Free-Standing and Heteroatoms-Doped Carbon Nanofiber Networks as a Binder-Free Flexible Electrode for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Heteroatoms-Doped Activated Carbon Nanofiber Networks (ACFNs)
2.2. Characterization
3. Results and Discussion
3.1. Morphology and Structure
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Xu, B.; Gong, J.; Wen, J.; Hua, T.; Kan, C.W.; Deng, J. Design of High-Performance Wearable Energy and Sensor Electronics from Fiber Materials. ACS Appl. Mater. Interfaces 2019, 11, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-State supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhi, C. Functional flexible and wearable supercapacitors. J. Phys. D Appl. Phys. 2017, 50, 273001. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Wu, W. Recent progress in printed flexible solid-State supercapacitors for portable and wearable energy storage. J. Power Sour. 2019, 410–411, 69–77. [Google Scholar] [CrossRef]
- Liu, L.; Tian, Q.; Yao, W.; Li, M.; Li, Y.; Wu, W. All-Printed ultraflexible and stretchable asymmetric in-Plane solid-State supercapacitors (ASCs) for wearable electronics. J. Power Sour. 2018, 397, 59–67. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Ramasamy, K.; Gupta, R.; Gupta, A. Flexible Supercapacitors: A Materials Perspective. Front. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Sumboja, A.; Liu, J.; Zheng, W.G.; Zong, Y.; Zhang, H.; Liu, Z. Electrochemical energy storage devices for wearable technology: A rationale for materials selection and cell design. Chem. Soc. Rev. 2018, 47, 5919–5945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Rogers, J.A. Mechanics of stretchable batteries and supercapacitors. Curr. Opin. Solid State Mater. Sci. 2015, 19, 190–199. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Beirne, S.; Razal, J.; Chen, J. Recent Development of Fabricating Flexible Micro-Supercapacitors for Wearable Devices. Adv. Mater. Technol. 2018, 3, 1800028. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.H.; Kang, F.; Yang, Q.H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-Doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Cao, Q.; Wang, X.; Jing, B.; Peng, X.; Tang, X. Nitrogen-Doped hierarchical porous carbon for supercapacitor with well electrochemical performances. J. Solid State Electrochem. 2015, 19, 1591–1597. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, X.; Huang, B.; Yuan, K.; Chen, Y. Nitrogen-Doped Hierarchically Porous Carbon Materials with Enhanced Performance for Supercapacitor. ChemElectroChem 2018, 5, 515–522. [Google Scholar] [CrossRef]
- Kim, D.K.; Bong, S.; Jin, X.; Seong, K.d.; Hwang, M.; Kim, N.D.; You, N.H.; Piao, Y. Facile in Situ Synthesis of Multiple-Heteroatom-Doped Carbons Derived from Polyimide Precursors for Flexible All-Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.; Sun, L.; Wan, P.; Zhang, X.; Qiu, J. Sustainable synthesis of phosphorus and nitrogen-Co-Doped porous carbons with tunable surface properties for supercapacitors. J. Power Sour. 2013, 239, 81–88. [Google Scholar] [CrossRef]
- Yu, X.; Kang, Y.; Park, H.S. Sulfur and phosphorus co-Doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance. Carbon 2016, 101, 49–56. [Google Scholar] [CrossRef]
- Zhou, M.; Pu, F.; Wang, Z.; Guan, S. Nitrogen-Doped porous carbons through KOH activation with superior performance in supercapacitors. Carbon 2014, 68, 185–194. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wu, Q. Nitrogen and phosphorus dual-Doped hierarchical porous carbon with excellent supercapacitance performance. Electrochim. Acta 2017, 247, 1140–1146. [Google Scholar] [CrossRef]
- Prasannan, K.; Rajalakshmi, N.; Dhathathreyan, K.S. Phosphorus-Doped Exfoliated Graphene for Supercapacitor Electrodes. J. Nanosci. Nanotechnol. 2013, 13, 1746–1751. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Fan, X.; Jia, X.; Yu, Y.; Yang, X. Nitrogen/phosphorus co-Doped nonporous carbon nanofibers for high-Performance supercapacitors. J. Power Sour. 2014, 248, 745–751. [Google Scholar] [CrossRef]
- Wang, B.; Lu, G.; Luo, Q.P.; Wang, T. Free-Standing Porous Carbon Nanofiber Networks from Electrospinning Polyimide for Supercapacitors. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Dar, S.U.; Zhao, Y.; Akram, R.; Zhang, X.; Jin, S.; Wu, Z.; Wu, D. Polyphosphazene-Derived heteroatoms-Doped carbon materials for supercapacitor electrodes. Carbon 2018, 129, 420–427. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Harrison, I.; Huang, N.M.; Andou, Y.; Chong, K.F.; Pandikumar, A. Electrospun nanofiber membranes as ultrathin flexible supercapacitors. RSC Adv. 2017, 7, 12033–12040. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Miyauchi, M.; Simmons, T.; Dordick, J.; Linhardt, R. Electrospinning of Nanomaterials and Applications in Electronic Components and Devices. J. Nanosci. Nanotechnol. 2010, 10, 5507–5519. [Google Scholar] [CrossRef]
- Miao, Y.E.; Yan, J.; Huang, Y.; Fan, W.; Liu, T. Electrospun polymer nanofiber membrane electrodes and an electrolyte for highly flexible and foldable all-Solid-State supercapacitors. RSC Adv. 2015, 5, 26189–26196. [Google Scholar] [CrossRef]
- Liu, H.; Song, H.; Chen, X.; Zhang, S.; Zhou, J.; Ma, Z. Effects of nitrogen and oxygen-Containing functional groups of activated carbon nanotubes on the electrochemical performance in supercapacitors. J. Power Sour. 2015, 285, 303–309. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Shrestha, S.; Asheghi, S.; Timbro, J.; Mustain, W.E. Temperature controlled surface chemistry of nitrogen-Doped mesoporous carbon and its influence on Pt ORR activity. Appl. Cata A Gen. 2013, 464–465, 233–242. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of Few-Layer Graphite Flakes with FeCl3: Raman Determination of Fermi Level, Layer by Layer Decoupling, and Stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, C.; Zhang, Z.J.; Xie, D.H.; Deng, X.; Liu, J.W. Nitrogen-Doped porous carbon for supercapacitor with long-Term electrochemical stability. J. Power Sour. 2013, 230, 50–58. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.H.; Lu, G.Q. Nitrogen-Enriched Nonporous Carbon Electrodes with Extraordinary Supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Jeon, J.W.; Sharma, R.; Meduri, P.; Arey, B.W.; Schaef, H.T.; Lutkenhaus, J.L.; Lemmon, J.P.; Thallapally, P.K.; Nandasiri, M.I.; McGrail, B.P.; et al. In Situ One-Step Synthesis of Hierarchical Nitrogen-Doped Porous Carbon for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7214–7222. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, D.; Jia, M.; Cao, G.; Yang, Y. Nitrogen-Doped porous carbon simply prepared by pyrolyzing a nitrogen-Containing organic salt for supercapacitors. Electrochim. Acta 2013, 98, 176–182. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Chen, X.; Fu, R. Nitrogen-Enriched Nanocarbons with a 3-D Continuous Mesopore Structure from Polyacrylonitrile for Supercapacitor Application. J. Phys. Chem. C 2010, 114, 8581–8586. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Kodiweera, N.K.A.C.; Stallworth, P.E.; Greenbaum, S.; Bandosz, T.J. Effect of surface phosphorus functionalities of activated carbons containing oxygen and nitrogen on electrochemical capacitance. Carbon 2009, 47, 1576–1584. [Google Scholar] [CrossRef] [Green Version]
- Nasini, U.B.; Bairi, V.G.; Ramasahayam, S.K.; Bourdo, S.E.; Viswanathan, T.; Shaikh, A.U. Phosphorous and nitrogen dual heteroatom doped mesoporous carbon synthesized via microwave method for supercapacitor application. J. Power Sour. 2014, 250, 257–265. [Google Scholar] [CrossRef]
- Paniego, A.R. Reporting physisorption data for gas solid systems with special reference to the determination of surface-Area and porosity. Quim. Serv. Quim. Fis. Quim. Tec. 1989, 85, 386–399. [Google Scholar]
- Zhang, H.; Zhang, X.; Ma, Y. Enhanced capacitance supercapacitor electrodes from porous carbons with high mesoporous volume. Electrochim. Acta 2015, 184, 347–355. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, K.P.; Teng, H. A simplified preparation of mesoporous carbon and the examination of the carbon accessibility for electric double layer formation. Carbon 2005, 43, 559–566. [Google Scholar] [CrossRef]
- Xia, K.; Gao, Q.; Jiang, J.; Hu, J. Hierarchical porous carbons with controlled micropores and mesopores for supercapacitor electrode materials. Carbon 2008, 46, 1718–1726. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-Performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Cho, S.; Shin, K.H.; Jang, J. Enhanced Electrochemical Performance of Highly Porous Supercapacitor Electrodes Based on Solution Processed Polyaniline Thin Films. ACS Appl. Mater. Interfaces 2013, 5, 9186–9193. [Google Scholar] [CrossRef]
- Zhu, G.; He, Z.; Chen, J.; Zhao, J.; Feng, X.; Ma, Y.; Fan, Q.; Wang, L.; Huang, W. Highly conductive three-Dimensional MnO2–Carbon nanotube–Graphene–Ni hybrid foam as a binder-Free supercapacitor electrode. Nanoscale 2014, 6, 1079–1085. [Google Scholar] [CrossRef]

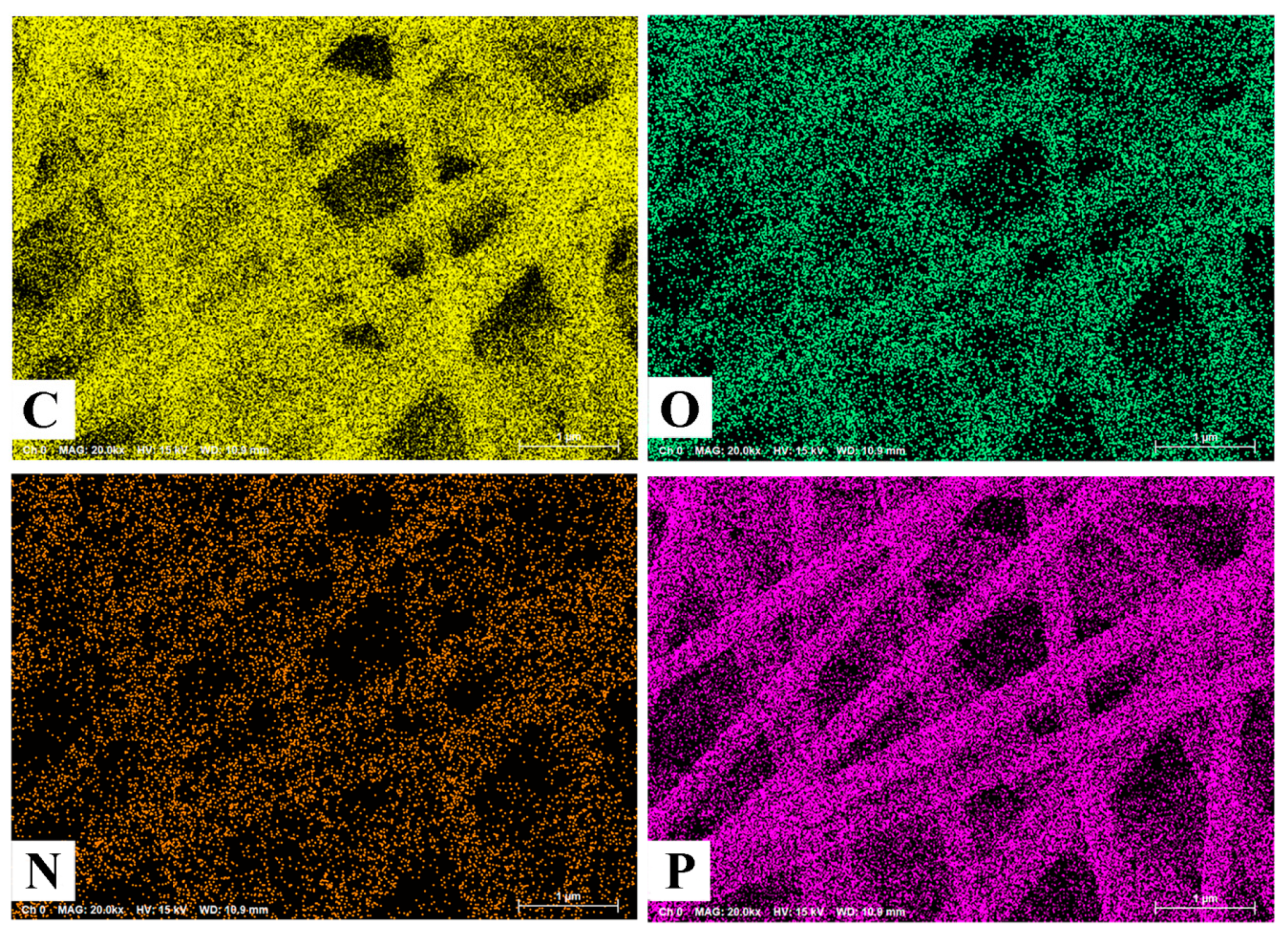
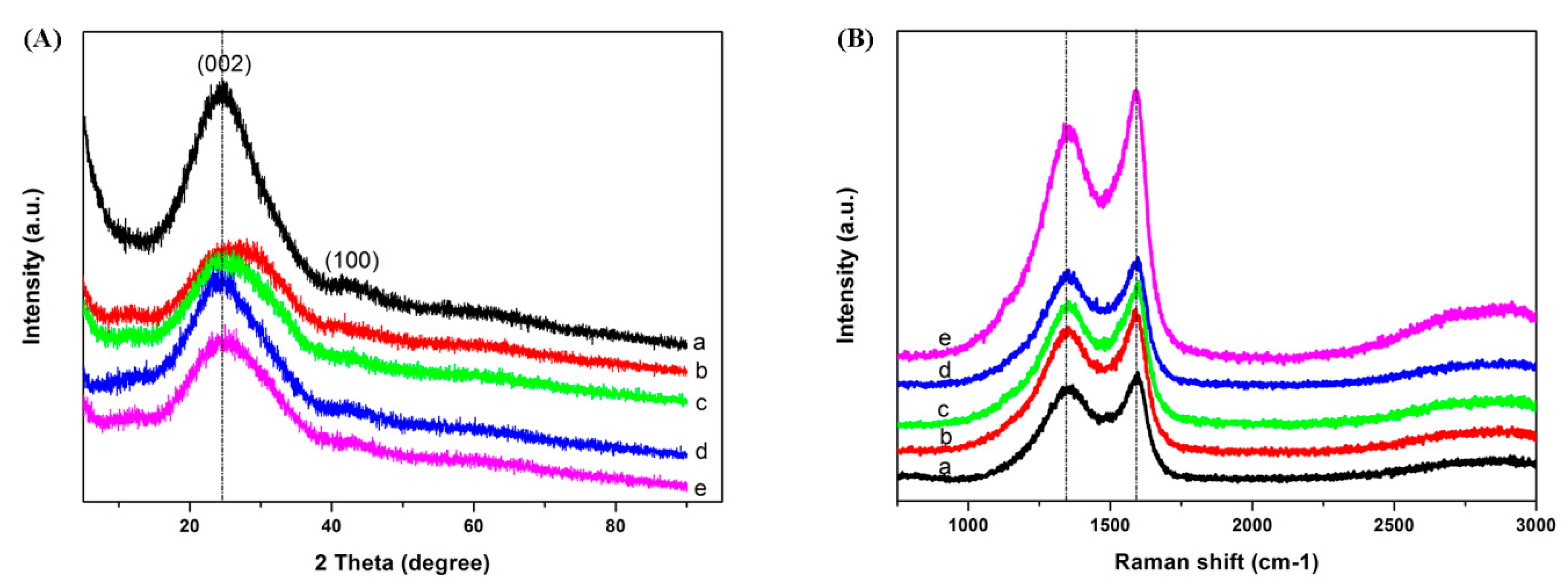



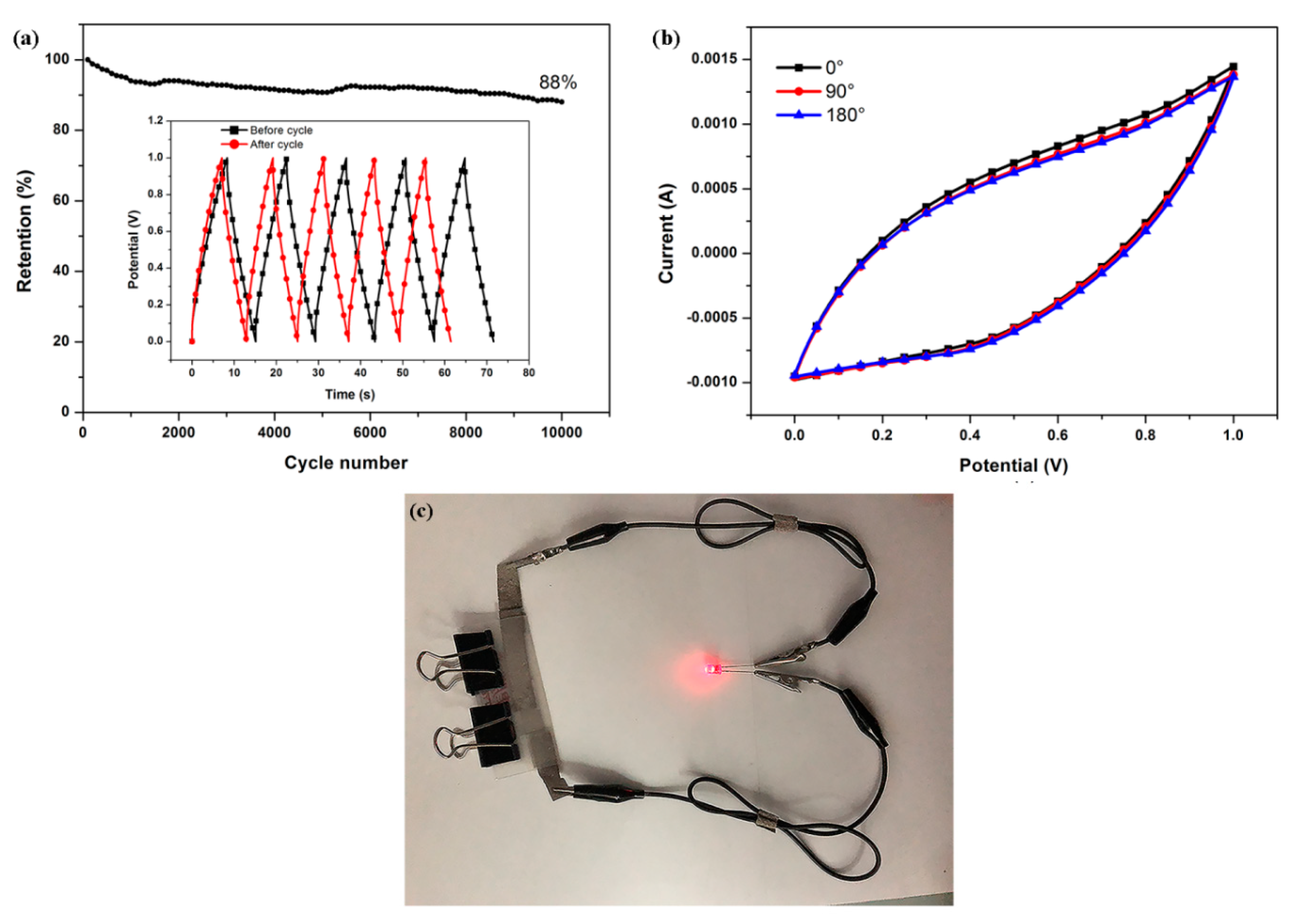
| Sample | (a) ACF-0 | (b) ACF-3.9% PDPP | (c) ACF-6.4% PDPP | (d) ACF-10.3% PDPP | (e) ACF-12.9% PDPP |
|---|---|---|---|---|---|
| ID/IG | 0.88 | 0.90 | 0.91 | 0.94 | 0.91 |
| C | O | N | P | N1s (at %) | ||
|---|---|---|---|---|---|---|
| (at %) | N-6 | N-5 | N-Q | |||
| 82.09 | 13.32 | 4.14 | 0.45 | 1.58 | 1.92 | 0.64 |
| Elements | C (at %) | O (at %) | N (at %) | P (at %) | |
|---|---|---|---|---|---|
| Sample | |||||
| PI-12.9% PDPP | 77.38 | 15.25 | 6.72 | 0.65 | |
| CF-12.9% PDPP | 85.9 | 10.14 | 3.41 | 0.55 | |
| ACF-12.9% PDPP | 81.67 | 14.92 | 2.98 | 0.44 | |
| Elements | C (at %) | O (at %) | N (at %) | P (at %) | |
|---|---|---|---|---|---|
| Sample | |||||
| ACF-0 | 82 | 12.34 | 5.66 | ||
| ACF-3.9% PDPP | 82.78 | 11.32 | 5.65 | 0.25 | |
| ACF-6.4% PDPP | 81.32 | 13.98 | 4.25 | 0.45 | |
| ACF-10.3% PDPP | 82.09 | 13.32 | 4.14 | 0.45 | |
| ACF-12.9% PDPP | 81.67 | 14.92 | 2.98 | 0.44 | |
| Sample | SBET (m2·g−1) | Smicro (m2·g−1) | Sext (m2·g−1) | Vtotal (m2·g−1) | APD a (nm) |
|---|---|---|---|---|---|
| ACF-0 | 814.2 | 672.7 | 141.5 | 0.37 | 1.8 |
| ACF-3.9% PDPP | 12.6 | 9.7 | 2.9 | 0.011 | 3.3 |
| ACF-6.4% PDPP | 16.5 | 12.8 | 3.7 | 0.012 | 2.9 |
| ACF-10.3% PDPP | 130.6 | 90.6 | 40 | 0.073 | 2.2 |
| ACF-12.9% PDPP | 54.6 | 30.2 | 24.4 | 0.035 | 2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; You, H.; Liu, W.; Wang, X.; Wu, D. Free-Standing and Heteroatoms-Doped Carbon Nanofiber Networks as a Binder-Free Flexible Electrode for High-Performance Supercapacitors. Nanomaterials 2019, 9, 1189. https://doi.org/10.3390/nano9091189
Yan X, You H, Liu W, Wang X, Wu D. Free-Standing and Heteroatoms-Doped Carbon Nanofiber Networks as a Binder-Free Flexible Electrode for High-Performance Supercapacitors. Nanomaterials. 2019; 9(9):1189. https://doi.org/10.3390/nano9091189
Chicago/Turabian StyleYan, Xiaona, Hanjing You, Wei Liu, Xiaodong Wang, and Dezhen Wu. 2019. "Free-Standing and Heteroatoms-Doped Carbon Nanofiber Networks as a Binder-Free Flexible Electrode for High-Performance Supercapacitors" Nanomaterials 9, no. 9: 1189. https://doi.org/10.3390/nano9091189




