Homogeneous Embedding of Magnetic Nanoparticles into Polymer Brushes during Simultaneous Surface-Initiated Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
2.4. Synthesis of Poly(APTAC) Brushes
2.5. Synthesis of Poly(APTAC) Brushes with Embedded SPIONs (Poly(APTAC)+SPIONs)
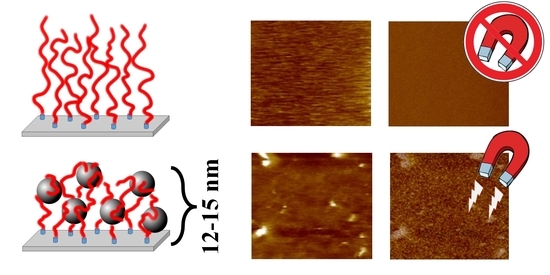
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Han, J.; Yang, B. Structural Fabrication and Functional Modulation of Nanoparticle–Polymer Composites. Adv. Funct. Mater. 2010, 20, 1533–1550. [Google Scholar] [CrossRef]
- Al-Hussein, M.; Koenig, M.; Stamm, M.; Uhlmann, P. The Distribution of Immobilized Platinum and Palladium Nanoparticles within Poly (2-vinylpyridine) Brushes. Macromol. Chem. Phys. 2014, 215, 1679–1685. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, K.; Chan-Park, M.B.; Wuc, H.; Wang, D.; Xu, R. Development of high refractive ZnS/PVP/PDMAA hydrogel nanocomposites for artificial cornea implants. Acta Biomater. 2014, 10, 1167–1176. [Google Scholar] [CrossRef]
- Zhu, X.; Loh, X.J. Layer-by-layer assemblies for antibacterial applications. Biomater. Sci. 2015, 3, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Kim, D.H. Nanoparticle polymer composites on solid substrates for plasmonic sensing applications. Nano Today 2016, 11, 415–434. [Google Scholar] [CrossRef]
- Yan, J.; Ye, Q.; Wang, X.; Yua, B.; Zhou, F. CdS/CdSe quantum dot co-sensitized graphene nanocomposites via polymer brush templated synthesis for potential photovoltaic applications. Nanoscale 2012, 4, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Long, D.X.; Choi, E.Y.; Noh, Y.Y. Manganese oxide nanoparticle as a new p-type dopant for high-performance polymer field-effect transistors. ACS Appl. Mater. Interfaces 2017, 9, 24763–24770. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.L.; Su, Y.K.; Chen, R.T. Hybrid light-emitting diodes from anthracene-contained polymer and CdSe/ZnS core/shell quantum dots. Nanoscale Res. Lett. 2014, 9, 611. [Google Scholar] [CrossRef]
- Basly, B.; Alnasser, T.; Aissou, K.; Fleury, G.; Pecastaings, G.; Hadziioannouu, G.; Duguet, E.; Goglio, G.; Mornet, S. Optimization of Magnetic Inks Made of L10-Ordered FePt Nanoparticles and Polystyrene-block-Poly(ethylene oxide) Copolymers. Langmuir 2015, 31, 6675–6680. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Li, G.; Wang, L.; Zhang, X. Nanocomposites of polymer brush and inorganic nanoparticles: Preparation, characterization and application. Polym. Chem. 2016, 7, 753–769. [Google Scholar] [CrossRef]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Douadi-Masrouki, S.; Frka-Petesic, B.; Save, M.; Charleux, B.; Cabuil, V.; Sandre, V. Incorporation of magnetic nanoparticles into lamellar polystyrene-b-poly-(n-butyl methacrylate) diblock copolymer films: Influence of the chain end-groups on nanostructuration. Polymer 2010, 51, 4673–4685. [Google Scholar] [CrossRef]
- Oren, R.; Liang, Z.; Barnard, J.S.; Warren, S.C.; Wiesner, U.; Huck, W.T.S. Organization of nanoparticles in polymer brushes. J. Am. Chem. Soc. 2009, 131, 1670–1671. [Google Scholar] [CrossRef]
- Choi, W.S.; Koo, H.Y.; Kim, J.Y.; Huck, W.T.S. Collective behavior of magnetic nanoparticles in polyelectrolyte brushes. Adv. Mater. 2008, 20, 4504–4508. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, J.; Wang, J.; Cui, F.; Chen, W.; Xu, F.; Wang, Z.; Zhang, K.; Yang, B. CdS-Nanoparticle/Polymer Composite Shells Grown on Silica Nanospheres by Atom-Transfer Radical Polymerization. Adv. Funct. Mater. 2005, 15, 481–486. [Google Scholar] [CrossRef]
- Benetti, E.M.; Sui, X.; Zapotoczny, S.; Vancso, G.J. Surface-Grafted Gel-Brush/Metal Nanoparticle Hybrids. Adv. Funct. Mater. 2010, 20, 939–944. [Google Scholar] [CrossRef]
- Aissou, K.; Fleury, G.; Pecastaings, G.; Alnasser, T.; Mornet, S.; Goglio, G.; Hadziioannou, G. Hexagonal-to-Cubic Phase Transformation in Composite Thin Films Induced by FePt Nanoparticles Located at PS/PEO Interfaces. Langmuir 2011, 27, 14481–14488. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, H.; Gao, L.; Xiao, G. Synthesis and Characterization of Film-forming Polymer/SiO2 Nanocomposite via Surfactant-Free Emulsion Polymerization. Asian J. Chem. 2013, 25, 5347–5350. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Kowal, J.; Zapotoczny, S. Self-templating surface-initiated polymerization: A route to synthesize conductive brushes. J. Mater. Chem. 2012, 22, 20179–20181. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Zapotoczny, S. Enhanced stability of conductive polyacetylene in ladder-like surface-grafted brushes. Polym. Chem. 2016, 7, 5664–5670. [Google Scholar] [CrossRef]
- Schüwer, N.; Klok, H.A. A potassium-selective quartz crystal microbalance sensor based on crown-ether functionalized polymer brushes. Adv. Mater. 2010, 22, 3251–3255. [Google Scholar] [CrossRef]
- Psarra, E.; Foster, E.; König, U.; You, J.; Ueda, Y.; Eichhorn, K.J.; Müller, M.; Stamm, M.; Revzin, A.; Uhlmann, P. Growth factor-bearing polymer brushes-versatile bioactive substrates influencing cell response. Biomacromolecules 2015, 16, 3530–3542. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Pomorska, A.; Uchacz, T.; Gut, A.; Łapok, Ł.; Zapotoczny, S. Photoactive Surface-Grafted Polymer Brushes with Phthalocyanine Bridging Groups as an Advanced Architecture for Light-Harvesting. Eur. J. Chem. 2017, 23, 11239–11243. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Kopeć, M.; Zapotoczny, S. Ordered photo- and electroactive thin polymer layers. Eur. Polym. J. 2015, 65, 155–170. [Google Scholar] [CrossRef]
- Taccola, S.; Pensabene, V.; Fujie, T.; Takeoka, S.; Pugno, N.M.; Mattoli, V. On the injectability of free-standing magnetic nanofilms. Biomed. Microdevices 2017, 19, 51. [Google Scholar] [CrossRef]
- Le Ouay, B.; Guldin, S.; Luo, Z.; Allegri, S.; Stellacci, F. Freestanding Ultrathin Nanoparticle Membranes Assembled at Transient Liquid–Liquid Interfaces. Adv. Mater. Interfaces 2016, 3, 1600191. [Google Scholar] [CrossRef]
- Boyaciyan, D.; Braun, L.; Löhmann, O.; Silvi, L.; Schneck, E.; von Klitzing, R. Gold nanoparticle distribution in polyelectrolyte brushes loaded at different pH conditions. J. Chem. Phys. 2018, 149, 163322. [Google Scholar] [CrossRef]
- Tamai, T.; Watanabe, M.; Ikeda, S.; Kobayashi, Y.; Fujiwara, Y.; Matsukawa, K.J. A Photocurable Pd Nanoparticle/Silica Nanoparticle/Acrylic Polymer Hybrid Layer for Direct Electroless Copper Deposition on a Polymer Substrate. J. Photopolym. Sci. Technol. 2012, 25, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Porel, S.; Singh, S.; Harsha, S.; Rao, D.N.; Radhakrishnan, T.P. Nanoparticle-embedded polymer: In situ synthesis, free-standing films with highly monodisperse silver nanoparticles and optical limiting. Chem. Mater. 2005, 17, 9–12. [Google Scholar] [CrossRef]
- Lachowicz, D.; Kaczyńska, A.; Wirecka, R.; Kmita, A.; Szczerba, W.; Bodzoń-Kułakowska, A.; Sikora, M.; Karewicz, A.; Zapotoczny, S. A Hybrid System for Magnetic Hyperthermia and Drug Delivery: SPION Functionalized by Curcumin Conjugate. Materials 2018, 11, 2388. [Google Scholar] [CrossRef]
- Szpak, A.; Kania, G.; Skórka, T.; Tokarz, W.; Zapotoczny, S.; Nowakowska, M. Stable aqueous dispersion of superparamagnetic iron oxide nanoparticles protected by charged chitosan derivatives. J. Nanopart. Res. 2013, 15, 1372. [Google Scholar] [CrossRef]
- Kania, G.; Kwolek, U.; Nakai, K.; Yusa, S.I.; Bednar, J.; Wójcik, T.; Chłopicki, S.; Skórka, T.; Szuwarzyński, M.; Szczubiałka, K.; et al. Stable polymersomes based on ionic–zwitterionic block copolymers modified with superparamagnetic iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 5523–5531. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative mapping of the elastic modulus of soft materials with HarmoniX and PeakForce QNM AFM modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Hall, D.J.; Tortorelli, P.F. Hardness and modulus measurements on oxide scales. Mater. High Temp. 1994, 12, 141–150. [Google Scholar] [CrossRef]
- Christau, S.; Möller, T.; Yenice, Z.; Genzer, J.; von Klitzing, R. Brush/gold nanoparticle hybrids: Effect of grafting density on the particle uptake and distribution within weak polyelectrolyte brushes. Langmuir 2014, 30, 13033–13041. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górka, W.; Kuciel, T.; Nalepa, P.; Lachowicz, D.; Zapotoczny, S.; Szuwarzyński, M. Homogeneous Embedding of Magnetic Nanoparticles into Polymer Brushes during Simultaneous Surface-Initiated Polymerization. Nanomaterials 2019, 9, 456. https://doi.org/10.3390/nano9030456
Górka W, Kuciel T, Nalepa P, Lachowicz D, Zapotoczny S, Szuwarzyński M. Homogeneous Embedding of Magnetic Nanoparticles into Polymer Brushes during Simultaneous Surface-Initiated Polymerization. Nanomaterials. 2019; 9(3):456. https://doi.org/10.3390/nano9030456
Chicago/Turabian StyleGórka, Weronika, Tomasz Kuciel, Paula Nalepa, Dorota Lachowicz, Szczepan Zapotoczny, and Michał Szuwarzyński. 2019. "Homogeneous Embedding of Magnetic Nanoparticles into Polymer Brushes during Simultaneous Surface-Initiated Polymerization" Nanomaterials 9, no. 3: 456. https://doi.org/10.3390/nano9030456
APA StyleGórka, W., Kuciel, T., Nalepa, P., Lachowicz, D., Zapotoczny, S., & Szuwarzyński, M. (2019). Homogeneous Embedding of Magnetic Nanoparticles into Polymer Brushes during Simultaneous Surface-Initiated Polymerization. Nanomaterials, 9(3), 456. https://doi.org/10.3390/nano9030456