Nanoencapsulation Enhances Anticoagulant Activity of Adenosine and Dipeptide IleTrp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mice
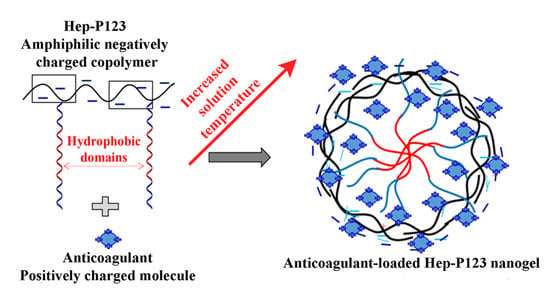
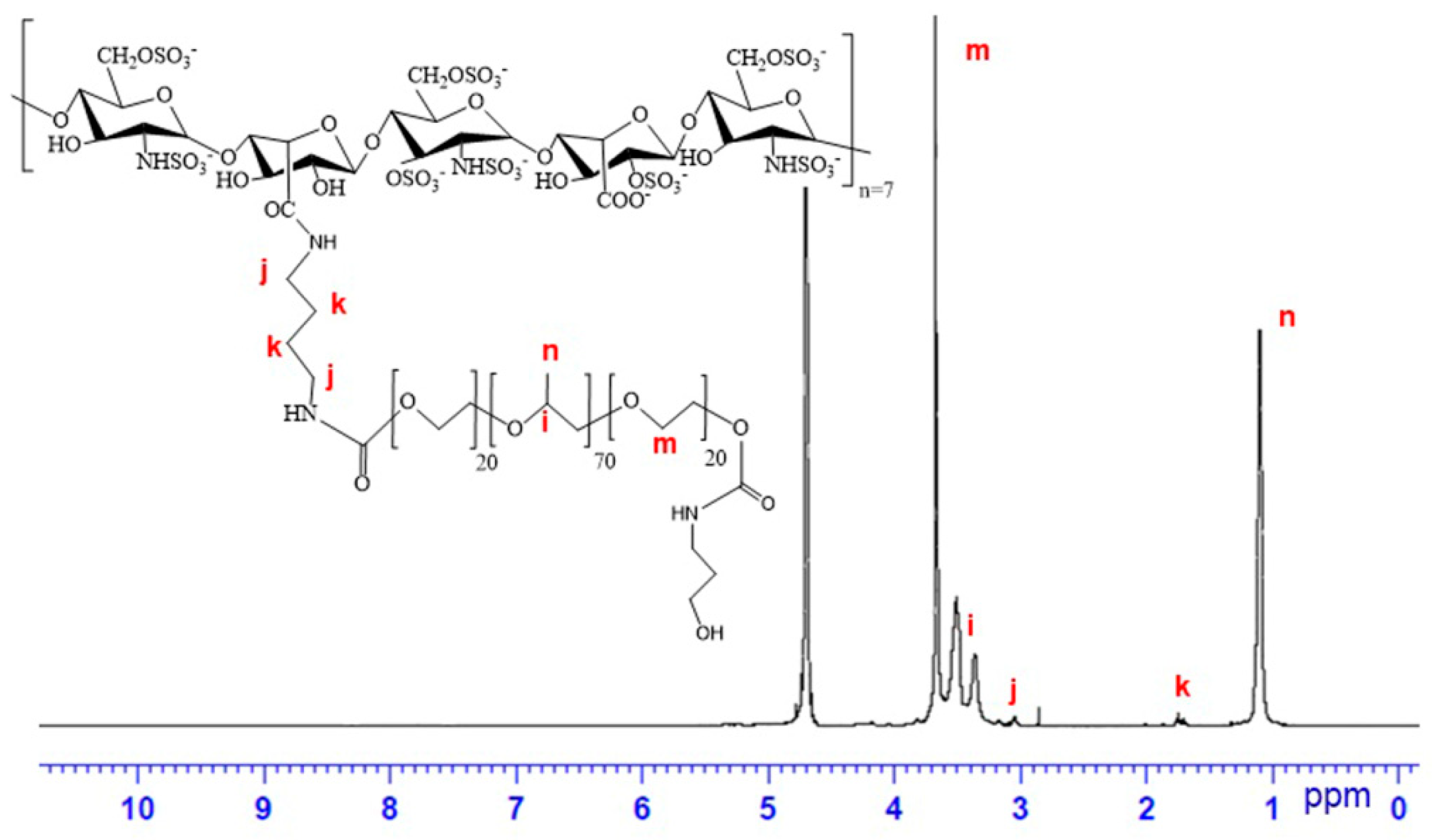
2.3. Preparation of Pluronic P123-grafted Heparin (Hep-P123)
2.4. Preparation of IW and Ado loaded Hep-P123 Nanogels
2.5. Study of IW and Ado Release Behavior
2.6. Determination of Anticoagulant Activity
2.7. Statistical Analysis
3. Results
3.1. Characterization of Hep-P123 Copolymer
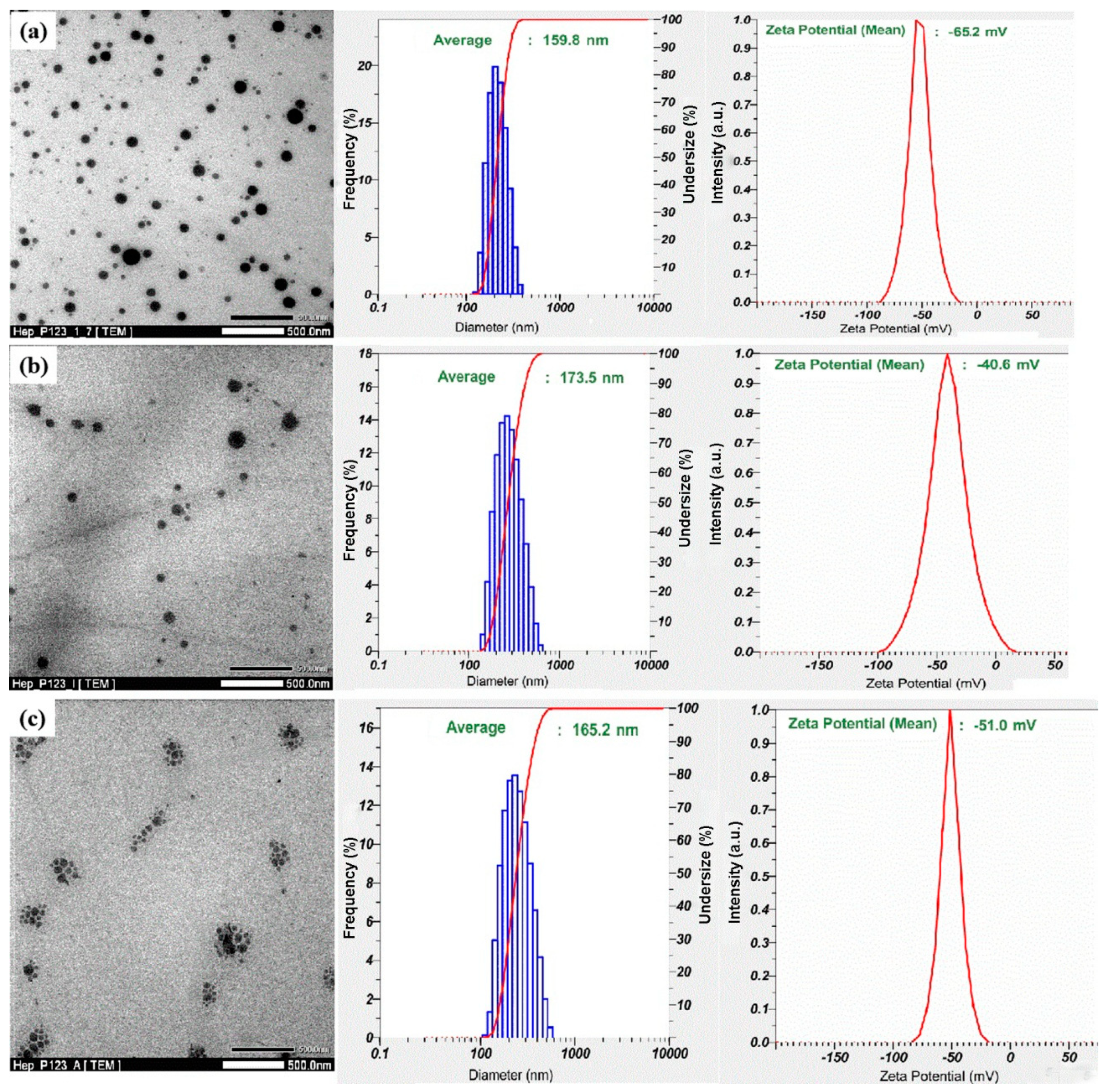
3.2. Preparation of IW and Ado Anticoagulants Encapsulated in Hep-P123 Nanogels
3.3. Release Profiles of Ado and IW
3.4. Studies of Anticoagulant Activity
3.4.1. Influence on Blood Coagulation in vitro
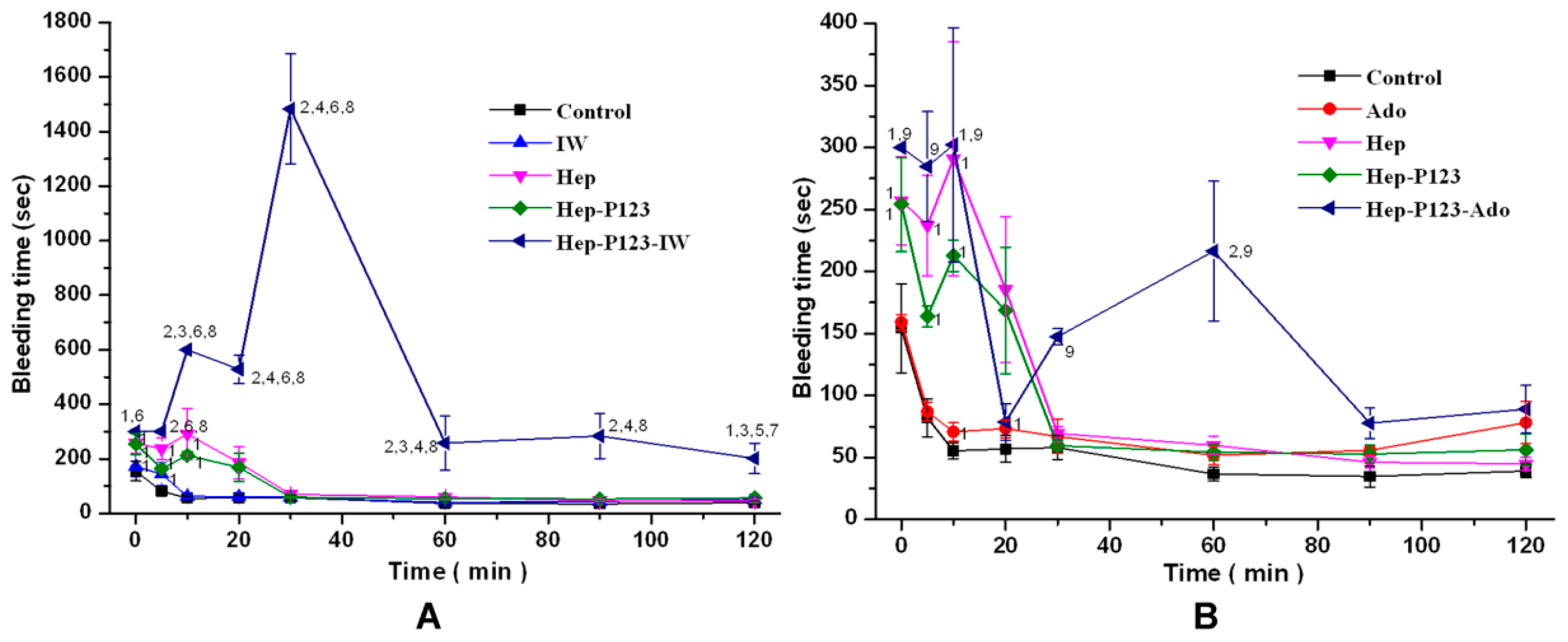
3.4.2. Influence on Bleeding Time in vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galdiero, S.; Gomes, P.A.C. Peptide-Based Drugs and Drug Delivery Systems. Molecules 2017, 22, 2185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, N.N.T.; Tran, N.T.N.; Le, P.N.; Nguyen, T.B.T.; Nguyen, N.H.; Bach, L.G.; Doan, V.N.; Tran, H.L.B.; Le, V.T.; et al. Synergic activity against MCF-7 breast cancer cell growth of nanocurcumin-encapsulated and cisplatin-complexed nanogels. Molecules 2018, 23, 3347. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.K.; Baboota, S.; Ahuja, A.; Hasan, S.; Ali, J. Recent advances in protein and peptide drug delivery systems. Curr. Drug Deliv. 2007, 4, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kovalainen, M.; Mönkäre, J.; Riikonen, J.; Pesonen, U.; Vlasova, M.; Salonen, J.; Lehto, V.P.; Järvinen, K.; Herzig, K.H. Novel delivery systems for improving the clinical use of peptides. Pharmacol. Rev. 2015, 67, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Galliani, M.; Tremolanti, C.; Signore, G. Nanocarriers for protein delivery to the cytosol: Assessing the endosomal escape of poly (lactide-co-glycolide)-poly (ethylene imine) nanoparticles. Nanomaterials 2019, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.; Huynh, C.K.; Tran, N.Q. Advances in thermosensitive polymer-grafted platforms for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1016–1103. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design, strategies and applications. J. Mater. Chem. B 2016, 4, 4060–4071. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric nanogels as drug delivery systems. Physiol. Res. 2018, 67, S305–S317. [Google Scholar] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Xu, P. Smart mesoporous silica nanoparticles for protein delivery. Nanomaterials 2019, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, H.; Liu, J.; Zhai, G. Advanced nanocarriers based on Heparin and its derivatives for cancer management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, C.K.; Tran, N.Q. Aquated cisplatin and heparin-pluronic nanocomplexes exhibiting sustainable release of active platinum compound and NCI-H460 lung cancer cell anti-proliferation. J. Biomater. Sci. Polym. Ed. 2016, 27, 709–720. [Google Scholar]
- Tong, N.N.A.; Cao, V.D.; Nguyen, C.K.; Nguyen, T.P.; Nguyen, X.T.D.T.; Tran, N.Q. Thermosensitive heparin-pluronic copolymer as effective dual anticancer drugs delivery system for combination cancer therapy. Int. J. Nanotechnol. 2018, 15, 174–187. [Google Scholar] [CrossRef]
- Argyo, C.; Cauda, V.; Engelke, H.; Rädler, J.; Bein, G.; Bein, T. Heparin-coated colloidal mesoporous silica nanoparticles efficiently bind to antithrombin as anticoagulant drug delivery system. Chem. Eur. J. 2012, 18, 428–432. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; He, C.; Xiao, Z.; Ye, J.; Li, Y.; Chen, A.; Zhang, H.; Li, X.; Lin, L.; et al. Comparative study of heparin-poloxamer hydrogel modified bFGF and aFGF for in vivo wound healing efficiency. ACS Appl. Mater. Interfaces 2016, 8, 18710–18721. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Jiang, X.; Xiao, J.; Lin, Q.; Yu, W.Z.; Tian, F.R.; Mao, K.L.; Yang, W.; Wong, H.L.; Lu, C.T. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016, 29, 71–80. [Google Scholar] [CrossRef]
- Choi, J.H.; Joung, Y.K.; Bae, J.W.; Choi, J.W.; Tran, N.Q.; Park, K.D. Self-assembled nanogel of pluronic-conjugated heparin as a versatile drug nanocarrier. Macromol. Res. 2011, 19, 180–188. [Google Scholar] [CrossRef]
- Smith, M.; Wakam, G.; Wakefield, T.; Obi, A. New Trends in Anticoagulation Therapy. Surg. Clin. N. Am. 2018, 98, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Pazzini, C.; Marcato, P.D.; Prado, L.B.; Alessio, A.M.; Höehr, N.F.; Montalvão, S.; Paixão, D.; Durán, N.; Annichino-Bizzacchi, J.M. Polymeric Nanoparticles of Enoxaparin as a Delivery System: In Vivo Evaluation in Normal Rats and in a Venous Thrombosis Rat Model. J. Nanosci. Nanotechnol. 2015, 15, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Hoang, A.N.; Nguyen, T.T.T.; Phung, T.V.; Nguyen, K.C.; Osipov, A.V.; Ivanov, I.A.; Tsetlin, V.I.; Utkin, Y.N. Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom. Toxins 2017, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, T.H.; Vu, M.T.; Le, V.T.; Nguyen, X.A.; Nguyen, T.C.; Nguyen, T.B.T. Novel amphiphilic heparin-pluronic P123 copolymers exhibiting a great potential for Cisplatin delivery. J. Mater. Sci. 2018, 53, 12692–12703. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Nguyen, N.T.; Nguyen, C.K.; Utkin, Y.N.; Tran, N.Q.; Hoang, N.A. Synthesis of nano encapsulated low-molecular weight anticoagulants. Vietnam J. Chem. 2019, 57, 322–327. [Google Scholar]
- Ankrum, J.A.; Miranda, O.R.; Ng, K.S.; Sarkar, D.; Xu, C.; Karp, J.M. Engineering cells with intracellular agent–loaded microparticles to control cell phenotype. Nat. Protoc. 2014, 9, 233–245. [Google Scholar] [CrossRef]
- Thakker, K.D.; Chern, W.H. Development and Validation of In Vitro Release Tests for Semisolid Dosage Forms—Case Study. Dissolut. Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Barker, L.F. The Clinical Diagnosis of Internal Diseases. In Monographic Medicine; Appleton Company: New York, NY, USA; London, UK, 1917; Volume III, p. 131. [Google Scholar]
- Liu, Y.; Jennings, N.L.; Dart, A.M.; Du, X.J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J. Exp. Med. 2012, 2, 30–36. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Dinh, V.T.; Dang, L.H.; Nguyen, D.N.; Giang, B.L.; Nguyen, C.T.; Nguyen, T.B.T.; Thu, L.V.; Tran, N.Q. Dual interactions of amphiphilic gelatin copolymer and nanocurcumin improving the delivery efficiency of the nanogels. Polymers 2019, 11, 814. [Google Scholar] [CrossRef]
- Park, K.; Kim, K.; Kwon, I.C.; Kim, S.K.; Lee, S.; Lee, D.Y.; Byun, Y. Preparation and characterization of self-assembled nanoparticles of heparin-deoxycholic acid conjugates. Langmuir 2014, 20, 11726–11731. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Action of nanoparticles on platelet activation and plasmatic coagulation. Curr. Med. Chem. 2016, 23, 408–430. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Hogwood, J.; Mulloy, B. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharmacol. 2012, 207, 43–61. [Google Scholar]
- Park, H.D.; Lee, W.K.; Ooya, T.; Park, K.D.; Kim, Y.H.; Yui, N. Anticoagulant activity of sulfonated polyrotaxanes as blood-compatible materials. J. Biomed. Mater. Res. 2002, 60, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; May, J.A.; Heptinstall, S.; Lowe, K.C. Effects of pluronic F-68 (poloxamer 188) on platelet aggregation in human whole blood. Thromb. Res. 1996, 81, 511–512. [Google Scholar] [CrossRef]
- Nguyen, T.T.C.; Nguyen, T.H.; Tran, N.Q.; Nguyen, C.K. Highly lipophilic pluronics-conjugated polyamidoamine dendrimer nanocarriers as potential delivery system for hydrophobic drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 992–999. [Google Scholar] [CrossRef]
- Le, P.N.; Nguyen, N.H.; Nguyen, C.K.; Tran, N.Q. Smart dendrimer-based nanogel for enhancing 5-fluorouracil loading efficiency against MCF7 cancer cell growth. Bull. Mater. Sci. 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A. Heparin-based nanoparticles: An overview of their applications. J. Nanomater. 2018, 2018, 9780489. [Google Scholar] [CrossRef]


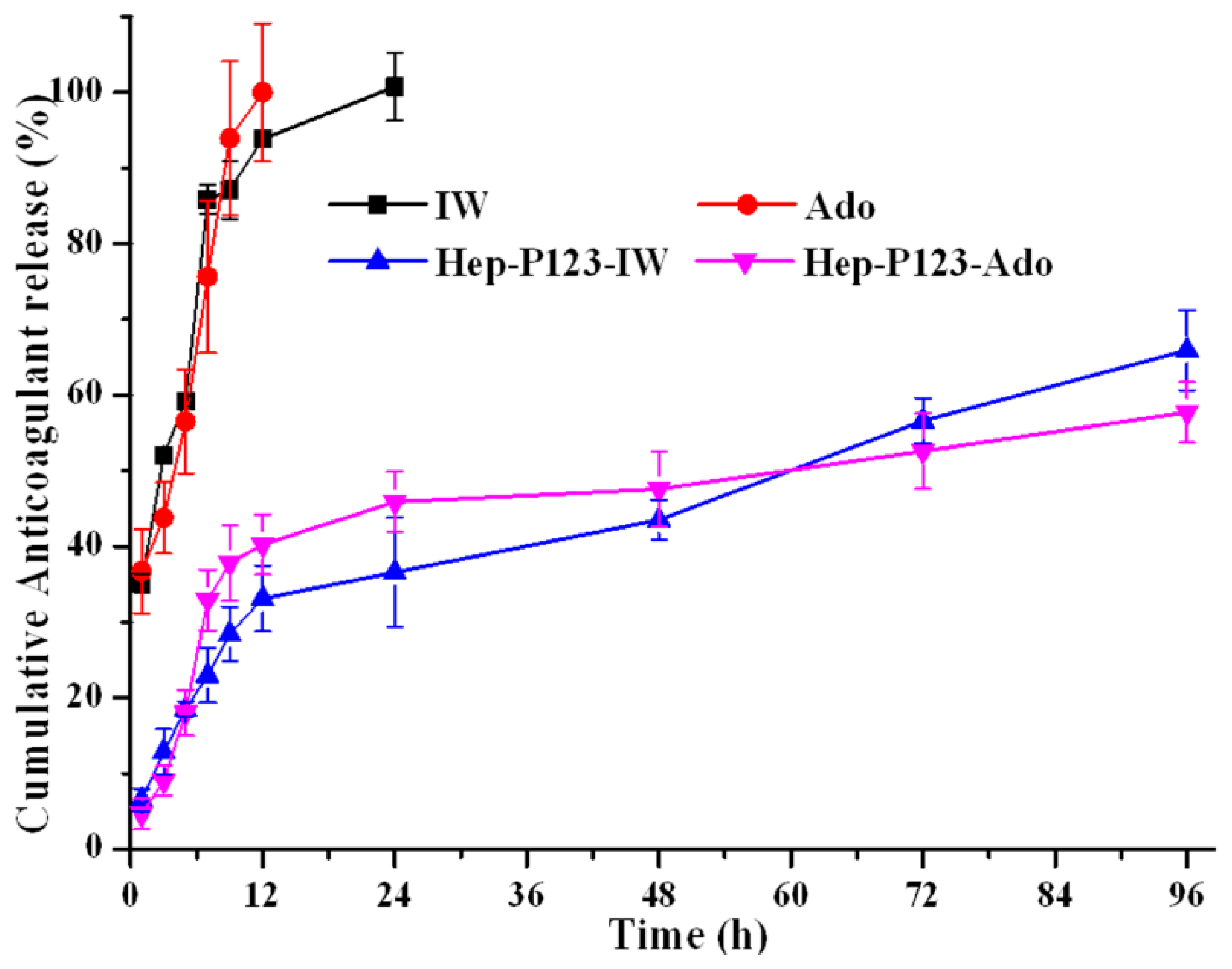

| Cumulative Anticoagulant Release (%) | ||||
|---|---|---|---|---|
| Time (h) | IW | Ado | Hep-P123-IW | Hep-P123-Ado |
| 1 | 35.17 ± 1.27 | 36.72 ± 5.54 | 6.48 ± 1.48 | 4.67 ± 2.40 |
| 3 | 52.08 ± 0.85 | 43.80 ± 4.73 | 12.87 ± 3.01 | 9.03 ± 2.03 |
| 5 | 59.20 ± 0.70 | 56.53 ± 6.84 | 18.52 ± 1.92 | 18.06 ± 3.61 |
| 7 | 85.86 ± 1.93 | 75.65 ± 10.10 | 22.95 ± 3.63 | 32.94 ± 4.52 |
| 9 | 87.13 ± 3.82 | 93.94 ± 10.14 | 28.44 ± 3.62 | 37.81 ± 5.03 |
| 12 | 93.84 ± 1.05 | 100.0 ± 9.10 | 33.11 ± 4.35 | 40.25 ± 4.04 |
| 24 | 100.0 ± 4.51 | - | 36.58 ± 7.26 | 45.87 ± 4.71 |
| 48 | - | - | 43.52 ± 2.64 | 47.59 ± 5.53 |
| 96 | - | - | 65.94 ± 5.28 | 57.74 ± 4.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Nguyen, T.N.; Nguyen, T.T.T.; Ivanov, I.A.; Nguyen, K.C.; Tran, Q.N.; Hoang, A.N.; Utkin, Y.N. Nanoencapsulation Enhances Anticoagulant Activity of Adenosine and Dipeptide IleTrp. Nanomaterials 2019, 9, 1191. https://doi.org/10.3390/nano9091191
Nguyen TD, Nguyen TN, Nguyen TTT, Ivanov IA, Nguyen KC, Tran QN, Hoang AN, Utkin YN. Nanoencapsulation Enhances Anticoagulant Activity of Adenosine and Dipeptide IleTrp. Nanomaterials. 2019; 9(9):1191. https://doi.org/10.3390/nano9091191
Chicago/Turabian StyleNguyen, Trung Dinh, The Ngoc Nguyen, Trang Thuy Thi Nguyen, Igor A. Ivanov, Khoa Cuu Nguyen, Quyen Ngoc Tran, Anh Ngoc Hoang, and Yuri N. Utkin. 2019. "Nanoencapsulation Enhances Anticoagulant Activity of Adenosine and Dipeptide IleTrp" Nanomaterials 9, no. 9: 1191. https://doi.org/10.3390/nano9091191