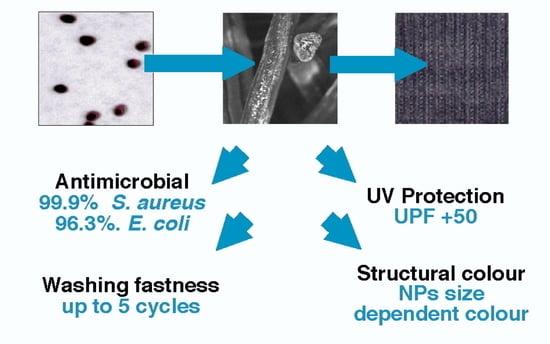
Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. AuNPs Synthesis
2.3. Chitosan Treatment
2.4. AuNPs Functionalization
2.5. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
2.6. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
2.7. UV-Visible Spectroscopy
2.8. Attenuated Total Reflectance-Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.9. X-ray Photoelectron Spectroscopy (XPS)
2.10. Thermal Gravimetric Analysis (TGA)
2.11. Differential Scanning Calorimeter (DSC) Analysis
2.12. Diffuse Reflectance Spectroscopy
2.13. UV-Light Protection
2.14. Colour Fastness
2.15. Antimicrobial Testing
3. Results and Discussion
3.1. Fabric Reflectance
3.2. AuNPs Characterization
3.3. AuNPs Functionalization
3.4. ATR-FTIR
3.5. XPS
3.6. Thermal Properties
3.7. Colour Fastness
3.8. UV-Light Protection
3.9. Antimicrobial Action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385. [Google Scholar] [CrossRef]
- Vu, N.K.; Zille, A.; Oliveira, F.R.; Carneiro, N.; Souto, A.P. Effect of Particle Size on Silver Nanoparticle Deposition onto Dielectric Barrier Discharge (DBD) Plasma Functionalized Polyamide Fabric. Plasma Process. Polym. 2013, 10, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Yao, Y.; Li, J.; Qin, S.; Zhu, H.; Kaur, J.; Chen, W.; Sun, L.; Wang, X. Functional Application of Noble Metal Nanoparticles In Situ Synthesized on Ramie Fibers. Nanoscale Res. Lett. 2015, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, M.; Jiang, S.-X.K.; Ding, F.; Wang, J. Coating fabrics with gold nanorods for colouring, UV-protection, and antibacterial functions. Nanoscale 2013, 5, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Sun, L.; Kaur, J.; Yu, Y.; Wang, X. In-situ synthesis of gold nanoparticles for multifunctionalization of silk fabrics. Dyes Pigments 2014, 103, 183–190. [Google Scholar] [CrossRef]
- Johnston, J.H.; Burridge, K.A.; Kelly, F.M. The Formation and Binding of Gold Nanoparticles onto Wool Fibres. AIP Conf. Proc. 2009, 1151, 189–192. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D.; Daniel, M. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 35, 293–346. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Lima, E.; Guerra, R.; Lara, V.; Guzmán, A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Cent. J. 2013, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, M.R.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Dong, B.H.; Hinestroza, J.P. Metal Nanoparticles on Natural Cellulose Fibers: Electrostatic Assembly and In Situ Synthesis. ACS Appl. Mater. Interfaces 2009, 1, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Radić, N.; Obradović, B.M.; Kostić, M.; Dojčinović, B.; Hudcová, M.; Kuraica, M.M.; Černák, M. Deposition of gold nanoparticles on polypropylene nonwoven pretreated by dielectric barrier discharge and diffuse coplanar surface barrier discharge. Plasma Chem. Plasma Process. 2013, 33, 201–218. [Google Scholar] [CrossRef]
- Tang, B.; Sun, L.; Li, J.; Kaur, J.; Zhu, H.; Qin, S.; Yao, Y.; Chen, W.; Wang, X. Functionalization of bamboo pulp fabrics with noble metal nanoparticles. Dyes Pigments 2015, 113, 289–298. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Vynias, D. Soybean fibre: A novel fibre in the textile industry. In Soybean-Biochemistry, Chemistry and Physiology; InTech: London, UK, 2011. [Google Scholar]
- Tusief, M.Q.; Amin, N.; Mahmood, N.; Ahmad, I.; Abass, M. Antimicrobial Studies of Knitted Fabrics from Bamboo, Soybean and Flax Fibers at Various Blends. J. Text. Sci. Eng. 2015, 5, 1. [Google Scholar]
- Yi-you, L. The Soybean Protein Fibre-A Healthy & Comfortable Fibre for the 21st Century. Fibres Textiles East. Eur. 2004, 12, 8–9. [Google Scholar]
- Avinc, O.; Yavas, A. Soybean: For Textile Applications and Its Printing. In Soybean-The Basis of Yield, Biomass and Productivity; InTech: London, UK, 2017. [Google Scholar] [Green Version]
- Jafari, S.; Izadan, H.; Khoddami, A.; Zarrebini, M. Investigation into the Dyeing of Soybean Fibres with Natural Dyes and their antimicrobial properties. J. Prog Color Color. Coat. 2014, 7, 2012–2018. [Google Scholar]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Shih, C.-M.; Shieh, Y.-T.; Twu, Y.-K. Preparation of gold nanopowders and nanoparticles using chitosan suspensions. Carbohydr. Polym. 2009, 78, 309–315. [Google Scholar] [CrossRef]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F. Size and aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by atmospheric DBD plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Cunha, M.; Felgueiras, H.P.; Gouveia, I.; Zille, A. Synergistically enhanced stability of laccase immobilized on synthesized silver nanoparticles with water-soluble polymers. Colloids Surf. Biointerfaces 2017, 154, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeb, A.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Guio, E.; Grassian, V.H. Silver nanoparticles in simulated biological media: A study of aggregation, sedimentation, and dissolution. J. Nanopart. Res. 2011, 13, 233–244. [Google Scholar] [CrossRef]
- Sarkar, A.; Kapoor, S.; Mukherjee, T. Preparation, characterization, and surface modification of silver nanoparticles in formamide. J. Phys. Chem. 2005, 109, 7698–7704. [Google Scholar] [CrossRef]
- Maqsood, H.S.; Bashir, U.; Zubair, M.; Wiener, J.; Militky, J. Cationization of cellulose fibers for composites. J. Text. Inst. 2017, 108, 1302–1307. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, S.-W.; Chen, D.-H. Magnetic chitosan nanoparticles: Studies on chitosan binding and adsorption of Co (II) ions. React. Funct. Polym. 2006, 66, 335–341. [Google Scholar] [CrossRef]
- Tang, B.; Lin, X.; Zou, F.; Fan, Y.; Li, D.; Zhou, J.; Chen, W.; Wang, X. In situ synthesis of gold nanoparticles on cotton fabric for multifunctional applications. Cellulose 2017, 24, 4547–4560. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Chen, X.; Xu, H.; Liu, J. Chitosan-capped gold nanoparticles for selective and colorimetric sensing of heparin. J. Nanopart. Res. 2013, 15, 1930. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yang, Z.; Ji, D.; Zhu, N.; Li, X.; Wan, L.; Zhang, K.; Guo, K. Novel synthesis of a soy-based polyol for a polyurethane rigid foam. RSC Adv. 2016, 6, 90771–90776. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.; Azadi, E. Poly (vinyl alcohol) chains grafted onto the surface of copper oxide nanoparticles: Application in synthesis and characterization of novel optically active and thermally stable nanocomposites based on poly (amide-imide) containing N-trimellitylimido-l-valine linkage. Int. J. Polym. Anal. Charact. 2015, 20, 82–97. [Google Scholar]
- Gannimani, R.; Perumal, A.; Krishna, S.; Sershen, M.; Mishra, A.; Govender, P. Synthesis and antibacterial activity of silver and gold nanoparticles produced using aqueous seed extract of Protorhus longifolia as a reducing agent. Dig. J. Nanomater. Biostruct. 2014, 9, 1669–1679. [Google Scholar]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yang, C.M.; Yuan, X.Y. Properties of soy protein isolate/poly (vinyl alcohol) blend “green” films: Compatibility, mechanical properties, and thermal stability. J. Appl. Polym. Sci. 2008, 110, 3706–3716. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, Z.R.; Wang, C.X.; Zang, C.F.; Zhang, Y.; Xu, L. Influence of DBD plasma pretreatment on the deposition of chitosan onto UHMWPE fiber surfaces for improvement of adhesion and dyeing properties. Appl. Surf. Sci. 2017, 396, 1571–1579. [Google Scholar] [CrossRef]
- Ding, L.; Hao, C.; Xue, Y.; Ju, H. A bio-inspired support of gold nanoparticles-chitosan nanocomposites gel for immobilization and electrochemical study of K562 leukemia cells. Biomacromolecules 2007, 8, 1341–1346. [Google Scholar] [CrossRef]
- Vynias, D.; Carr, C.M. Investigation into the flame retardant properties of soybean fabric treated with sulphamic acid. J. Appl. Polym. Sci. 2008, 109, 3590–3595. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhu, H.T.; Zhang, B.W.; Chen, J.; Ao, Q.; Wang, X.Y. XRD, SEM, and XPS Analysis of Soybean Protein Powders Obtained Through Extraction Involving Reverse Micelles. J. Am. Oil Chem. Soc. 2015, 92, 975–983. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. An XPS study on the attachment of triethoxsilylbutyraldehyde to two titanium surfaces as a way to bond chitosan. Appl. Surf. Sci. 2008, 254, 4599–4605. [Google Scholar] [CrossRef]
- Augusto, G.D.; Scarminio, J.; Silva, P.R.C.; de Siervo, A.; Rout, C.S.; Rouxinol, F.; Gelamo, R.V. Flexible metal-free supercapacitors based on multilayer graphene electrodes. Electrochim. Acta 2018, 285, 241–253. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.F.; Li, S.Q.; Fan, Z.X.; Warzywoda, J.; Fan, Z.Y. Soybean-derived hierarchical porous carbon with large sulfur loading and sulfur content for high-performance lithium-sulfur batteries. J. Mater. Chem. 2016, 4, 16507–16515. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Freire, C. Hybrid photochromic multilayer films based on chitosan and europium phosphomolybdate. J. Appl. Electrochem. 2014, 44, 655–665. [Google Scholar] [CrossRef]
- Dambies, L.; Vincent, T.; Guibal, E. Treatment of arsenic-containing solutions using chitosan derivatives: Uptake mechanism and sorption performances. Water Res. 2002, 36, 3699–3710. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M. Chitosan Bio-Based Organic-Inorganic Hybrid Aerogel Microspheres. Chem. Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef]
- Dambies, L.; Guimon, C.; Yiacoumi, S.; Guibal, E. Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloids Surf. Physicochem. Eng. Asp. 2001, 177, 203–214. [Google Scholar] [CrossRef]
- Feng, J.J.; Chen, S.S.; Chen, X.L.; Zhang, X.F.; Wang, A.J. One-pot fabrication of reduced graphene oxide supported dendritic core-shell gold@gold-palladium nanoflowers for glycerol oxidation. J. Colloid Interface Sci. 2018, 509, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Vasil’kov, A.Y.; Rubina, M.S.; Naumkin, A.V.; Zubavichus, Y.V.; Belyakova, O.A.; Maksimov, Y.V.; Imshennik, V.K. Metal-containing systems based on chitosan and a collagen-chitosan composite. Rus. Chem. Bull. 2015, 64, 1663–1670. [Google Scholar] [CrossRef]
- Manna, A.; Imae, T.; Aoi, K.; Okada, M.; Yogo, T. Synthesis of dendrimer-passivated noble metal nanoparticles in a polar medium: Comparison of size between silver and gold particles. Chem. Mater. 2001, 13, 1674–1681. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Richardson, M.J.; Johnston, J.H. Sorption and binding of nanocrystalline gold by Merino wool fibres—An XPS study. J. Colloid Interface Sci. 2007, 310, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, G.; Felgueiras, H.P.; Ribeiro, A.I.; Seventekin, N.; Zille, A.; Souto, A.P. Dyed poly (styrene-methyl methacrylate-acrylic acid) photonic nanocrystals for enhanced structural color. ACS Appl. Mater. Interfaces 2018, 10, 23285–23294. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.; Sampaio, A.; Silva, C.J.; Felgueiras, H.P.; Amorim, M.T.P.; Zille, A. Antibacterial Electrospun Poly (vinyl alcohol)/Enzymatic Synthesized Poly (catechol) Nanofibrous Midlayer Membrane for Ultrafiltration. ACS Appl. Mater. Interfaces 2017, 9, 33107–33118. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Dairi, N. Green nanocomposite films based on cellulose acetate and biopolymer-modified nanoclays: Studies on morphology and properties. Iran. Polym. J. 2014, 23, 917–931. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Bhattacharya, R.; Bone, N.; Lee, Y.K.; Patra, C.R.; Wang, S.; Lu, L.; Secreto, C.; Banerjee, P.C.; Yaszemski, M.J. Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): Enhancing apoptosis. J. Nanobiotechnology 2007, 5, 4. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/poly (vinyl alcohol) blended films: Mechanical, thermal and surface investigations. J. Chem. 2011, 8, 91–96. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, M.; Yu, S.; Sui, G.; Yang, X. Studies on soy protein isolate/polyvinyl alcohol hybrid nanofiber membranes as multi-functional eco-friendly filtration materials. Mater. Sci. Eng. 2016, 214, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Q.; Jiang, S.; Jiang, S. Fabrication and Characterization of PVA/SPI/SiO2 Hybrid Fibres via Electrospinning Technique. Polym. Polym. Compos. 2012, 20, 621–628. [Google Scholar] [CrossRef]
- Oboh, J.; Okafor, J.; Kovo, A.; Abdulrahman, A. Investigation of eco-friendly cellulosic nanoparticles potential as reinforcement agent in the production of natural rubber composites. Niger. J. Technol. 2017, 36, 1078–1087. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Keer, L.M. Influence of surface modification on the microstructure and thermo-mechanical properties of bamboo fibers. Materials 2015, 8, 6597–6608. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Green synthesis of AuNPs for eco-friendly functionalization of cellulosic substrates. Appl. Surf. Sci. 2016, 389, 118–125. [Google Scholar] [CrossRef]
- Reinert, G.; Fuso, F.; Hilfiker, R.; Schmidt, E. UV-protecting properties of textile fabrics and their improvement. Text. Chem. Color. 1997, 29, 36–43. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Lawrence, R.; Tripathi, P.; Jeyakumar, E. Isolation, Purification and Evaluation of Antibacterial Agents from Aloe vera. Braz. J. Microbiol. 2009, 40, 906–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Yang, X. Chitosan mediated assembly of gold nanoparticles multilayer. Colloids Surf. Physicochem. Eng. Asp. 2003, 226, 77–86. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Morales, J.; Amariei, G.; Letón, P.; Rosal, R. Antimicrobial activity of poly (vinyl alcohol)-poly (acrylic acid) electrospun nanofibers. Colloids Surf. B Biointerfaces 2016, 146, 144–151. [Google Scholar] [CrossRef] [PubMed]








| Chemical Composition (%) | Atomic Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | C1s | N1s | O1s | Si2p | S2p | Au4f | O/C | N/C |
| Soy | 86.66 | 1.59 | 9.58 | 1.98 | 0.19 | - | 0.11 | 0.02 |
| Soy + Chitosan | 72.55 | 3.63 | 23.15 | 0.55 | 0.12 | - | 0.32 | 0.05 |
| Soy + AuNPs | 80.58 | 2.26 | 14.63 | 2.34 | 0.14 | 0.05 | 0.18 | 0.03 |
| Soy + Chitosan + AuNPs | 70.53 | 4.34 | 23.85 | 0.30 | 0.15 | 0.74 | 0.34 | 0.06 |
| Relative Area Corresponding to Different Chemical Bonds (%) | |||||
|---|---|---|---|---|---|
| BE (eV) | Soy | Soy + Chitosan | Soy + AuNPs | Soy + Chitosan + AuNPs | |
| C1s | 285.0 | 66.0 | 49.0 | 74.6 | 48.5 |
| 287.1 | 5.7 | - | - | - | |
| 287.9 | - | 11.2 | 4.9 | 10.9 | |
| 285.9 | 25.5 | - | - | - | |
| 286.5 | - | 36.7 | 17.9 | 38.1 | |
| 288.5 | 2.8 | - | 2.6 | - | |
| 288.9 | - | 2.8 | - | 2.5 | |
| O1s | 534.5 | 6.6 | 4.6 | - | 2.6 |
| 532.3 | 93.4 | - | 100 | - | |
| 532.8 | - | 84.7 | - | - | |
| 531.3 | - | 10.7 | - | - | |
| 532.9 | - | - | - | 85.2 | |
| 531.5 | - | - | - | 12.2 | |
| N1s | 400.0 | 100.0 | 100.0 | 100.0 | 84.8 |
| 402.0 | - | - | - | 15.2 | |
| Au4f | 87.2 | - | - | 44.4 | - |
| 87.5 | - | - | - | 40.1 | |
| 83.6 | - | - | 55.6 | - | |
| 83.8 | - | - | - | 51.4 | |
| 85.0 | - | - | - | 5.1 | |
| 88.6 | - | - | - | 3.5 | |
| Samples | Tm (°C) | ΔH (J g−1) | T Peaks of 1st Derivative (°C) | Residue (%) at 600 °C |
|---|---|---|---|---|
| Soy | 76.4; 231.6; 275.8; | 159.4; 65.5; 58.8 | 295.1 | 10.3 |
| Soy + Chitosan | 78.0; 235.4; 291.4 | 127.5; 65.0; 90.6 | 324.5; 352.9 | 14.3 |
| Soy + AuNPs | 77.1; 234.0; 277.0 | 130.5; 53.4; 61.7 | 292.3 | 9.8 |
| Soy + Chitosan + AuNPs | 80.5; 233.2; 284.2 | 312.5; 70.7; 33.3 | 348.3; 424.1 | 15.3 |
| Unwashed | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| L* | 65.42 | 62.83 | 64.93 | 63.29 | 62.29 | 64.67 |
| a * | 1.80 | 2.86 | 2.80 | 2.95 | 3.15 | 2.83 |
| b * | 2.63 | −2.87 | −2.76 | −2.44 | −2.91 | −2.82 |
| ΔE * | - | 6.17 | 5.50 | 5.60 | 6.50 | 5.59 |
| Samples | UPF ± SD | UPF Classification | Average UVB Transmittance (%) | Average UVA Transmittance (%) |
|---|---|---|---|---|
| Soy | 7.3 ± 3.8 | +5 | 12.3 | 21.3 |
| Soy + AuNPs | 14.6 ± 2.4 | +10 | 6.2 | 8.2 |
| Soy + Chitosan | 62.2 ± 2.3 | +50 | 1.1 | 3.8 |
| Soy + Chitosan + AuNPs | 288.7 ± 0.1 | +50 | 0.2 | 0.5 |
| Samples | S. Aureus | E. Coli | ||
|---|---|---|---|---|
| % ± SD | log10 | % ± SD | log10 | |
| Soy | 51.79 ± 4.17 | 0.32 | 13.82 ± 8.27 | 0.06 |
| Soy+Chitosan | 97.02 ± 0.88 | 1.53 | 52.85 ± 9.86 | 0.33 |
| Soy+AuNPs | 98.70 ± 0.23 | 1.89 | 69.43 ± 10.7 | 0.51 |
| Soy+Chitosan+AuNPs | 99.94 ± 0.02 | 3.23 | 96.26 ± 0.51 | 1.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.S.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials 2019, 9, 1064. https://doi.org/10.3390/nano9081064
Silva IO, Ladchumananandasivam R, Nascimento JHO, Silva KKOS, Oliveira FR, Souto AP, Felgueiras HP, Zille A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials. 2019; 9(8):1064. https://doi.org/10.3390/nano9081064
Chicago/Turabian StyleSilva, Iris O., Rasiah Ladchumananandasivam, José Heriberto O. Nascimento, Késia Karina O.S. Silva, Fernando R. Oliveira, António P. Souto, Helena P. Felgueiras, and Andrea Zille. 2019. "Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles" Nanomaterials 9, no. 8: 1064. https://doi.org/10.3390/nano9081064