Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer
Abstract
:1. Introduction
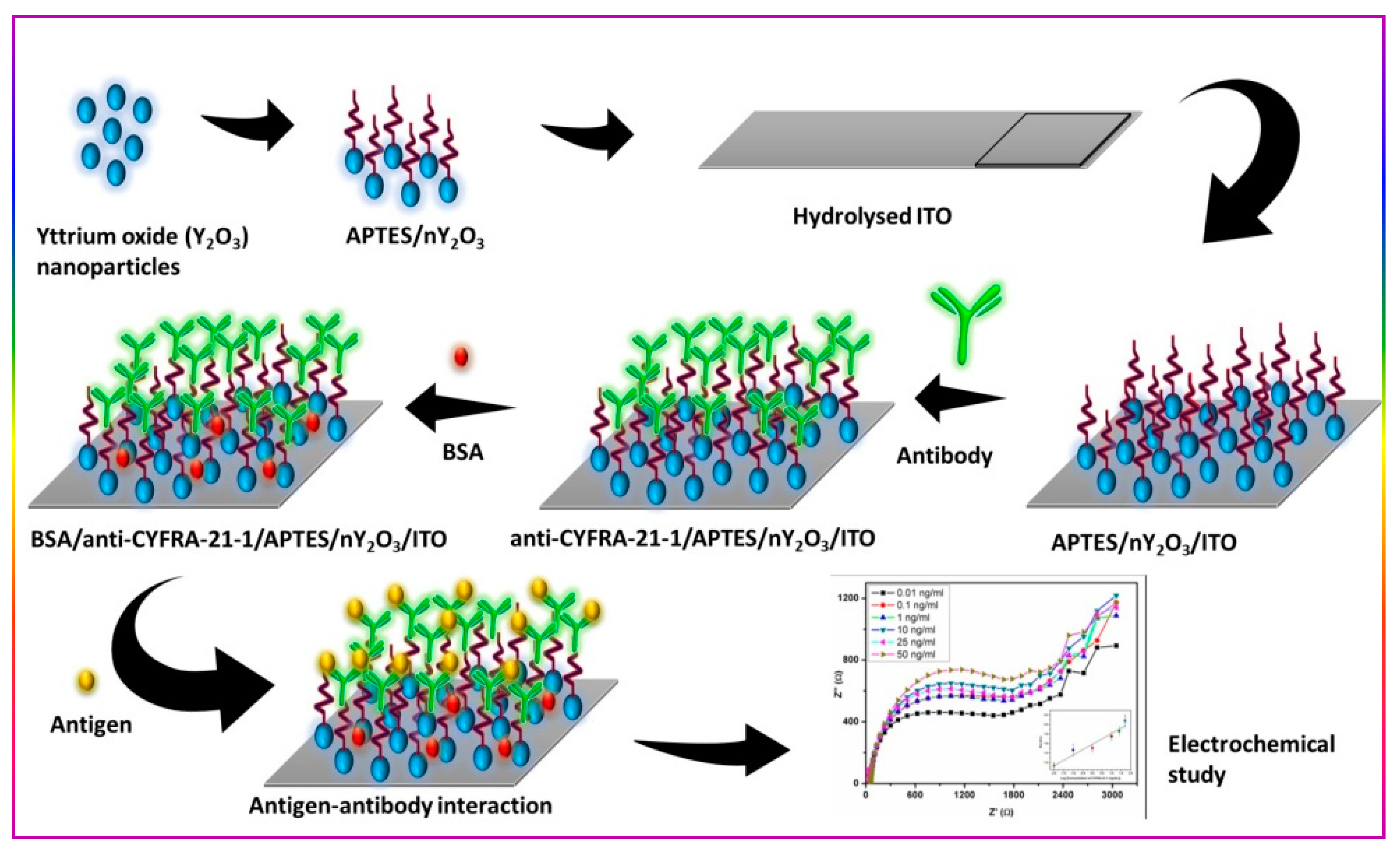
2. Experimental Section
2.1. Preparation and Functionalization of Nanostructured Yttrium Oxide (nY2O3)
2.2. Fabrication of Impedometric Biosensing Platform
2.3. Biocompatibility Studies of nY2O3
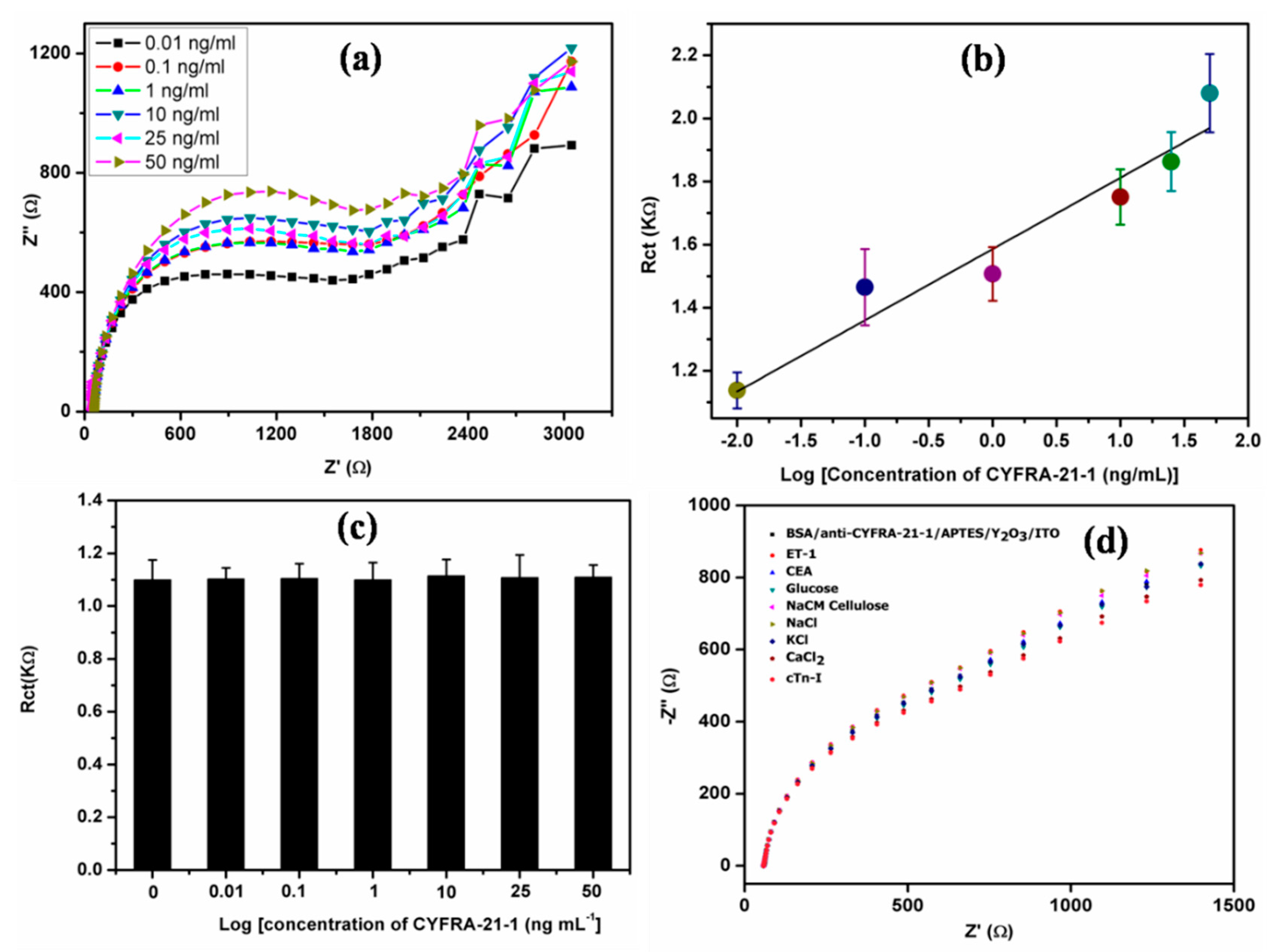
3. Results and Discussion
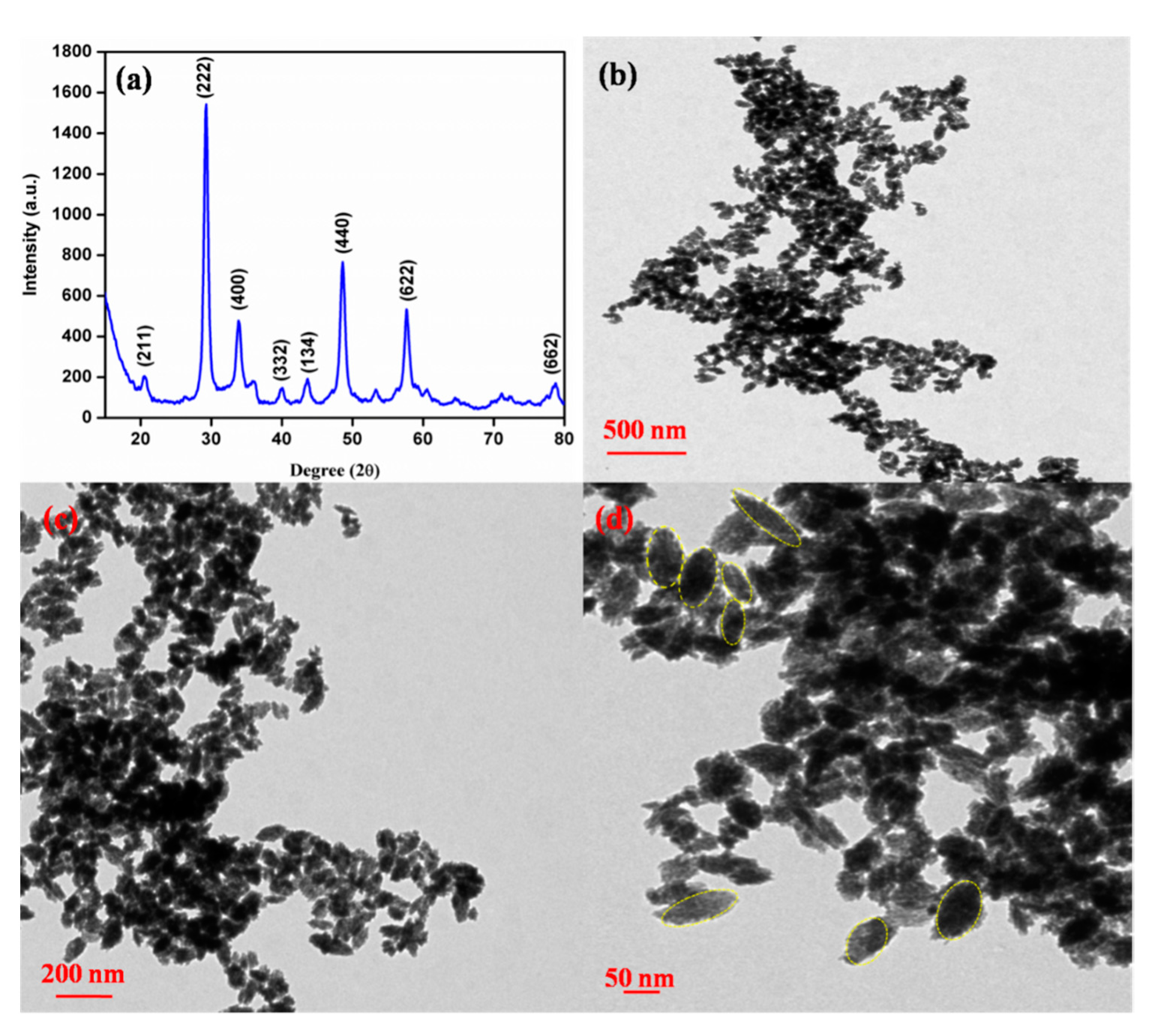
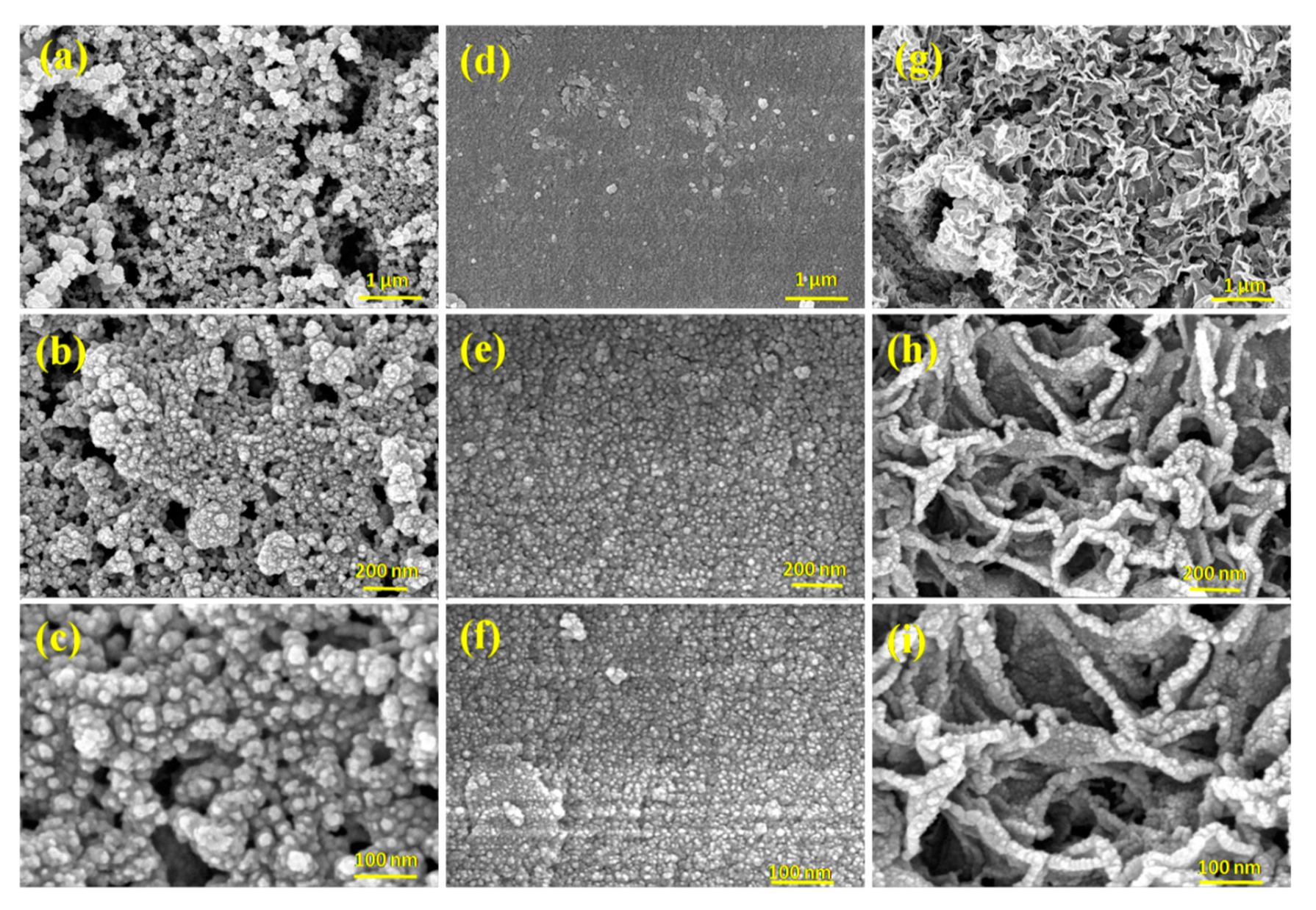
3.1. Structural and Morphological Studies
3.2. Fourier Transform-Infrared (FTIR) Spectroscopy Study
3.3. Biocompatibility Studies
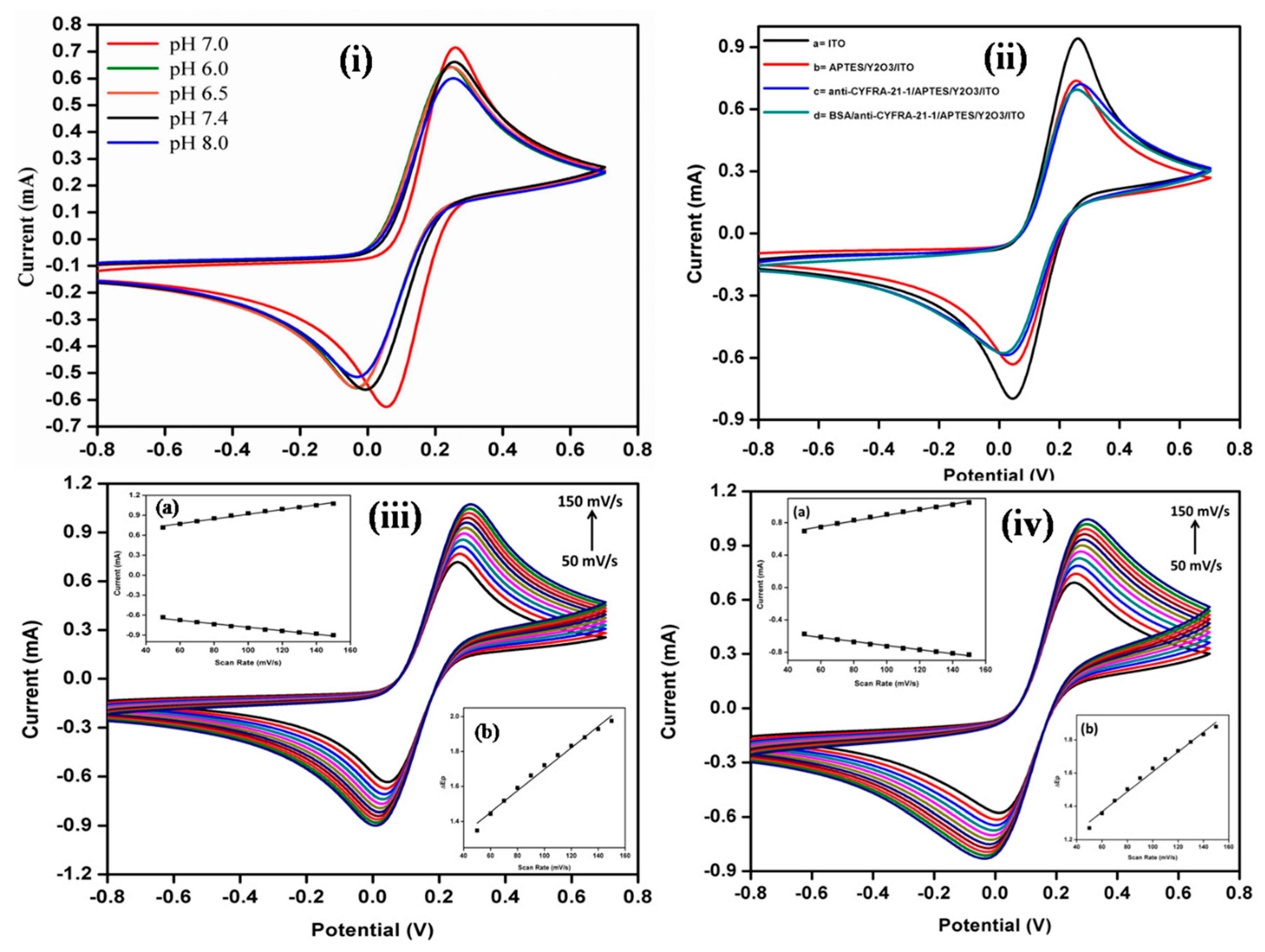
3.4. Electrochemical Characterization
3.5. Electrochemical Impedometric Response, Control, Interferent, Reproducibility,and Shelf Life Studies
3.6. Oral Cancer Patient Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ecsedy, J.; Hunter, D. The Origin of Cancer; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 November 2018).
- Silverman, S. Oral Cancer; PMPH-USA: Raleigh, NC, USA, 2003; Volume 1. [Google Scholar]
- Winn, D.M.; Blot, W.J.; Shy, C.M.; Pickle, L.W.; Toledo, A.; Fraumeni, J.F., Jr. Snuff dipping and oral cancer among women in the southern united states. N. Engl. J. Med. 1981, 304, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.L.; Epstein, J.B.; Kerr, A.R. Adjunctive techniques for oral cancer examination and lesion diagnosis: A systematic review of the literature. J. Am. Dent. Assoc. 2008, 139, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.; Cretin, S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1989, 67, 621–627. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. Ca A Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Alevizos, I.; Mahadevappa, M.; Zhang, X.; Ohyama, H.; Kohno, Y.; Posner, M.; Gallagher, G.T.; Varvares, M.; Cohen, D.; Kim, D. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene 2001, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Q.; Wang, L.Y.; Su, F.H.; Hao, P.Y.; Wang, H.; Lv, Y.K. A gate-opening controlled metal-organic framework for selective solid-phase microextraction of aldehydes from exhaled breath of lung cancer patients. Microchim. Acta 2018, 185, 307. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Urs, A.B.; Chakravarti, A.; Kumar, S.; Gupta, V.; Mahajan, B. Correlation of cyfra 21-1 levels in saliva and serum with ck19 mrna expression in oral squamous cell carcinoma. Tumor Biol. 2016, 37, 9263–9271. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, K.; Ramya, R.; Nandhini, G.; Rajashree, P.; Ramesh Kumar, A.; Nirmala Anandan, S. Salivary and serum level of cyfra 21–1 in oral precancer and oral squamous cell carcinoma. Oral Dis. 2015, 21, 90–96. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Srivastava, S.; Srivastava, M.; Yadav, B.K.; Kumar, S.; Tran, T.T.; Dewan, A.K.; Mulchandani, A. Biofunctionalized nanostructured zirconia for biomedical application: A smart approach for oral cancer detection. Adv. Sci. 2015, 2, 1500048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. A biocompatible serine functionalized nanostructured zirconia based biosensing platform for non-invasive oral cancer detection. RSC Adv. 2016, 6, 77037–77046. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly sensitive protein functionalized nanostructured hafnium oxide based biosensing platform for non-invasive oral cancer detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Augustine, S.; Yadav, S.; Yadav, B.K.; Chauhan, R.P.; Dewan, A.K.; Malhotra, B.D. Effect of brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018, 102, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosens. Bioelectron. 2016, 89, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. Npg Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.J.; Min, D.H. Emerging approaches for graphene oxide biosensor. Anal. Chem. 2016, 89, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Fregnani, J.H.T.G.; Carvalho, A.L.; Oliveira Jr, O.N. Carbon nanotube matrix for highly sensitive biosensors to detect pancreatic cancer biomarker ca19-9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef]
- Othman, A.; Karimi, A.; Andreescu, S. Functional nanostructures for enzyme based biosensors: Properties, fabrication and applications. J. Mater. Chem. B 2016, 4, 7178–7203. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, L.; Hao, Y.; Guo, Q.; Mu, F.; Yan, Z. Application of tubular tetrapod magnesium oxide in a biosensor for hydrogen peroxide. Microchim. Acta 2012, 178, 439–445. [Google Scholar] [CrossRef]
- Shi, X.; Gu, W.; Li, B.; Chen, N.; Zhao, K.; Xian, Y. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta 2014, 181, 1–22. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim. Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Iron and iron-oxide magnetic nanoparticles as signal-amplification elements in electrochemical biosensing. Trac. Trends Anal. Chem. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Bhansali, S. Electrochemical immunosensor for label free epidermal growth factor receptor (egfr) detection. Biosens. Bioelectron. 2013, 39, 300–305. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Nikoleli, G.P.; Pingarrón, J.; Nikolelis, D. Hybrid 2d-nanomaterials-based electrochemical immunosensing strategies for clinical biomarkers determination. Biosens. Bioelectron. 2017, 89, 269–279. [Google Scholar] [CrossRef]
- Solanki, S.; Pandey, C.M.; Soni, A.; Sumana, G.; Biradar, A.M. An amperometric bienzymatic biosensor for the triglyceride tributyrin using an indium tin oxide electrode coated with electrophoretically deposited chitosan-wrapped nanozirconia. Microchim. Acta 2016, 183, 167–176. [Google Scholar] [CrossRef]
- Casero, E.; Alonso, C.; Petit-Domínguez, M.D.; Vázquez, L.; Parra-Alfambra, A.M.; Merino, P.; Álvarez-García, S.; de Andrés, A.; Suárez, E.; Pariente, F. Lactate biosensor based on a bionanocomposite composed of titanium oxide nanoparticles, photocatalytically reduced graphene, and lactate oxidase. Microchim. Acta 2014, 181, 79–87. [Google Scholar] [CrossRef]
- Huang, G.; Zhanglian, H.; Shizhu, Z.; Pengyue, Z.; Xianping, F. Synthesis of yttrium oxide nanocrystal via solvothermal process. J. Rare Earths 2006, 24, 47–50. [Google Scholar] [CrossRef]
- Li, N.; Yanagisawa, K. Yttrium oxide nanowires. In Nanowires Science and Technology; InTech: London, UK, 2010. [Google Scholar]
- Tang, Q.; Liu, Z.; Li, S.; Zhang, S.; Liu, X.; Qian, Y. Synthesis of yttrium hydroxide and oxide nanotubes. J. Cryst. Growth 2003, 259, 208–214. [Google Scholar] [CrossRef]
- Curtis, C. Properties of yttrium oxide ceramics. J. Am. Ceram. Soc. 1957, 40, 274–278. [Google Scholar] [CrossRef]
- Razavi, R.S.; Ahsanzadeh-Vadeqani, M.; Barekat, M.; Naderi, M.; Hashemi, S.H.; Mishra, A.K. Effect of sintering temperature on microstructural and optical properties of transparent yttria ceramics fabricated by spark plasma sintering. Ceram. Int. 2016, 42, 7819–7823. [Google Scholar] [CrossRef]
- Mori, T.; Lee, J.H.; Li, J.; Ikegami, T.; Auchterlonie, G.; Drennan, J. Improvement of the electrolytic properties of y2o3-based materials using a crystallographic index. Solid State Ion. 2001, 138, 277–291. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Radhakrishnan, T.; Shihabudeen, P.; Sandhyarani, N. Reduced graphene oxide-yttria nanocomposite modified electrode for enhancing the sensitivity of electrochemical genosensor. Biosens. Bioelectron. 2016, 83, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Nanocomposites in electrochemical sensors. In Handbook of Gas Sensor Materials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–235. [Google Scholar]
- Tsutsumi, T. Dielectric properties of y2o3 thin films prepared by vacuum evaporation. Jpn. J. Appl. Phys. 1970, 9, 735. [Google Scholar] [CrossRef]
- Dutta, P.K.; Szabo, N.F.; Du, H.; Akbar, S.A. Potentiometric Nox Sensors Based on Yttria-Stabilized Zirconia with Zeolite Modified Electrode. Google Patents No. US6,843,900B2, 18 January 2005. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Liu, G.; Lin, Y. Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal. Chem. 2005, 77, 5894–5901. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Augustine, S.; Malhotra, B.D. Protein functionalized nanostructured zirconia based electrochemical immunosensor for cardiac troponin i detection. J. Mater. Res. 2017, 32, 2966–2972. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 48. [Google Scholar]
- Srivastava, S.; Kumar, V.; Ali, M.A.; Solanki, P.R.; Srivastava, A.; Sumana, G.; Saxena, P.S.; Joshi, A.G.; Malhotra, B. Electrophoretically deposited reduced graphene oxide platform for food toxin detection. Nanoscale 2013, 5, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Singh, C.; Mondal, K.; Srivastava, S.; Sharma, A.; Malhotra, B.D. Mesoporous few-layer graphene platform for affinity biosensing application. ACS Appl. Mater. Interfaces 2016, 8, 7646–7656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Guo, Y.; Wu, X.; Yang, W.; Xu, L.; Chen, J.; Fu, F. A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microrna. Biosens. Bioelectron. 2013, 45, 108–113. [Google Scholar] [CrossRef] [PubMed]


| Biomarker | Labeled | Detection Technique | Materials Used | Linear Detection Range | Sensitivity | Lower Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|
| has-miR-200a (mi-RNA) | Yes | CV | Gold | 1 aM–10 fM | ----- | ----- | [51] |
| CYFRA-21-1 (Protein) | No | CV | APTES/nZrO2 | 2–16 ng mL−1 | 2.2 µA mL ng−1 | 0.08 ng mL−1 | [14] |
| APTES/nHfO2 | 2–18 ng mL−1 | 9.28 µA mL ng−1 cm−2 | 0.21 ng mL−1 | [16] | |||
| DPV | APTES/nZrO2-RGO | 2–22 ng mL−1 | 0.756 µA mL ng−1 | 0.122 ng mA−1 | [13] | ||
| Serine/nZrO2 | 0.01–29 ng mL−1 | 0.295 µA mL ng−1 | 0.01 ng mL−1 | [15] | |||
| Cys-La(OH)3 | 0.001–10.2 ng mL−1 | 12.044 µA mL ng−1 cm−2 | 0.001 ng mL−1 | [18] | |||
| APTES/nHfO2@RGO | 0–30 ng mL−1 | 18.24 µA mL ng−1 | 0.164 ng mL−1 | [17] | |||
| EIS | APTES/nY2O3 | 0.01–50 ng mL−1 | 226.0 Ω mL ng−1 | 0.33 ng mL−1 | Present Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Panwar, S.; Kumar, S.; Augustine, S.; Malhotra, B.D. Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer. Nanomaterials 2019, 9, 1190. https://doi.org/10.3390/nano9091190
Kumar S, Panwar S, Kumar S, Augustine S, Malhotra BD. Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer. Nanomaterials. 2019; 9(9):1190. https://doi.org/10.3390/nano9091190
Chicago/Turabian StyleKumar, Suveen, Shweta Panwar, Saurabh Kumar, Shine Augustine, and Bansi D. Malhotra. 2019. "Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer" Nanomaterials 9, no. 9: 1190. https://doi.org/10.3390/nano9091190
APA StyleKumar, S., Panwar, S., Kumar, S., Augustine, S., & Malhotra, B. D. (2019). Biofunctionalized Nanostructured Yttria Modified Non-Invasive Impedometric Biosensor for Efficient Detection of Oral Cancer. Nanomaterials, 9(9), 1190. https://doi.org/10.3390/nano9091190






