org/10.3390/antibiotics3020216
APA StylePignatello, R., Leonardi, A., Petronio, G. P., Ruozi, B., Puglisi, G., & Furneri, P. M. (2014). Preparation and Microbiological Evaluation of Amphiphilic Kanamycin-Lipoamino Acid Ion-Pairs. Antibiotics, 3(2), 216-232. https://doi.org/10.3390/antibiotics3020216






