Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template
Abstract
:1. Introduction
2. Materials and Methods
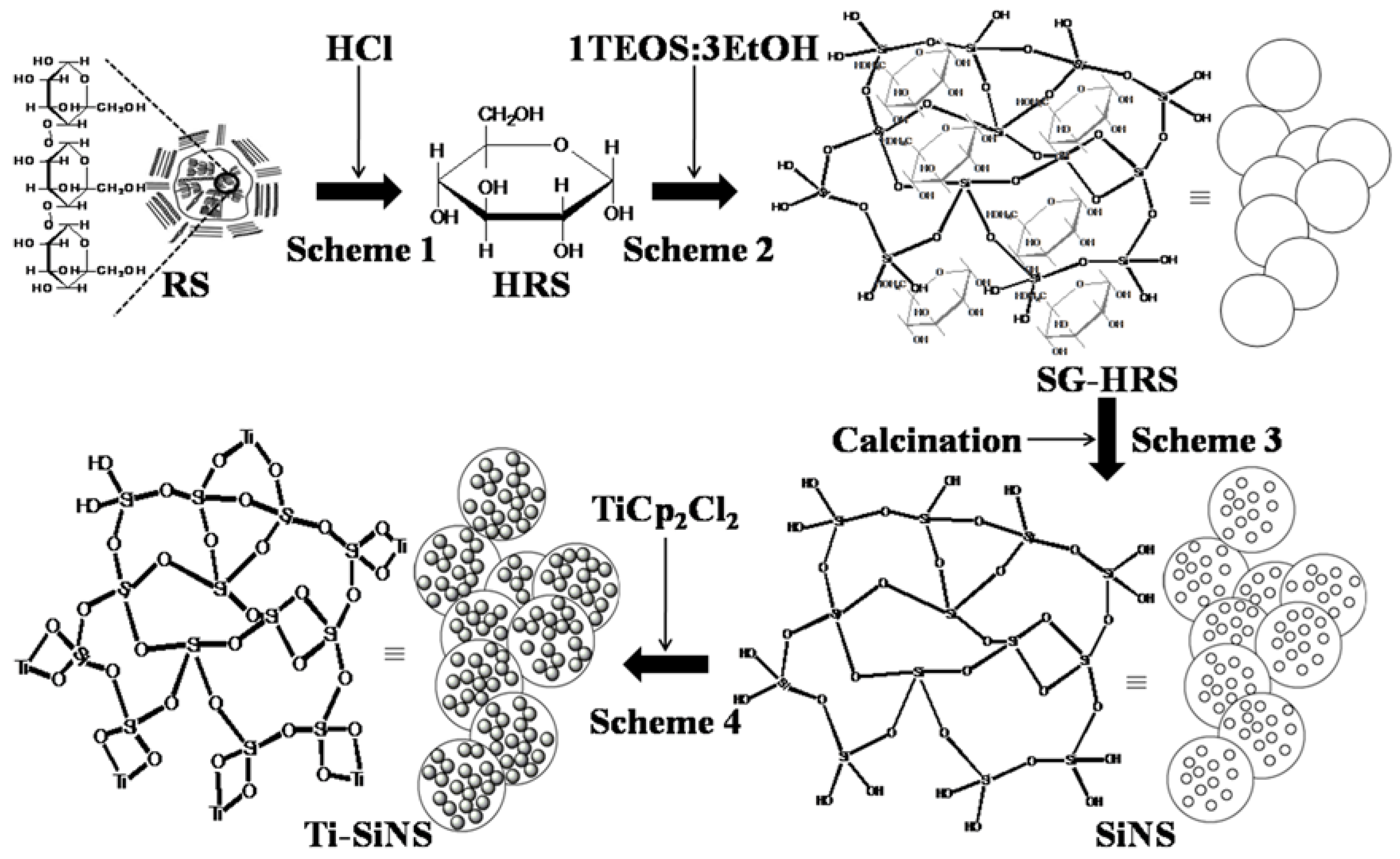
2.1. Preparation of Nanostructured-Silica (SiNS) and Nanostructured Titania-Silica (Ti-SiNS)
2.2. Characterization
2.3. Catalytic Experiment
3. Results and Discussion
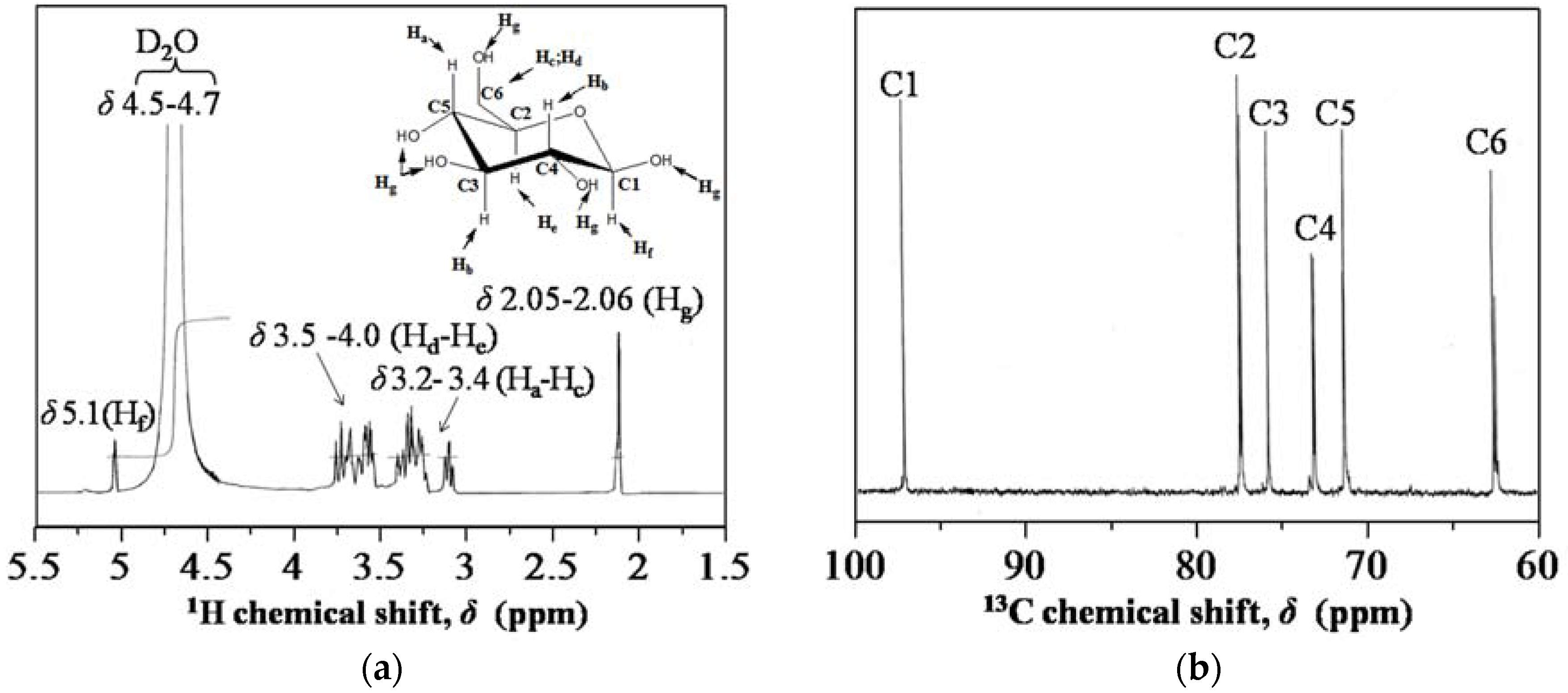
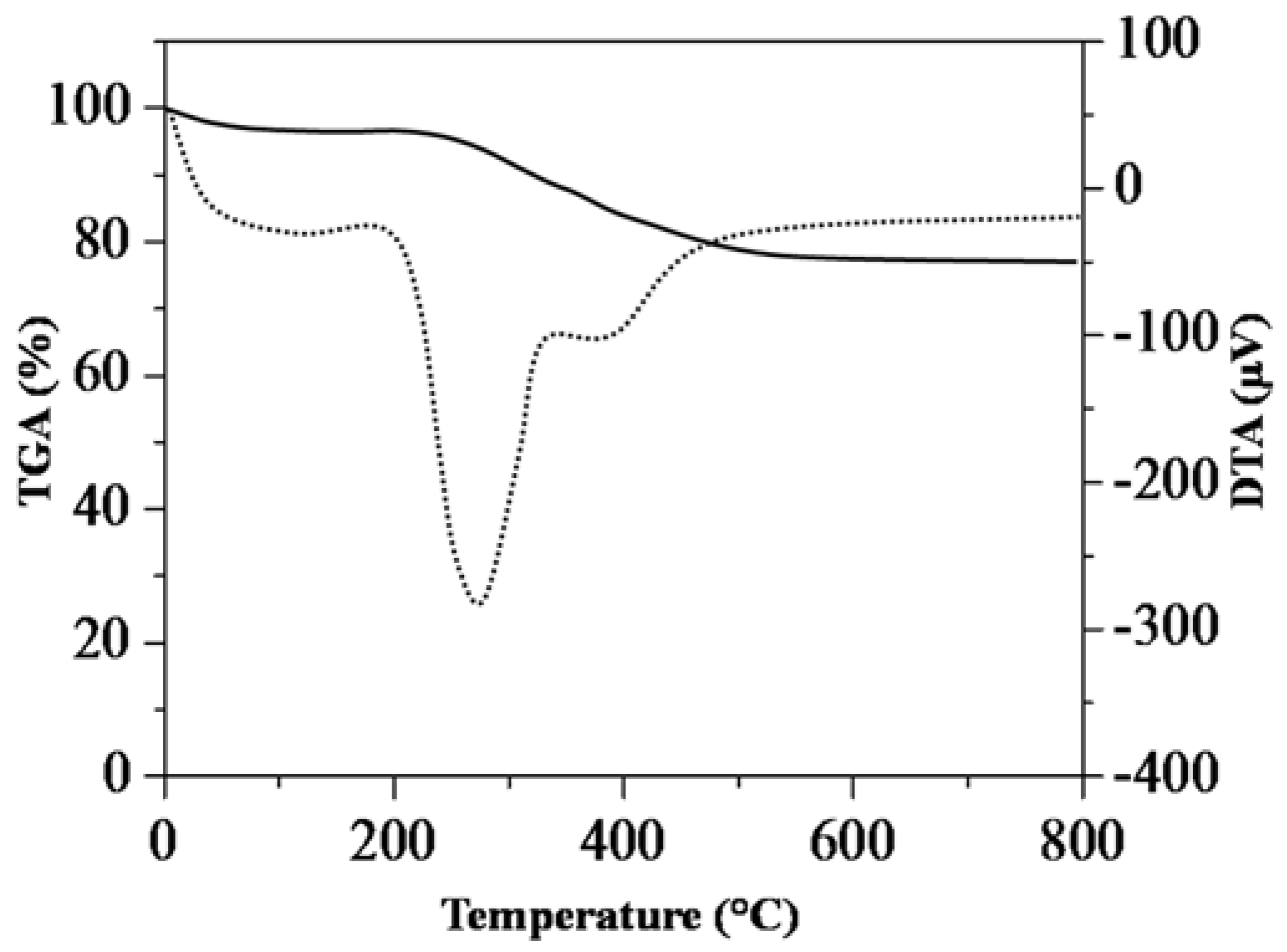
3.1. Characterization of Hydrolysis of Rice Starch (HRS) and SG-HRS
3.2. Characterization of SiNS
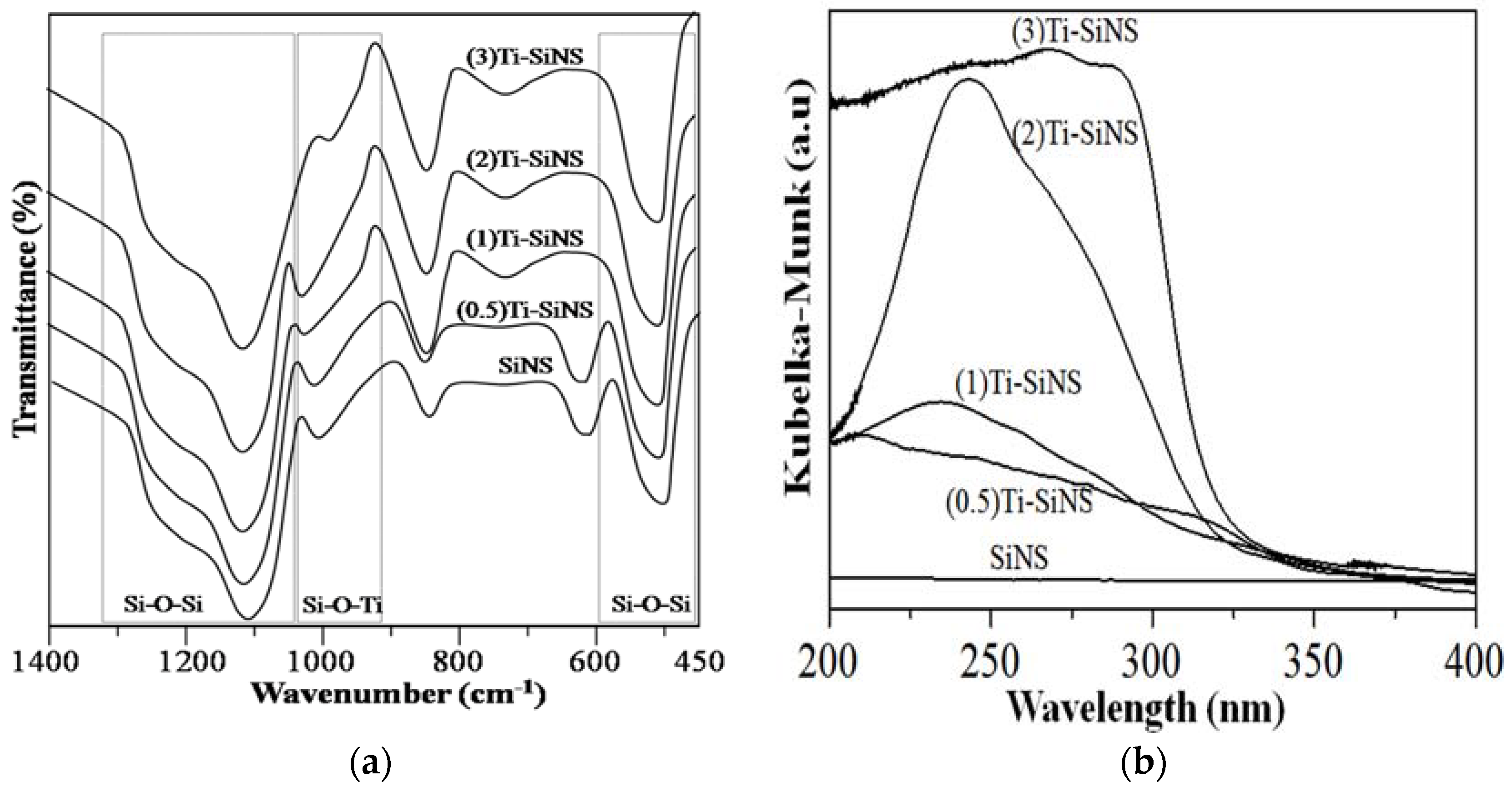
3.2.1. Fourier Transform Infrared (FTIR) Study
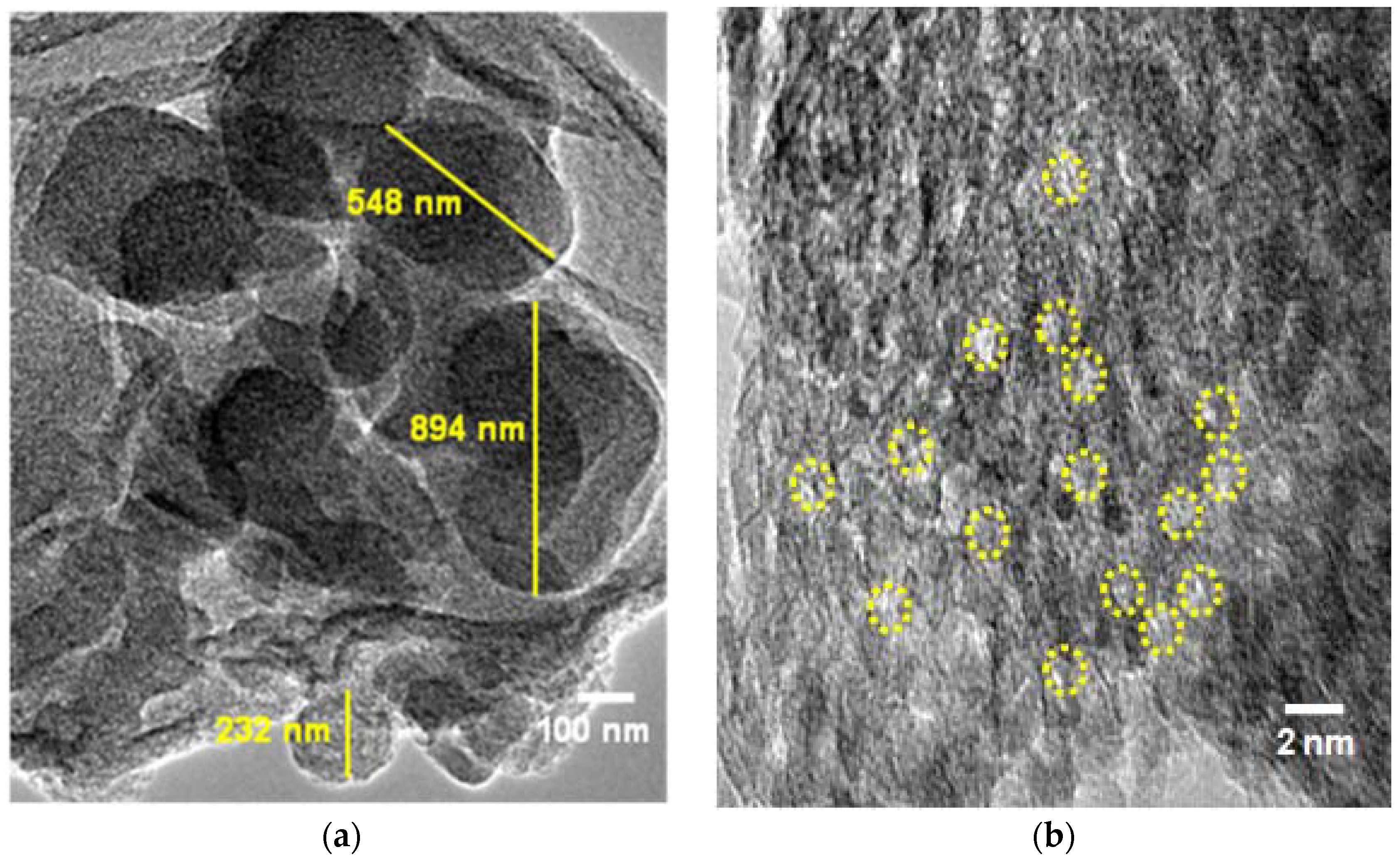
3.2.2. Electron Microscopy
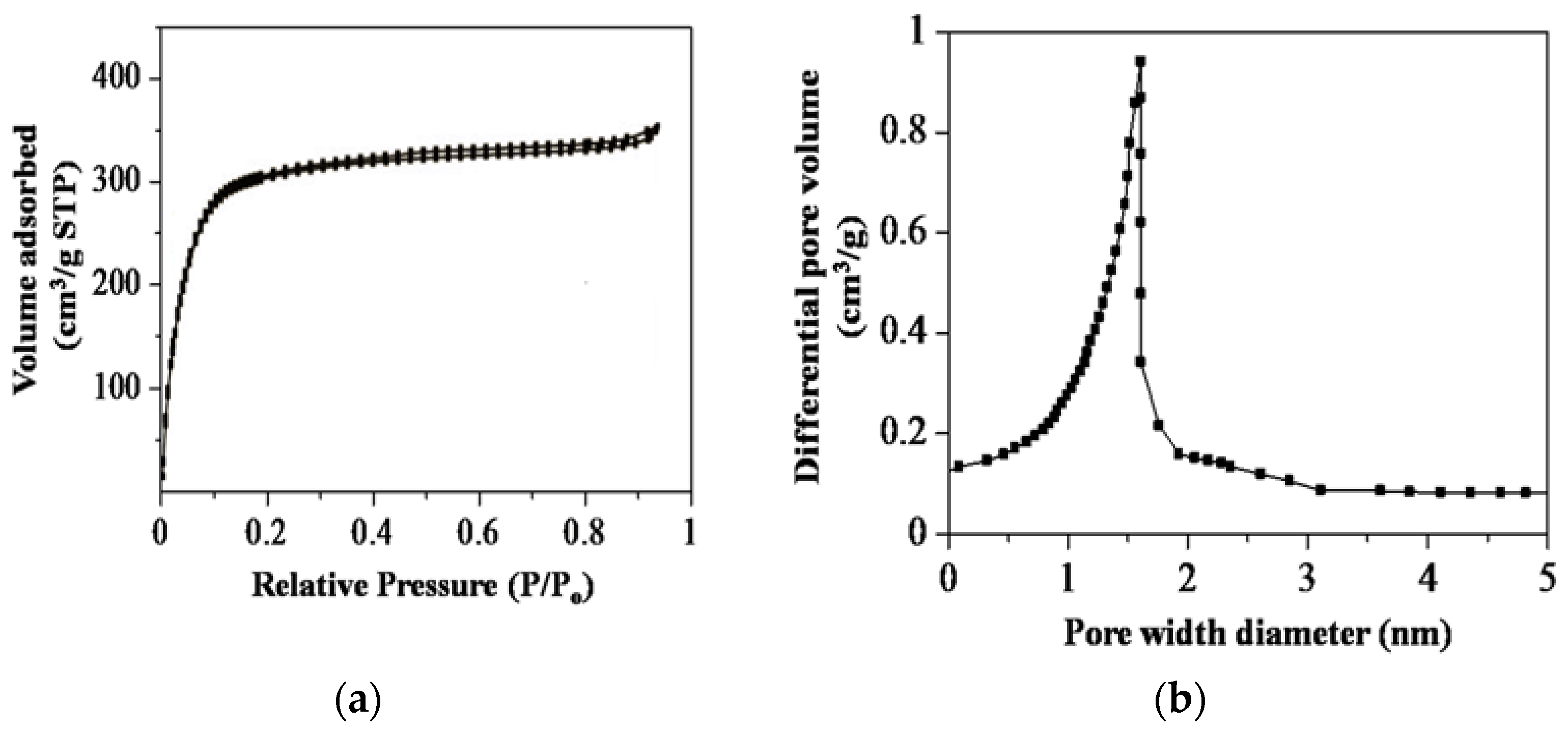
3.2.3. Physisorption Measurements
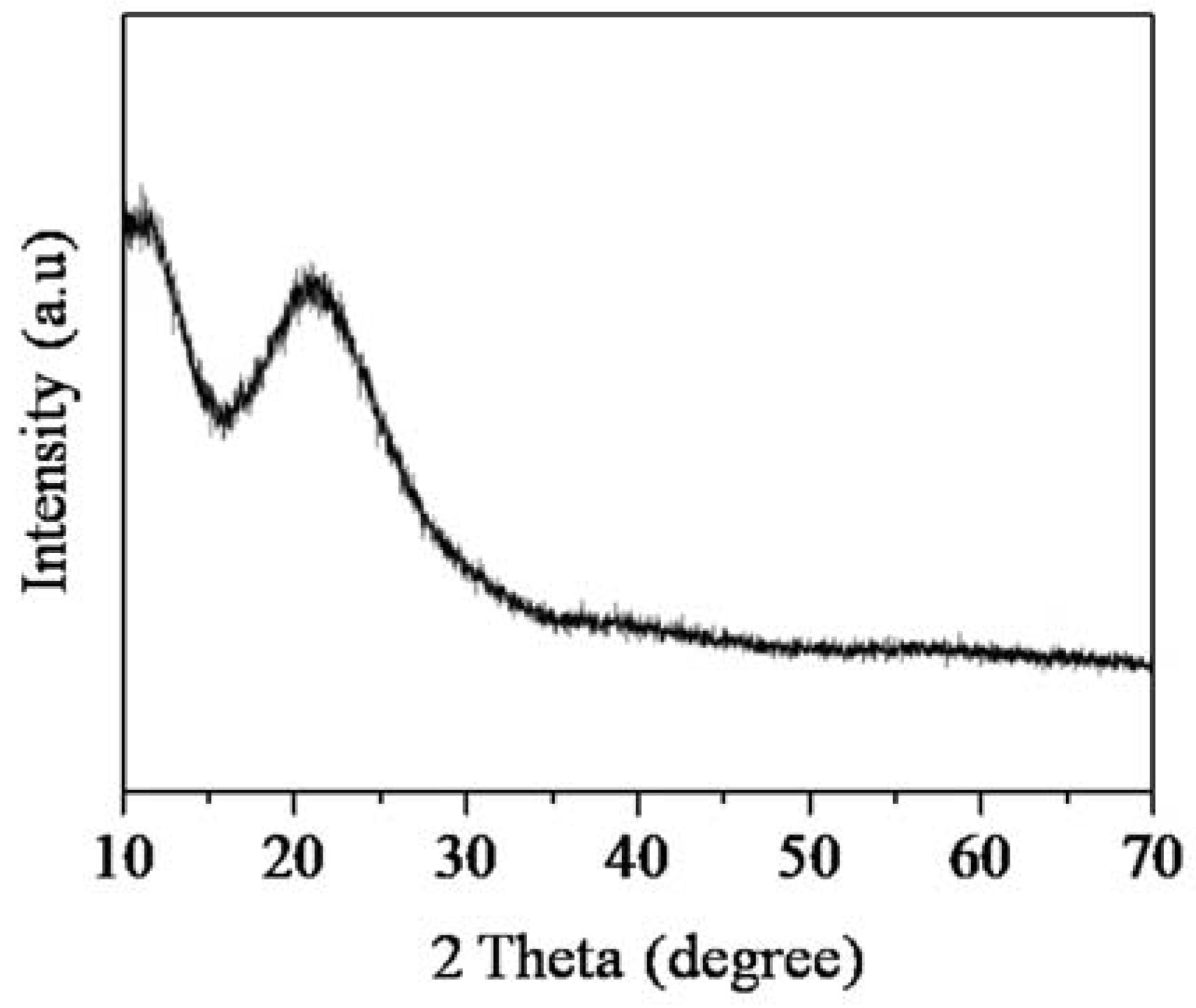
3.2.4. X-ray Diffraction (XRD) Analysis
3.3. Characterization of Ti-SiNS
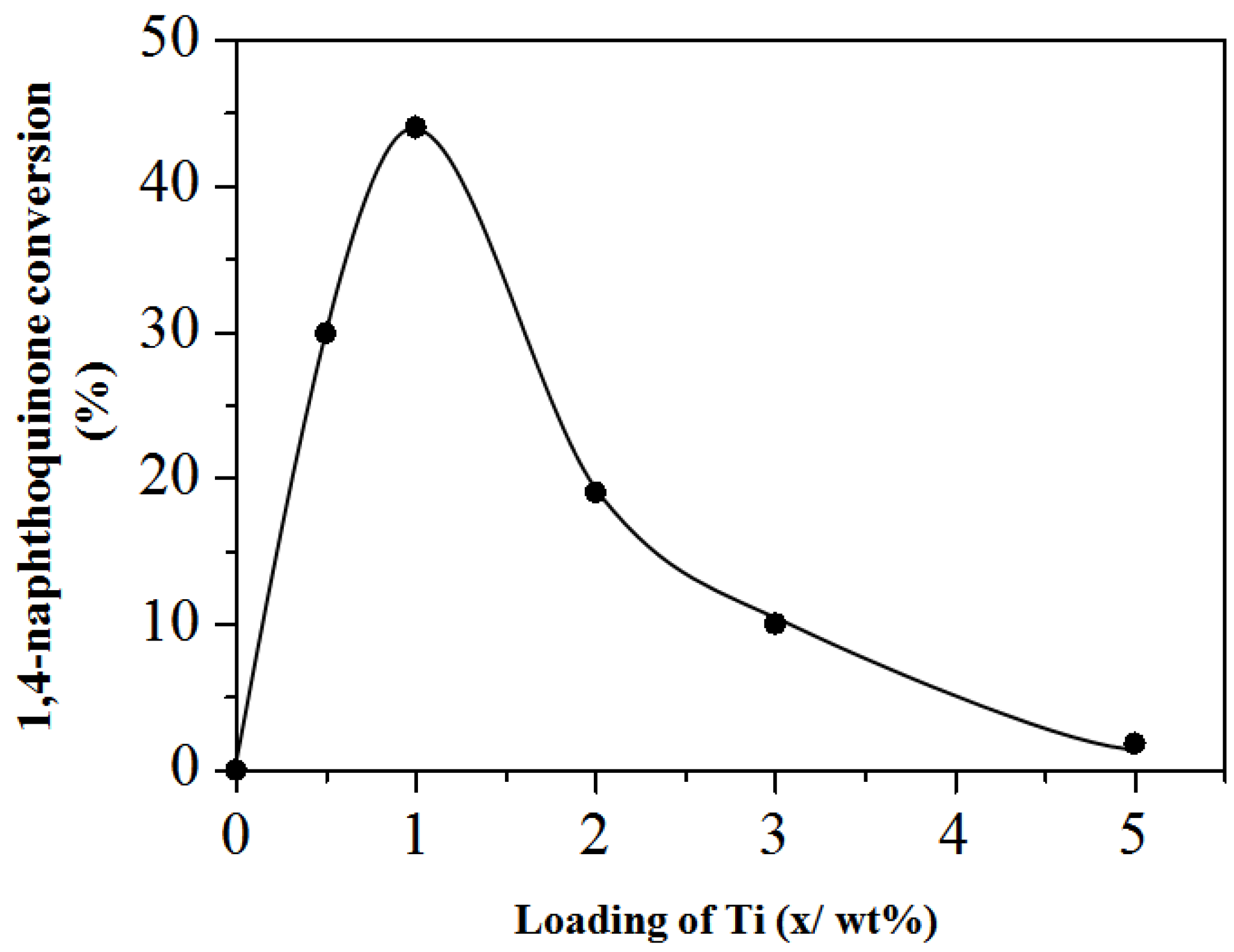
Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Oleske, K.W.; Barteau, K.P.; Turker, M.Z.; Beaucage, P.A.; Estroff, L.A.; Wiesner, U. Block Copolymer Directed Nanostructured Surfaces as Templates for Confined Surface Reactions. Macromolecules 2017, 50, 542–549. [Google Scholar] [CrossRef]
- Huczko, A. Template-based Synthesis of Nanomaterials. Appl. Phys. A 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Martin, C.R. A General Template-Based Method for the Preparation of Nanomaterials. J. Mater. Chem. 1997, 7, 1075–1087. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Wet-Chemical Synthesis and Applications of Non-Layer Structured Two-Dimensional Nanomaterials. Nat. Commun. 2015, 6, 7873. [Google Scholar] [CrossRef] [PubMed]
- Epps, T.H., III; O’Reilly, R.K. Block Copolymers: Controlling Nanostructure to Generate Functional Materials–Synthesis, Characterization, and Engineering. Chem. Sci. 2016, 7, 1674–1689. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.; Li, X.; Wang, J. Silica-Based Nanocapsules: Synthesis, Structure Control and Biomedical Applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, M.; Sathishkumar, Y.; Lee, Y.S.; Yoo, D.J.; Kim, A.R. Ni–Co Alloy Nanostructures Anchored on Mesoporous Silica Nanoparticles for Non-Enzymatic Glucose Sensor Applications. RSC Adv. 2015, 5, 57804–57814. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Zhang, H. Synthesis, Functionalization, and Applications of Morphology-Controllable Silica-Based Nanostructures: A Review. Prog. Solid State Chem. 2016, 44, 1–19. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactant-templated Nanomaterials Synthesis. J. Colloid Interface Sci. 2004, 274, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Linssen, T.; Cassiers, K.; Cool, P.; Vansant, E.F. Mesoporous Templated Silicates: An Overview of their Synthesis, Catalytic Activation and Evaluation of the Stability. Adv. Colloid Interface Sci. 2003, 103, 121–147. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Yoo, J.C.; Pruski, M.; Lin, V.S.Y. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Penfold, J.; Staples, E.; Thompson, L.; Tucker, I.; Hines, J.; Thomas, R.K.; Lu, J.R.; Warren, N. Structure and Composition of Mixed Surfactant Micelles of Sodium Dodecyl Sulfate and Hexaethylene Glycol Monododecyl Ether and of Hexadecyltrimethylammonium Bromide and Hexaethylene Glycol Monododecyl Ether. J. Phys. Chem. B 1999, 103, 5204–5211. [Google Scholar] [CrossRef]
- El-Safty, S.A. Instant Synthesis of Mesoporous Monolithic Materials with Controllable Geometry, Dimension and Stability: A Review. J. Porous Mater. 2011, 18, 259–287. [Google Scholar] [CrossRef]
- Patarin, J.; Lebeau, B.; Zana, R. Recent Advances in the Formation Mechanisms of Organized Mesoporous Materials. Curr. Opin. Colloid Interface Sci. 2002, 7, 107–115. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An Overview of Global Rice Production, Supply, Trade, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Zhang, B.; Davis, S.A.; Mann, S. Starch Gel Templating of SpongelikeMacroporousSilicalite Monoliths and Mesoporous Films. Chem. Mater. 2002, 14, 1369–1375. [Google Scholar] [CrossRef]
- Shi, W.; Liang, P.; Ge, D.; Wang, J.; Zhang, Q. Starch-Assisted Synthesis of Polypyrrole Nanowires by a Simple Electrochemical Approach. Chem. Commun. 2007, 23, 2414–2416. [Google Scholar] [CrossRef]
- Qiao, Y.; Lin, Y.; Yang, Z.; Chen, H.; Zhang, S.; Yan, Y.; Huang, J. Unique Temperature-Dependent Supramolecular Self-Assembly: From Hierarchical 1D Nanostructures to Super Hydrogel. J. Phys. Chem. B 2010, 114, 11725–11730. [Google Scholar] [CrossRef] [PubMed]
- Koohsaryan, E.; Anbia, M. Nanosized and Hierarchical Zeolites: A Short Review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Hu, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, S. Development of a Novel Starch-Derived Porous Silica Monolith for Enhancing the Dissolution Rate of Poorly Water Soluble Drug. Mater. Sci. Eng. C 2012, 32, 201–206. [Google Scholar] [CrossRef]
- Du, X.; He, J. Spherical Silica Micro/Nanomaterials with Hierarchical Structures: Synthesis and Applications. Nanoscale 2011, 3, 3984–4002. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.C.; Isabettini, S.; Verboekend, D.; Rodrigues, E.G.; Pérez-Ramírez, J. Hierarchical High-Silica Zeolites as Superior Base Catalysts. Chem. Sci. 2014, 5, 677–684. [Google Scholar] [CrossRef]
- Ryoo, R.; Ko, C.H.; Kruk, M.; Antochshuk, V.; Jaroniec, M. Block-Copolymer-Templated Ordered Mesoporous Silica: Array of Uniform Mesopores or Mesopore−Micropore Network? J. Phys. Chem. B 2000, 104. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and Functional Properties of Rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and Analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.J.; Mark, H.B., Jr.; Reilley, C.N. Simultaneous Spectrophotometric Determination of Fructose and Glucose Mixtures by Differential Reaction Rates. Application to Blood Serum Analysis. Anal. Chem. 1962, 34, 1443–1446. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Studies on MCM-41: Effect of Sulfate on Nitration of Phenol. J. Mol. Catal. A-Chem. 2006, 258, 381–387. [Google Scholar] [CrossRef]
- Jermy, B.R.; Pandurangan, A. Al-MCM-41 as an Efficient Heterogeneous Catalyst in the Acetalization of Cyclohexanone with Methanol, Ethylene Glycol and Pentaerythritol. J. Mol. Catal. A-Chem. 2006, 256, 184–192. [Google Scholar] [CrossRef]
- Palani, A.; Gokulakrishnan, N.; Palanichamy, M.; Pandurangan, A. Transesterification of Dimethyl Carbonate with Diethyl Carbonate over Al-Zn-MCM-41 and Al-MCM-41 Molecular Sieves. Appl. Catal. A Appl. 2006, 304, 152–158. [Google Scholar] [CrossRef]
- Martínez, A.; Haavik, J.; Flatmark, T.; Arrondo, J.L.R.; Muga, A. Conformational Properties and Stability of Tyrosine Hydroxylase Studied by Infrared Spectroscopy Effect of Iron/Catecholamine Binding and Phosphorylation. J. Biol. Chem. 1996, 271, 19737–19742. [Google Scholar] [CrossRef] [PubMed]
- De Man, A.J.; Sauer, J. Coordination, Structure, and Vibrational Spectra of Titanium in Silicates and Zeolites in Comparison with Related Molecules. An Ab Initio Study. J. Phys. Chem. A 1996, 100, 5025–5034. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Soliman, E.A.; Furuta, M. Influence of Phase Behavior and Miscibility on Mechanical, Thermal and Micro-Structure of Soluble Starch-Gelatin Thermoplastic Biodegradable Blend Films. Food Nutr. Sci. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Wheelwright, W.; Cooney, R.P.; Ray, S.; Zujovic, Z.; de Silva, K. Ultra-High Surface Area Nano-Porous Silica from Expanded Perlite: Formation and Characterization. Ceram. Int. 2017, 43, 11495–11504. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of Micro-and mesoporous Solids by Physisorption Methods and Pore-Size Analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A Facile Method for Production of High-Purity Silica Xerogels from Bagasse Ash. Adv. Powder Technol. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Ding, T.; Shih, W.H.; Liu, X.; Cheng, S.Z.; Fu, Q. A Non-Surfactant Templating Route to Mesoporous Silica Materials. Adv. Mater. 1998, 10, 313–316. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and Condensation of Silicates: Effects on Structure. J. Non-Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Jung, J.H.; Kobayashi, H.; Masuda, M.; Shimizu, T.; Shinkai, S. Helical Ribbon Aggregate Composed of a Crown-Appended Cholesterol Derivative which Acts as an Amphiphilic Gelator of Organic Solvents and as a Template for Chiral Silica Transcription. J. Am. Chem. Soc. 2001, 123, 8785–8789. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, D.M. Synthesis of Phosphorus-Free Mesoporous Titania via Templating with Amine Surfactants. Microporous Mesoporous Mater. 1999, 30, 315–319. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of Mesoporous Molecular Sieves by Nonionic Polyethylene Oxide Surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Tanev, P.T.; Pinnavaia, T.J. Mesoporous Silica Molecular Sieves Prepared by Ionic and Neutral Surfactant Templating: A Comparison of Physical Properties. Chem. Mater. 1996, 8, 2068–2079. [Google Scholar] [CrossRef]
- Gao, H.; Lu, W.; Chen, Q. Characterization of Titanium Silicalite-1 Prepared from Aqueous TiCl3. Microporous Mesoporous Mater. 2000, 34, 307–315. [Google Scholar] [CrossRef]
- Trukhan, N.N.; Panchenko, A.A.; Roduner, E.; Mel’guno, M.S.; Kholdeeva, O.A.; Mrowiec-Białoń, J.; Jarzebski, A.B. FTIR Spectroscopic Study of Titanium-Containing Mesoporous Silicate Materials. Langmuir 2005, 21, 10545–10554. [Google Scholar] [CrossRef] [PubMed]
- Marchese, L.; Gianotti, E.; Dellarocca, V.; Maschmeyer, T.; Rey, F.; Coluccia, S.; Thomas, J.M. Structure–Functionality Relationships of Grafted Ti-MCM41 Silicas. Spectroscopic and Catalytic Studies. Phys. Chem. Chem. Phys. 1999, 1, 585–592. [Google Scholar] [CrossRef]
- Oldroyd, R.D.; Thomas, J.M.; Maschmeyer, T.; MacFaul, P.A.; Snelgrove, D.W.; Ingold, K.U.; Wayner, D.D. The Titanium (IV)-Catalyzed Epoxidation of Alkenes by Tert-Alkyl Hydroperoxides. Angew. Chem. Int. Ed. 1996, 35, 2787–2790. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]


| Elements | Atomic Percentage |
|---|---|
| Silica | 47.31 |
| Carbon | 30.65 |
| Oxygen | 19.27 |
| Titanium | 2.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matmin, J.; Affendi, I.; Endud, S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials 2018, 8, 514. https://doi.org/10.3390/nano8070514
Matmin J, Affendi I, Endud S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials. 2018; 8(7):514. https://doi.org/10.3390/nano8070514
Chicago/Turabian StyleMatmin, Juan, Irwan Affendi, and Salasiah Endud. 2018. "Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template" Nanomaterials 8, no. 7: 514. https://doi.org/10.3390/nano8070514






