Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Pretreatment on ABS Substrate
2.2. Catalytic Activity for H2O2 Decomposition
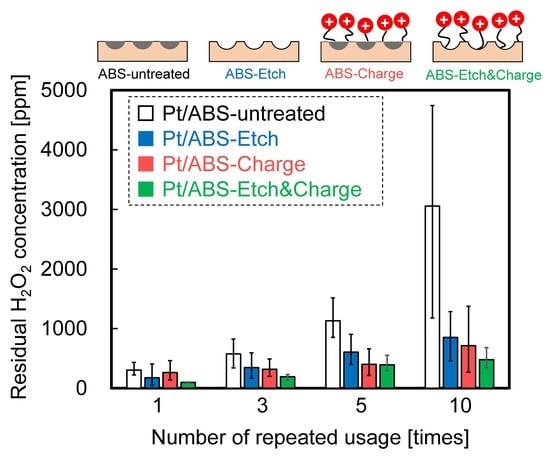
2.3. Catalytic Durability in H2O2 Decomposition
3. Materials and Methods
3.1. Pretreatment and Synthesis of Pt-Particle/ABS
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muntz, A.; Subbaraman, L.N.; Sorbara, L.; Jones, L. Tear exchange and contact lenses: A review. J. Optom. 2015, 8, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musgrave, C.S.A.; Fang, F. Contact lens materials: A materials science perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.J. Continuing upward trends in daily disposable prescribing and other key segments maintained a healthy industry. Contact Lens Spectr. 2018, 33, 20–25. [Google Scholar]
- Paugh, J.R.; Brennan, N.A.; Efron, N. Ocular response to hydrogen peroxide. Am. J. Optom. Physiol. Opt. 1988, 65, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Radiolytic synthesis of Pt-particle/ABS catalysts for H2O2 decomposition in contact lens cleaning. Nanomaterials 2017, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Carberry, J.J.; Fleisch, T.H.; Wolf, E.E. CO oxidation activity and XPS studies of PtCu γ-Al2O3 bimetallic catalysts. J. Catal. 1982, 74, 307–316. [Google Scholar] [CrossRef]
- Mohamed, R.M. Characterization and catalytic properties of nano-sized Pt metal catalyst on TiO2-SiO2 synthesized by photo-assisted deposition and impregnation methods. J. Mater. Process. Technol. 2009, 209, 577–583. [Google Scholar] [CrossRef]
- Rahsepar, M.; Pakshir, M.; Piao, Y.; Kim, H. Preparation of highly active 40 wt % Pt on multiwalled carbonnanotube by improved impregnation method for fuel cell applications. Fuel Cells 2012, 12, 827–834. [Google Scholar] [CrossRef]
- Toshima, N.; Wang, Y. Preparation and catalysis of novel colloidal dispersions of copper/noble metal bimetallic clusters. Langmuir 1994, 10, 4574–4580. [Google Scholar] [CrossRef]
- Toshima, N.; Wang, Y. Polymer-protected Cu/Pd bimetallic clusters. Adv. Mater. 1994, 6, 245–247. [Google Scholar] [CrossRef]
- Daimon, H.; Kurobe, Y. Size reduction of PtRu catalyst particle deposited on carbon support by addition of non-metallic elements. Catal. Today 2006, 111, 182–187. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of platinum nanoparticles by sonochemical reduction of the Pt(II) ion. Langmuir 1999, 15, 2733–2737. [Google Scholar] [CrossRef]
- Nitani, H.; Yuya, M.; Ono, T.; Nakagawa, T.; Seino, S.; Okitsu, K.; Mizukoshi, Y.; Emura, S.; Yamamoto, T.A. Sonochemically synthesized core-shell structured Au-Pd nanoparticles supported on γ-Fe2O3 particles. J. Nanopart. Res. 2006, 8, 951–958. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Tsuru, Y.; Tominaga, A.; Seino, S.; Masahashi, N.; Tanabe, S.; Yamamoto, T.A. Sonochemical immobilization of noble metal nanoparticles on the surface of maghemite: Mechanism and morphological control of the products. Ultrason. Sonochem. 2008, 15, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Supporting of pristine TiO2 with noble metals to enhance the oxidation and mineralization of paracetamol by sonolysis and sonophotolysis. Appl. Catal. B Environ. 2015, 172–173, 7–17. [Google Scholar] [CrossRef]
- Seino, S.; Kinoshita, T.; Nakagawa, T.; Kojima, T.; Taniguchi, R.; Okuda, S.; Yamamoto, T.A. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high energy electron beam. J. Nanopart. Res. 2008, 10, 1071–1076. [Google Scholar] [CrossRef]
- Kageyama, S.; Sugano, Y.; Hamaguchi, Y.; Kugai, J.; Ohkubo, Y.; Seino, S.; Nakagawa, T.; Ichikawa, S.; Yamamoto, T.A. Pt/TiO2 composite nanoparticles synthesized by electron beam irradiation for preferential CO oxidation. Mater. Res. Bull. 2013, 48, 1347–1351. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Seino, S.; Kageyama, S.; Kugai, J.; Nakagawa, T.; Ueno, K.; Yamamoto, T.A. Effect of decrease in the size of Pt nanoparticles using sodium phosphinate on electrochemically active surface area. J. Nanopart. Res. 2014, 16, 2237. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kageyama, S.; Seino, S.; Nakagawa, T.; Kugai, J.; Ueno, K.; Yamamoto, T.A. Mass production of highly loaded and highly dispersed PtRu/C catalysts for methanol oxidation using an electron-beam irradiation reduction method. J. Exp. Nanosci. 2016, 11, 123–137. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: Application to catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Kanao, Y. Japan Patent P2010-159457A. Available online: https://www.j-platpat.inpit.go.jp/web/PU/JPA_H22159457/57BCE0F5B3F81A184B6B6C63C93F1815 (accessed on 22 July 2010).









| Sample ID | Etching | Surface Charge Modification | EBIRM (EB Irradiation) |
|---|---|---|---|
| ABS-untreated | ― | ― | ― |
| ABS-Etch | 〇 | ― | ― |
| ABS-Charge | ― | 〇 | ― |
| ABS-Etch&Charge | 〇 | 〇 | ― |
| Pt/ABS-untreated | ― | ― | 〇 |
| Pt/ABS-Etch | 〇 | ― | 〇 |
| Pt/ABS-Charge | ― | 〇 | 〇 |
| Pt/ABS-Etch&Charge | 〇 | 〇 | 〇 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2019, 9, 342. https://doi.org/10.3390/nano9030342
Ohkubo Y, Aoki T, Seino S, Mori O, Ito I, Endo K, Yamamura K. Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials. 2019; 9(3):342. https://doi.org/10.3390/nano9030342
Chicago/Turabian StyleOhkubo, Yuji, Tomonori Aoki, Satoshi Seino, Osamu Mori, Issaku Ito, Katsuyoshi Endo, and Kazuya Yamamura. 2019. "Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning" Nanomaterials 9, no. 3: 342. https://doi.org/10.3390/nano9030342