Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain)
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
| Compound | Use | Structure | pKa [17,18] | Kow * |
|---|---|---|---|---|
| Atenolol | Antihypertensive |  | 9.2 | 0.16 |
| Acetaminophen | Analgesic |  | 9.9 | 0.46 |
| Norfloxacin | Antibiotic |  | 6.4 | 0.46 |
| Ciprofloxacin | Antibiotic |  | 6.4 | 0.28 |
| Carbamazepine | Antiepileptic |  | 13.9 | 2.45 |
| Ketoprofen | Anti-inflammatory |  | 4.4 | 4.23 |
| Diclofenac | Anti-inflammatory |  | 4.2 | 4.51 |
2.2. Sample Collection
2.3. Chromatographic Conditions
| Compound | Precursor ion (m/z) | Cone voltage (V) | Fragment ions (collision potential) | Ion mode |
|---|---|---|---|---|
| Atenolol | 267.0 | 52 | 145 (23.5) *, 190 (16.5) | ESI + |
| Acetaminophen | 152.0 | 56 | 109.9 (13), 92.8 (20) | ESI + |
| Norfloxacin | 320.1 | 56 | 301.9 (19), 230.8 (37) | ESI + |
| Ciprofloxacin | 332.1 | 52 | 313.9 (19), 230.8 (36) | ESI + |
| Carbamazepine | 237.1 | 40 | 194 (13.5), 179.2 (29.5) | ESI + |
| Ketoprofen | 255.1 | 52 | 209 (10), 104.9 (18.5) | ESI + |
| Diclofenac | 295.9 | 32 | 214.0 (30), 250.0 (11) | ESI − |
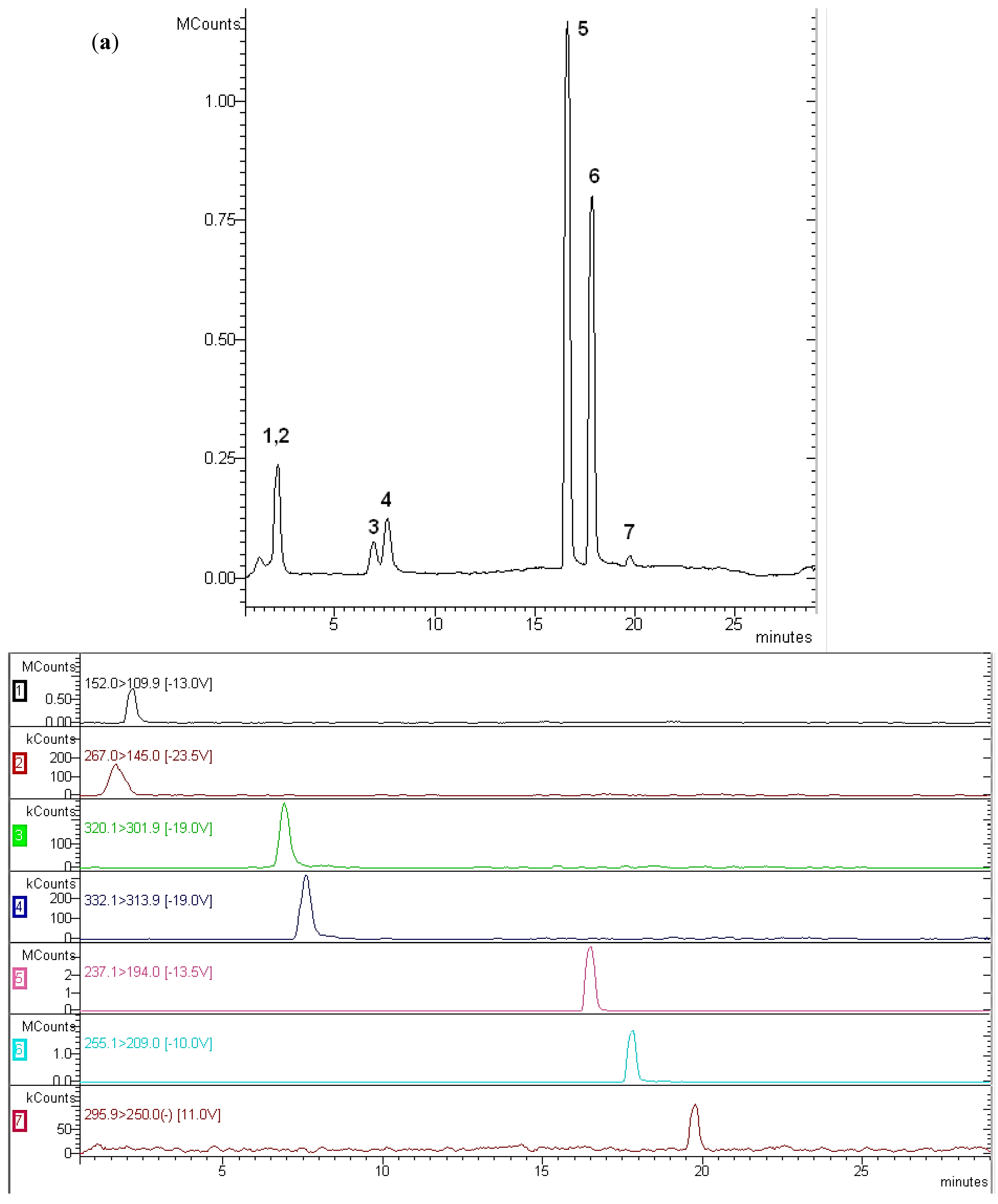
2.4. Solid-Phase Extraction
3. Results and Discussion
3.1. Optimisation of Solid-Phase Extraction
3.2. Analytical Parameters
| LOD a (ng·L−1) | LOQ b (ng·L−1) | RSD (%) | Recovery (%) | LDR c (µg·L−1) | r2 | |
|---|---|---|---|---|---|---|
| Atenolol | 0.1 | 0.3 | 6.5 | 78.3 | 0.5–600 | 0.9986 |
| Acetaminophen | 0.6 | 2.0 | 6.4 | 95.7 | 0.5–600 | 0.9906 |
| Norfloxacin | 2.8 | 9.3 | 11.8 | 80.2 | 0.5–600 | 0.9964 |
| Ciprofloxacin | 1.0 | 3.4 | 10.7 | 81.4 | 0.5–600 | 0.9935 |
| Carbamazepine | 0.9 | 2.9 | 6.0 | 96.0 | 0.5–600 | 0.9926 |
| Ketoprofen | 0.1 | 0.3 | 1.7 | 94.8 | 0.5–600 | 0.9962 |
| Diclofenac | 1.4 | 4.6 | 9.8 | 98.2 | 0.5–600 | 0.9936 |
3.3. Evaluation of Pharmaceutical Compounds in Seawater
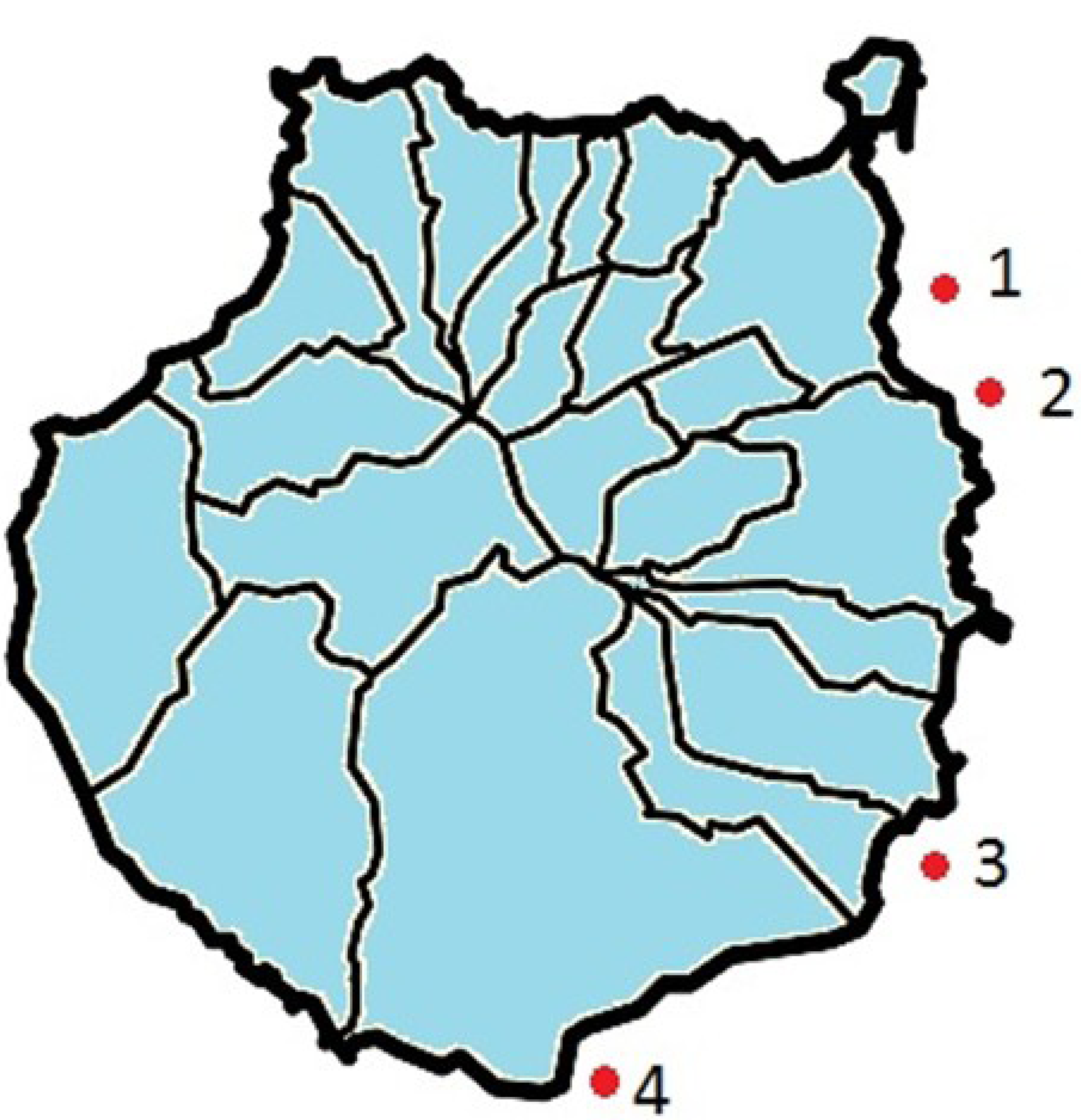
| Outfall | Date | Acetaminophen | Norfloxacin | Ciprofloxacin | Ketoprofen | Diclofenac |
|---|---|---|---|---|---|---|
| Las Palmas de Gran Canaria | Jan-2011 | nd b | nd | nd | nd | nd |
| Feb-2011 | nd | 3551.7 ± 315.0 | 303.6 ± 8.6 | 67.8 ± 5.3 | nd | |
| Mar-2011 | 297.0 ± 26.8 | 1653.0 ± 141.2 | 123.3 ± 5.4 | nd | nd | |
| Apr-2011 | nd | 77.9 ± 0.5 | nd | nd | nd | |
| May-2011 | nd | nd | nd | nd | nd | |
| Jun-2011 | nd | 1248.8 ± 62.3 | 95.3 ± 0.4 | nd | nd | |
| Jul-2011 | nd | nd | 40.1 ± 4.9 | nd | nd | |
| Aug-2011 | nd | 325.5 ± 44.9 | 18.9 ± 0.2 | nd | nd | |
| Sep-2011 | nd | nd | 29.64 ± 3.1 | nd | 47.9 ± 3.5 | |
| Oct-2011 | nd | 1380.74 ± 197.2 | 65.9 ± 9.2 | nd | 28.4 ± 4.0 | |
| Nov-2011 | 29.7 ± 1.4 | 69.4 ± 10.3 | 60.4 ± 7.9 | nd | nd | |
| Dic-2011 | nd | nd | nd | 41.6 ± 5.0 | nd | |
| Jan-2012 | 21.5 ± 2.0 | 11.3 ± 0.7 | nd | 49.0 ± 3.8 | nd | |
| Jinámar | Jan-2011 | nd | nd | 17.4 ± 1.6 | nd | nd |
| Feb-2011 | nd | 3179.1 ± 106.2 | 303.4 ± 12.0 | 106.3 ± 6.4 | nd | |
| Mar-2011 | nd | 1130.1 ± 106.6 | 140.7 ± 21.1 | nd | nd | |
| Apr-2011 | nd | 92.1 ± 6.0 | nd | nd | nd | |
| May-2011 | nd | nd | nd | nd | nd | |
| Jun-2011 | nd | 808.6 ± 71.9 | 66.7 ± 2.0 | nd | nd | |
| Jul-2011 | nd | 191.6 ± 7.1 | 70.2 ± 7.9 | nd | nd | |
| Aug-2011 | nd | nd | 9.0 ± 0.2 | nd | nd | |
| Sep-2011 | nd | 591.3 ± 15.8 | 52.3 ± 0.2 | nd | nd | |
| Oct-2011 | nd | 1565.9 ± 210.0 | 121.6 ± 15.8 | nd | 28.4 ± 2.9 | |
| Nov-2011 | nd | 2250 ± 112.5 | 119.9 ± 14.9 | nd | nd | |
| Dic-2011 | nd | nd | nd | nd | nd | |
| Jan-2012 | nd | 18.0 ± 1.0 | 17.7 ± 1.4 | nd | nd | |
| Punta Salina | Jan-2011 | nd | nd | nd | nd | nd |
| Feb-2011 | - | - | - | - | - | |
| Mar-2011 | nd | nd | nd | nd | nd | |
| Apr-2011 | - | - | - | - | - | |
| May-2011 | nd | nd | nd | nd | nd | |
| Jun-2011 | nd | nd | nd | nd | nd | |
| Jul-2011 | nd | 185.3 ± 22.8 | 18.8 ± 2.6 | nd | nd | |
| Aug-2011 | nd | nd | nd | nd | 56.7 ± 5.3 | |
| Sep-2011 | nd | 634.5 ± 52.0 | 64.9 ± 10.3 | nd | 343.6 ± 51.1 | |
| Oct-2011 | nd | 1681.5 ± 244.0 | 101.0 ± 5.7 | nd | 29.5 ± 1.4 | |
| Nov-2011 | nd | 33.3 ± 2.4 | 30.8 ± 3.9 | nd | nd | |
| Dic-2011 | nd | nd | nd | nd | nd | |
| Jan-2012 | nd | 22.9 ± 2.1 | 26.3 ± 3.8 | nd | nd | |
| Los Cochinos | Jan-2011 | nd | nd | nd | nd | nd |
| Feb-2011 | - | - | - | - | - | |
| Mar-2011 | nd | nd | nd | nd | nd | |
| Apr-2011 | - | - | - | - | - | |
| May-2011 | nd | nd | nd | nd | nd | |
| Jun-2011 | nd | nd | nd | nd | nd | |
| Jul-2011 | nd | 134.3 ± 15.1 | 80.1 ± 12.2 | nd | nd | |
| Aug-2011 | nd | nd | nd | nd | 57.1 ± 0.8 | |
| Sep-2011 | nd | 899.9 ± 130.8 | 72.0 ± 8.8 | nd | 160.0 ± 24.4 | |
| Oct-2011 | nd | nd | 4.4 ± 0.5 | nd | 23.7 ± 0.4 | |
| Nov-2011 | nd | 20.8 ± 3.2 | 15.8 ± 1.4 | nd | nd | |
| Dic-2011 | nd | 17.4 ± 1.9 | 12.5 ± 0.2 | nd | nd | |
| Jan-2012 | nd | 17.0 ± 1.3 | 13.5 ± 0.1 | nd | nd |
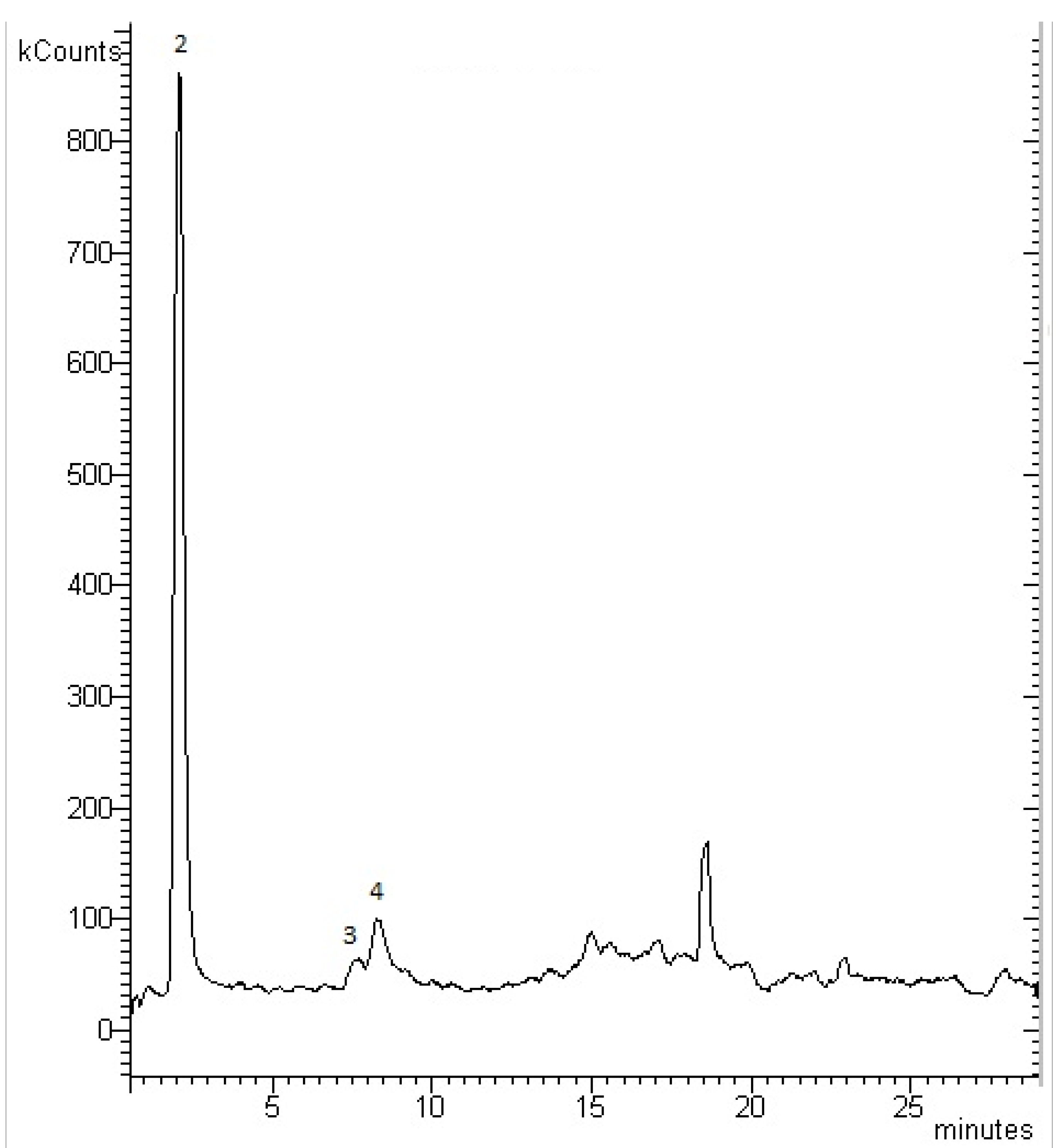
4. Conclusions
Acknowledgments

Conflict of Interest
References and Notes
- Fuerhacker, M. EU water framework directive and Stockholm convention. Environ. Sci. Pollut. Res. 2009, 16, 92–97. [Google Scholar] [CrossRef]
- Vargas, F. La contaminación ambiental como factor determinante de la salud. Rev. Esp. Salud. Publica 2005, 79, 117–127. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/EC of the European Parliament and of the council 23 October 2000 establishing a framework for community action in the field of water policy. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:02000L0060-20090113:EN:NOT (accessed on 22 December 2000).
- Wille, K.; de Brander, H.F.; de Wulf, E.; van Caeter, P.; Janssen, C.R.; Vanhaecke, L. Coupled chromatograhic and mass-spectrometric techniques for the analysis of emerging pollutants in the aquatic environment. Trac-Trend. Anal. Chem. 2012, 35, 87–108. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Analysis of anti-inflammatory, analgesic, stimulant and antidepressant drugs in purified water from wastewater treatment plants using SPE-LC tandem mass spectrometry. J. Environ. Sci. Heal. A 2012, 47, 887–895. [Google Scholar]
- Petrovic, M.; Farré, M.; Lopez de Alda, M.; Perez, S.; Postigo, C.; Köck, M.; Radjenovic, J.; Gros, M.; Barcelo, D. Recent trends in the liquid chromatography-mass spectrometry analysis of organic contaminants in environmental samples. J. Chromatogr. A 2010, 1217, 4004–4017. [Google Scholar] [CrossRef]
- Richardson, S.D. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2007, 79, 4295–4324. [Google Scholar] [CrossRef]
- Petrovic, M.; Gonzalez, S.; Damia, B. Analysis and removal of emerging contaminants in wastewater and drinking water. Trac-Trend. Anal. Chem. 2003, 22, 685–696. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhou, J.L. Simultaneous determination of various pharmaceutical compounds in water by solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar]
- Lopez-Serna, R.; Petrovic, M.; Barcelo, D. Occurrence and distribution of multi-class pharmaceuticals and their active metabolites and transformation products in the Ebro River basin (NE Spain). Sci. Total. Environ. 2012, 440, 280–289. [Google Scholar] [CrossRef]
- Wille, K.; Noppe, H.; Verheyden, K.; Vanden, J.; de Wulf, E.; van Caeter, P.; Janssen, C.; de Brabander, H.F.; Vanhaecke, L. Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal. Bioanal. Chem. 2010, 397, 1797–1808. [Google Scholar] [CrossRef]
- Bueno, M.J.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernandez-Alba, A. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Fatta, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Ahmed, N.; Pauzi, M.; Ismail, M.; Surif, S. Multi-residue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 6791–6806. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Multi-residue analysis of pharmaceutical compounds in aqueous samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar]
- Mastroianni, N.; Lopez, M.; Barcelo, D. Emerging organic contaminants in aquatic environments: State-of-the-art and recent scientific contributions. Contrib. Sci. 2010, 6, 193–197. [Google Scholar]
- Nödler, K.; Licha, T.; Bester, K.; Sauter, M. Development of a multi-residue analytical method, based on liquid chromatography-tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A 2010, 1217, 6511–6521. [Google Scholar]
- Bones, J.; Thomas, K.; Nesterenko, P.; Paull, B. On-line preconcentration of pharmaceutical residues from large volume water samples using short reversed-phase monolithic cartridges coupled to LC-UV-ESI-MS. Talanta 2006, 70, 1117–1128. [Google Scholar]
- Nebot, C.; Gibb, S.W.; Boyd, K.G. Quantification of human pharmaceuticals in water samples by high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2007, 598, 87–94. [Google Scholar]
- Grabic, R.; Fick, J.; Lindberg, R.; Fedeorova, G.; Tysklind, M. Multi-residue method for trace level determination of pharmaceuticals in environmental samples using liquid chromatography coupled to triple quadrupole mass spectrometry. Talanta 2012, 100, 183–195. [Google Scholar]
- Fang, T.; Nan, F.; Chin, T.; Feng, H. The occurrence and distribution of pharmaceutical compounds in the effluents of a major sewage treatment plant in Northern Taiwan and the receiving coastal waters. Mar. Pollut. Bull. 2012, 64, 1435–1444. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Comparison of solid phase extraction using micellar desorption combined with LC-FD and LC-MS/MS in the determination of antibiotics fluoroquinolonas residues in sewage samples. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2081–2096. [Google Scholar]
- Golet, E.M.; Alder, A.C.; Giger, W. Environmental Exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Glatt Valley Watershed, Switzerland. Environ. Sci. Technol. 2002, 36, 3645–3651. [Google Scholar] [CrossRef]
- Sifrtová, M.; Pena, A.; Lino, C.M.; Solich, P. Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal. Bioanal. Chem. 2008, 391, 799–805. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Safe. 2012, 80, 208–215. [Google Scholar]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef]
- Beausse, J. Selected drugs in solid matrices: A review of environmental determination, occurrence and properties of principal substances. Trac-Trend. Anal. Chem. 2004, 23, 753–761. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Afonso-Olivares, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics 2013, 2, 274-287. https://doi.org/10.3390/antibiotics2020274
Afonso-Olivares C, Torres-Padrón ME, Sosa-Ferrera Z, Santana-Rodríguez JJ. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics. 2013; 2(2):274-287. https://doi.org/10.3390/antibiotics2020274
Chicago/Turabian StyleAfonso-Olivares, Cristina, Mª Esther Torres-Padrón, Zoraida Sosa-Ferrera, and José Juan Santana-Rodríguez. 2013. "Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain)" Antibiotics 2, no. 2: 274-287. https://doi.org/10.3390/antibiotics2020274





